Abstract
We used three-colour cytometry to analyse intracellular cytokine production in activated whole blood cultures derived from patients with HIV-1 infection. We assessed mitogen-induced IL-2, IL-4 and IFN-γ production from T cells as possible markers of immune dysfunction. The percentages of T cells staining for IL-2 were significantly reduced in stimulated cultures from HIV+ individuals relative to normal controls (P < 0.0001); this reduction was observed in both the CD4+ and the CD8+ subsets. IL-2 production was significantly reduced in CD4+ T cells from HIV+ individuals clinically classified as symptomatics compared with HIV+ asymptomatics (P < 0.001); in addition, production of IL-2 inversely correlated with viral load (r2 = 0.832). On the other hand, HIV+ individuals showed significantly more T cells staining positive for IFN-γ (P < 0.0001); subset analysis identified these T cells as CD8+. Increased IFN-γ production in the CD8+ T cell subset of HIV+ individuals correlated neither with clinical status nor with plasma viral load. IL-4 staining in activated T cells was low (< 5%) and no differences were observed between HIV+ and control groups. Three-colour FACS analysis of whole blood provides a sensitive, rapid and relatively easy means to detect cytokine profiles within T cell subpopulations. Only small volumes of blood are required (0.5 ml), since there is no need for cell isolation, making it more practical than ELISA or reverse transcriptase-polymerase chain reaction (RT-PCR) for the analysis of immune function in HIV+ individuals. This technique could therefore play a role in mapping the dynamics and extent of immune recovery in AIDS patients undergoing triple combination therapy.
Keywords: HIV, cell-mediated immunity, cytokines, flow cytometry, whole blood cultures
INTRODUCTION
Infection with HIV is associated with changes in host cell-mediated immunity which can be identified by culturing cells from infected individuals in vitro. T helper cell hyporesponsiveness is manifested in vitro by a sequential loss of proliferative responses to recall antigen, allo-antigen and mitogen [1]; strong cytotoxic T lymphocyte (CTL) responses to HIV-derived peptides in vitro are thought to correlate with protective cell-mediated immunity [2], and disease progression is marked by increased levels of spontaneous and activation-induced programmed cell death [3].
Peripheral blood mononuclear cells (PBMC) isolated from HIV-infected individuals also exhibit imbalanced production of cytokines associated with cell-mediated immunity. For example, reduced IL-2 production has been described both with [4, 5] and without [6, 7] prior in vitro stimulation. Also, raised mitogen-induced IFN-γ production has been reported in HIV infection [8], and flares of IFN-γ have been associated with oligoclonal anti-viral CTL activity during the acute phase of HIV infection [9, 10]. Conversely, others have described a decreased ability of PBMC from infected individuals to produce IFN-γ [5, 11].
Levels of cytokines can be measured directly either from unstimulated samples or in stimulated cultures. Although some might argue that the latter method is unnecessary and may bias the results by ‘artificially’ inducing cytokine production, levels of cytokines in serum are relatively low and are more easily affected by diurnal fluctuations in immune activity. Furthermore, cytokines, in contrast to hormones, for example, are soluble factors which generally exert their effects in the local cellular environment; in vitro stimulation of T cells hopefully gives a better picture of how T cells would react in terms of cytokine production when they are activated in vivo.
Most data on cytokine responses obtained to date have coupled in vitro mitogenic stimulation of PBMC with reverse transcriptase-polymerase chain reaction (RT-PCR) analysis (to show induction of cytokine messenger RNA) or ELISA (to detect cytokine release). However, there are significant drawbacks associated with such techniques. First, RT-PCR analysis requires PBMC isolation, precluding the analysis of small blood volumes. Second, translational control mechanisms are involved in regulating cytokine gene expression, as has been demonstrated for IL-2 [12], meaning that the levels of cytokine RNA may not accurately reflect the amount of protein synthesized. Third, cytokine levels measured by ELISA are net of protein synthesis, consumption and biodegradation. The use of multiparameter flow cytometry enables simultaneous labelling of samples to surface antigens and intracellular molecules, thus distinguishing the cytokine responses of phenotypically distinct populations within the same sample. We therefore decided to use three-colour flow cytometry to determine the primary cytokine responses to mitogen in CD4+ and CD8+ T cell populations from HIV+ and control individuals. To circumvent the problems associated with PBMC isolation, we stained activated whole blood cultures. We compared the results obtained with this technique with those obtained from ELISA analysis of supernatants from whole blood stimulated ex vivo.
Using these techniques we show that there is an abnormal response of T cells in whole blood from HIV-infected individuals to ex vivo stimulation in terms of cytokine production: altered cytokine responses can be detected in both CD4+ and CD8+ T cell subpopulations. These results are consistent with the hypothesis that HIV infection is associated with hyporesponsiveness in the T helper cell subset and a generalized state of activation in the CD8+ T cell subset. Thus, this relatively simple method using unfractionated whole blood provides a useful tool for studying immune dysfunction in HIV infection.
MATERIALS AND METHODS
Source of samples
Venous blood was collected in preservative-free heparin from 20 HIV-1+ individuals who gave their informed consent and were attending the Genito-Urinary Clinic at St George's (mean age 35 years; range 25–53 years). Of these, 15 were heterosexual men and women from Sub-Saharan Africa, whilst the other five were homosexual Caucasian men. Four patients had CD4 counts < 200/μl (mean 135/μl; range 10–190/μl), 11 had CD4 counts 200–499/μl (mean 299/μl; range 200–460/μl) and five had CD4 counts > 500/μl (mean 704/μl; range 500–960/μl). One patient was receiving antiretroviral therapy (AZT, ddI) at the time of analysis. Twenty age-matched laboratory volunteers (mean age 35 years; range 24–69 years) were studied in parallel.
Viral loads
Viral loads were also measured at the time of sample collection in 18 of the 20 HIV+ individuals using a commercially available RT-PCR kit from Roche Molecular Systems (Amplicor kit; Basel, Switzerland); the results are expressed as number of HIV RNA viral copies per ml of serum. Viral RNA was amplified using Version 1.5 primers (Roche Molecular Systems) which detect HIV-1 group M viruses (clades A–G). The threshold of the kit was 400 HIV copies/ml. Samples which fell below this detection limit were assigned a value of 200 to permit statistical analysis.
Monoclonal antibodies
MoAbs Leu-2a (CD8–FITC), Leu-4 (CD3–PerCP), clone 5344.11 (Anti-Hu IL-2 PE), clone 3010.21 (Anti-Hu IL-4 PE), clone 25723 (Anti-Hu IFN-γ PE) and platelet controls (mouse IgG1 and IgG2a controls; PE) were obtained from Becton Dickinson Immunocytometry Systems (BDIS: San Jose, CA). Mouse IgG1 control-FITC was obtained from Dako Ltd. (High Wycombe, UK).
Intracellular staining and flow cytometry
Whole blood was diluted (1:1) with RPMI 1640 (Sigma-Aldrich Co. Ltd, Poole, UK) supplemented with 2 mml-glutamine, 100 μg/ml penicillin and 100 μg/ml streptomycin (Life Technologies Ltd, Paisley, UK). Blood was stimulated with phorbol 12-myristate 13-acetate (PMA; Sigma; 2.5 ng/ml final concentration) and ionomycin (I; Sigma; 1 μg/ml final concentration) in the presence of 10 μg/ml Brefeldin A (BFA; Sigma), an inhibitor of intracellular protein transport [13]. Samples were incubated for 4 h at 37°C/5% CO2, conditions shown previously to result in maximal activation of T cells and accumulation of intracellular cytokine [14]. Stimulated and unstimulated (BFA alone) samples were then surface stained with CD3–PerCP and CD8–FITC at room temperature for 15 min. Erythrocytes were lysed by addition of 1 × FACS lysis buffer (BDIS) and lymphocytes were then washed in PBS–0.5% bovine serum albumin (BSA)–0.1% sodium azide and incubated in 1 × FACS permeabilization solution (BDIS) for 10 min. The permeabilized cells were washed once and stained using anti-cytokine antibodies for 30 min at room temperature. Finally, the samples were washed once, resuspended in 1% paraformaldehyde/PBS prior to acquisition on a FACScan (BDIS). For sample acquisition the threshold was set on FL3 to exclude non-CD3 cells and 5000–10 000 events were collected for analysis by Cellquest software (BDIS). The population of CD3+ T lymphocytes was gated by forward and side-scatter characteristics (to exclude non-specific CD3-staining platelets) and two-dimensional dot plots created of FL1 versus FL2 to show cytokine staining in CD8+ and CD8− T cell subsets. Data were first analysed for total T cell cytokine production (CD3+) before more detailed analysis of CD8+ and CD8− T cell subsets; for simplicity, the CD8− T cell subset is termed ‘CD4+’ in the text, though this population also included a small percentage of CD4−CD8− T cells. Isotype controls enabled correct compensation and confirmed antibody specificity, and the unstimulated samples were used to set the quadrants for two-dimensional dot plot analysis. Results were expressed as percentage of a particular T cell subtype which stained positive for cytokine. Intra-assay variability was assessed by independently activating, staining and analysing three aliquots of the same blood sample; in a typical assay the mean T cell staining in samples from a control individual was 58.1 ± 1.9% (for IL-2); 1.6 ± 0.2% (for IL-4) and 32.4 ± 1.2% (for IFN-γ).
Whole blood stimulation and ELISA
Blood was diluted (1:4) with RPMI 1640 supplemented as before and stimulated for 20 h with PMA/I (2.5 ng/ml and 1 μg/ml, respectively) at 37°C/5% CO2 in a 24-well plate (Nunc, Life Technologies). Cell-free supernatants were collected and stored in aliquots at −70°C which were discarded after a single use. Supernatants were assayed for IFN-γ using an assay procedure and reagents (α-cytokine capture MoAb, biotinylated α-cytokine detection MoAb and recombinant cytokine) provided by Pharmingen (Cambridge Bioscience, Cambridge, UK). IL-2 was detected using a kit from R&D Systems (Abingdon, UK) and by following the manufacturer's instructions exactly. Results are expressed as cytokine concentration (pg/ml).
Statistical analysis
Results were analysed using one way anova: percentages were arcsine-transformed before analysis; ELISA data were log transformed before analysis. In both cases P values < 0.05 were considered significant. Mean values are shown in the text plus or minus standard errors, where appropriate. Correlations between cytokine production and viral load were performed using least squares analysis, and correlation coefficients are indicated.
RESULTS
Cytokine responses of T cells to PMA/I stimulation in whole blood cultures
Intracellular staining and flow cytometry identified a heterologous pattern of cytokine-producing CD3+ T cells in PMA/I-stimulated whole blood cultures from both HIV-infected individuals and normal controls. Variable proportions of T cells stained positive for IL-2 (35.7 ± 2.8%; range 5.5–70.0%) and IFN-γ (48.8 ± 3.0%; range 11.0–82.2%), whilst only relatively low numbers of cells stained for IL-4 (3.3 ± 0.3%; range 0.0–9.1%). There was no staining of cells with cytokine-specific MoAbs in ‘unstimulated’ cultures, indicating that any constitutive cytokine expression was below the threshold of detection of the assay.
Co-staining with α-CD8 enabled CD3+ T cells to be subdivided into CD8+ and CD8− cells (Fig. 1). Those cells which did not stain for CD8 (left-hand quadrants) were assumed to represent the CD4+ T helper cell population. These cells could not be stained directly, since treatment of lymphocytes with phorbol esters leads to rapid and complete down-modulation of surface CD4 [15]. IL-2 was synthesized in CD4+T cells and T cells staining strongly positive for CD8+, but not from those cells which were weakly positive for CD8+ (Fig. 1, panels second from left). On the other hand, most weakly positive CD8+ T cells were strongly positive for IFN-γ (Fig. 1, right-hand panels). This phenomenon was seen in blood cultures from both HIV-infected and control individuals. Co-staining with MoAbs specific for T cell receptor (TCR) αβ or γδ heterodimers and the cytokine-specific MoAbs identified this population as CD3+ CD8± TCR γδ+ (data not shown). Thus this method identified three populations of CD3+ T cells: CD4+ and CD8+ TCR αβ+ T cells which had heterologous expression of IL-2, IL-4 and IFN-γ, and a subset of CD8+ T cells which were TCR γδ+, did not produce any detectable IL-2 but were positive for IFN-γ following PMA/I activation.
Fig. 1.
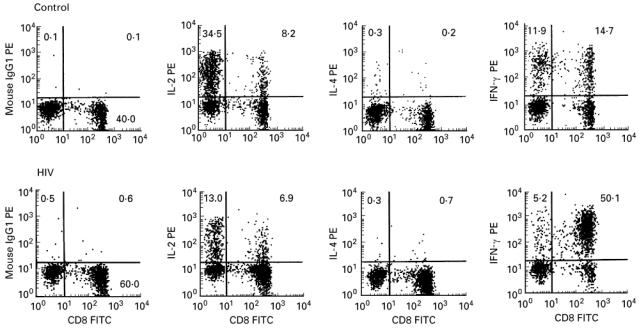
Three-colour FACS analysis of phorbol 12-myristate 13-acetate (PMA)/ionomycin (I)-stimulated whole blood. CD3+ gated lymphocyte populations were analysed on FL1 (FITC) versus FL2 (PE) two-dimensional plots to discriminate CD8+ and ‘CD4+’ T cell subsets staining positive for the relevant cytokines (upper right and upper left quadrants, respectively). The percentage of CD3+ events falling within the given quadrant are shown. For each sample, 5000 CD3+ gated events were analysed.
Altered cytokine responses are associated with HIV infection
A highly significant difference in PMA/I-induced IL-2 and IFN-γ synthesis was observed between HIV and control groups (Fig. 2). The proportion of T cells from HIV+ individuals producing IL-2 was much lower than the samples from control individuals (21.4 ± 2.2% versus 50 ± 2.3%; P < 0.0001; Fig. 2a). Conversely, more T cells from HIV individuals stained positive for IFN-γ than controls (63.4 ± 2.7% versus 34.2 ± 2.5%; P < 0.0001; Fig. 2b). There was no significant difference in the proportion of CD3+ T cells staining positive for IL-4 between HIV+ and control groups (2.8 ± 0.3% versus 3.8 ± 0.2%; P = 0.15; not shown).
Fig. 2.
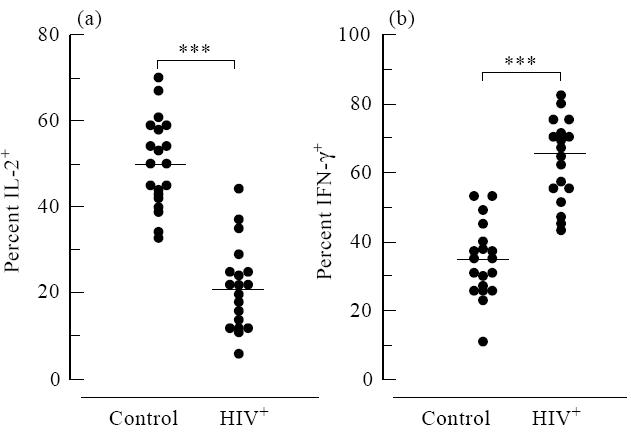
Stimulated T cells from HIV-infected individuals exhibit decreased intracellular staining for IL-2 (a) and increased IFN-γ staining (b) relative to control groups. The percentages of CD3+ T cells staining positive for IL-2 and IFN-γ were plotted for HIV+ and control groups (***P< 0.0001). Horizontal bars represent population means.
In order to disregard the possibility that the differences in cytokine staining between HIV+ and control samples described above were simply due to a decreased CD4:CD8 ratio, we normalized the data to show the percentage of CD4+ or CD8+ T cells synthesizing cytokine as a proportion of the total CD4+ or CD8+ population (Fig. 3). It is clear that in HIV-infected individuals there is an impaired ability of CD4+ T cells to synthesize IL-2 compared with healthy controls in response to stimulation with PMA/I (38.6 ± 3.1% versus 62.0 ± 2.0%; P < 0.0001; Fig. 3a, left-hand panel). The CD8+ T cell subsets of HIV-infected individuals' cells were also impaired in their ability to synthesize IL-2 (11.9 ± 1.6% versus 27.7 ± 3.0%; P < 0.0001; Fig. 3a, right-hand panel), but were more likely to produce IFN-γ than the corresponding cells in controls (78.6 ± 2.6% versus 49.2 ± 3.7%; P < 0.0001; Fig. 3b, right-hand panel). Since we showed above that the CD8± T cell population did not produce IL-2 but was strongly IFN-γ-positive following mitogenic stimulation, an increase in the proportion of these cells in the HIV-infected samples could explain our observations. However, we did not observe any changes in the proportions of CD8± T cells in the samples we analysed from HIV-infected individuals and normal controls, and the defective cytokine production occurred in the CD8+ (high) subset (as shown in the lower right panel of Fig. 1).
Fig. 3.
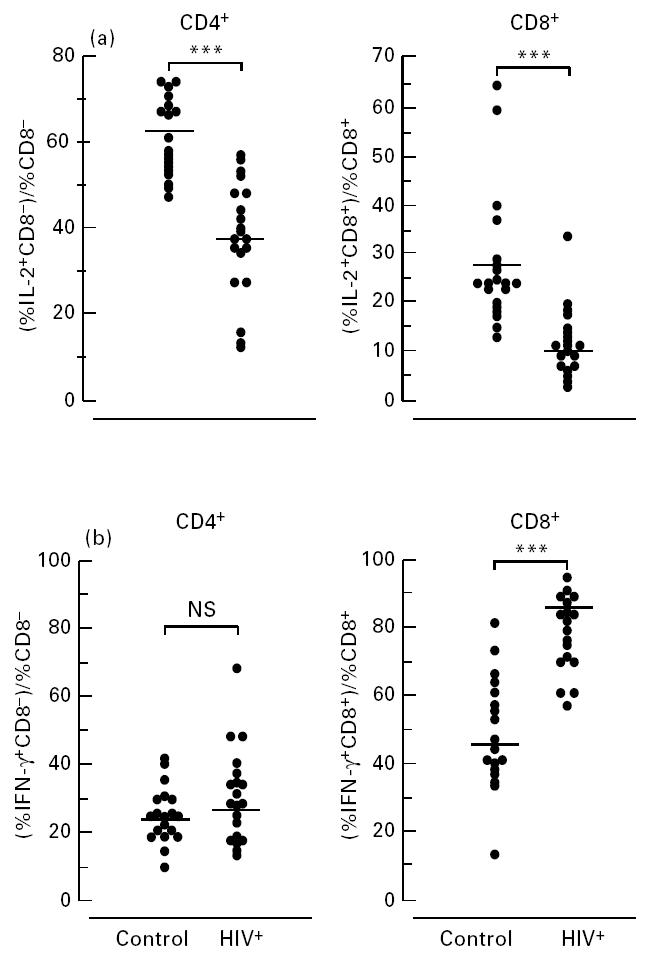
Altered cytokine responses exist in both CD4 and CD8 T cell subsets of HIV+ compared with healthy controls. Values for the percentages of CD4 and CD8 cells staining positive for IL-2 (a) or IFN-γ (b) were ‘normalized’ by dividing the percentages of the IL-2- and IFN-γ-staining CD4+ and CD8+ cells by the total percentages of CD4+ and CD8+ T cells in the CD3 population. ***P< 0.0001; NS, not significant.
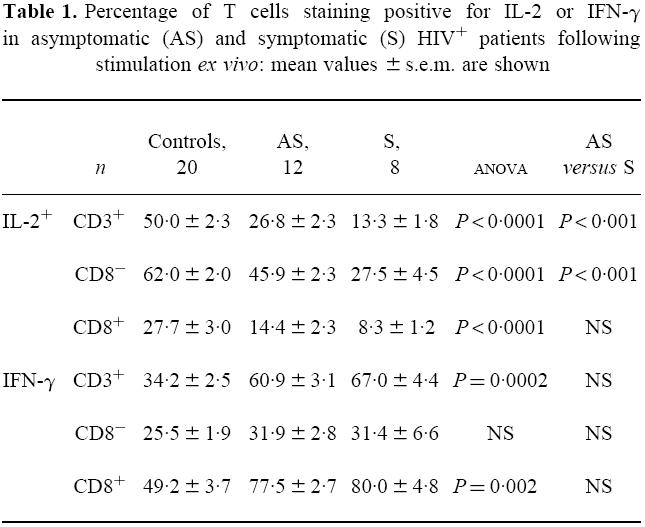
As shown in Table 1, T cells from both clinically asymptomatic and symptomatic HIV+ individuals had a reduced capacity to produce IL-2 and an increased IFN-γ response compared with controls. The IL-2 response in the CD4+ T cell subset was significantly lower in the symptomatic versus the asymptomatic HIV+ group (P < 0.001); IL-2 production was also reduced in the CD8+ T cells of symptomatics compared with asymptomatics, but the difference was not statistically significant. On the other hand, there was no difference between the two clinically distinct HIV+ groups in terms of IFN-γ production. Therefore, the activation of the CD8 T cell subset (as indicated by the high IFN-γ production) appears an early event in the course of HIV infection which is maintained throughout the course of the disease, whilst the loss of IL-2-mediated responses appears progressive and increases with the onset of symptomatic disease.
Table 1.
Percentage of T cells staining positive for IL-2 or IFN-γ in asymptomatic (AS) and symptomatic (S) HIV+ patients following stimulation ex vivo: mean values ± s.e.m. are shown
Correlation between IL-2 production and HIV RNA plasma viral load
As shown in Fig. 4, we found that there was a good correlation between the percentage of T cells producing IL-2 and the HIV RNA plasma viral load (r2 = 0.832). Furthermore, this correlation existed in both the CD4 and CD8 T cell subsets (r2 = 0.435 and r2 = 0.622, respectively). There was no observed correlation between IFN-γ over-production by T cells and T cell subsets, indicating that in our assay this parameter was independent of viral replication.
Fig. 4.
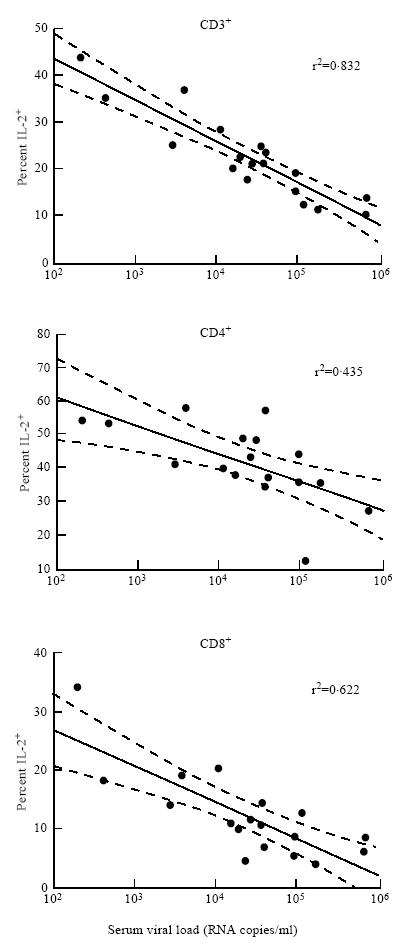
Association between the percentage of cells staining positive for IL-2 following activation in vitro and HIV plasma viral load. Dashed lines show 95% confidence limits.
ELISA results
There was a similar trend in reduction of IL-2 in the HIV group compared with controls (HIV 190 pg/ml, range 0–565 pg/ml, versus control 263 pg/ml, range 0–420 pg/ml), but the difference was not significant (P = 0.52). There was no difference in IFN-γ production between the two groups (HIV 4.6 ng/ml, range 1.5–40.2 ng/ml, versus control 4 ng/ml, range 1.4–39.2 ng/ml; P = 0.93).
DISCUSSION
Our results clearly show that there is a reduced ability of CD4+ and CD8+ T cells to produce IL-2, with a concomitant increase in CD8+ T cell IFN-γ production associated with HIV infection following ex vivo stimulation. The differences observed between samples from HIV-infected individuals and controls cannot simply be explained by the inversion in the CD4:CD8 ratio that occurs following HIV infection, since the results were normalized for the relative percentages of CD4 and CD8 cells in each sample. Our findings of a reduced ability of CD4+ T cells to produce IL-2 and a predisposition of CD8+ T cells to synthesize IFN-γ are consistent with the observations in HIV-infected individuals of CD4+T helper cell anergy and chronic CD8+ T cell activation, and both appear highly significant markers of HIV-associated immune dysfunction.
In contrast to the flow cytometry data, the ELISA data showed a non-significant trend towards decreased IL-2 production in the PMA/I-stimulated cultures of infected individuals. In addition, non-CD3+ cells, such as natural killer cells, are stimulated by PMA/I to produce IFN-γ, but cannot be distinguished from IFN-γ-producing T cells by ELISA. Three-colour FACS analysis, on the other hand, identified a significantly different IFN-γ response in the CD8+ T cell subset associated with HIV infection.
Our results extend two other studies on HIV-infected patients. First, Fan and colleagues analysed CD4+ and CD8+ sorted T cells from HIV-infected individuals by semiquantitative RT-PCR [6]: they identified reduced levels of IL-2 mRNA and increased levels of IFN-γ mRNA in the HIV-infected group. However, since translational control mechanisms regulate cytokine protein production [12], altered mRNA levels alone do not indicate the extent or functional significance of altered cytokine responses at the protein level. More recently, Hagiwara et al. used an ELISPOT assay to show that the frequency of IL-2-secreting cells was reduced in HIV infection and correlated with CD4 count, whilst there was an increase in IFN-γ-producing cells [7]. In our assay we have shown that changes in cytokine responses exist at the level of protein expression within individual cells and are apparent following in vitro stimulation, implying that there is a functional impairment of cell-mediated responses in the HIV-infected host. Furthermore, since the cytokine dysfunction we observed was so pronounced, we conclude that HIV induces a general defect in IL-2 and IFN-γ production within the host individual through infection of only a small proportion of cells: only 0.5% of CD4+ T cells in an infected asymptomatic individual harbour HIV DNA [16].
T helper cell hyporesponsiveness occurs in HIV-infected individuals in the context of a generalized state of activation. The levels of cell surface markers of activation are increased on both CD4+ and CD8+ T cells in HIV-infected individuals [17, 18]; their expression increases with disease severity. The proportion of CD4+ T cells expressing HLA-DR which are in the proliferative phase of the cell cycle is increased in HIV-infected individuals [19], implying that some of the activated T helper cell population are able to proliferate normally. On the other hand, our results show a significant decrease in the proportion of CD4+ T cells producing IL-2 in HIV+ symptomatics versus asymptomatics, and an inverse correlation between percentage IL-2+ CD4+ T cells and RNA viral load, suggesting that the defect in IL-2 production is progressive. It would therefore be interesting to investigate the mitogen-induced production of IL-2 from proliferating and non-proliferating CD4+ T cells using flow cytometry, since this may have relevance to the impaired antigen-specific immune responses in activated T helper cells from HIV-infected patients [20].
A number of non-cytopathic viruses encode proteins which can interfere with anti-viral host immunity and thus modulate the immune response in favour of maintaining a chronic viral persistence in the host [21]. The HIV-encoded envelope glycoprotein (gp160/120) has been implicated as having immunomodulatory activity from experiments carried out in vitro. Interactions between membrane-associated envelope (gp120/41) on HIV-infected antigen-presenting cells (APC) and CD4 on T helper cells can result in an incomplete activation signal which leads to T cell hyporesponsiveness [22] or apoptosis [23, 24] upon subsequent antigen stimulation. In agreement with previously published work [3], we found increased levels of lymphocyte apoptosis when PBMC from seven of the 20 HIV+ patients were activated in vitro (M. Westby and M. Guckian, unpublished observations). However, CD4-independent mechanisms of virus-induced immune dysfunction must exist to explain the suppression of IL-2 production and enhanced synthesis of IFN-γ which we observed in the CD8+ T cells of HIV-infected individuals.
Soluble factors released from HIV-infected APC may be involved in orchestrating a generalized immune dysregulation. Imbalancd production of IL-12/IL-10 has been described following infection of primary monocyte-derived macrophages in vitro, leading to suppression of IL-2 production from T cells co-cultured with the infected APC [25]. We observed significant reductions in lipopolysaccharide (LPS)-induced IL-10 production concomitant with increased tumour necrosis factor-alpha (TNF-α) production from the same cohort in a parallel study (M. Westby and J. B. Marriott, unpublished observations).
Alternatively, HIV infection of APC may lead to aberrant surface expression of costimulatory molecules, such as CD80, which could affect T cell responsiveness, as has been described [26]. In support of this, reduced surface expression of the T cell ligand for CD80, CD28, is associated with disease progression [27]. Interestingly, ex vivo CD4+ T cell proliferation in HIV+ patients can be enhanced as a result of CD28 costimulation [28]. Our results fit with the model that HIV infection leads to a defect in the costimulatory pathway to T cell activation: IL-2 production, which we have shown is down-regulated in the HIV+ group, requires two independent signals: one from the TCR complex and one from the CD28-associated intracellular signal transduction pathway. Furthermore, the high staining for IFN-γ that we observed in the CD8+ T cell subset is in agreement with a previous report where CD8+ CD28− cells were identified as the main producers of IFN-γ in HIV-infected individuals [29].
Few IL-4-producing cells were identified in either HIV+or control groups by flow cytometry, making it impossible to speculate on previous assertions of a Th1–Th2 switch in HIV infection [30].
The identification of immune dysfunction in whole blood at the single-cell level has significant implications for monitoring HIV infection longitudinally in a clinical setting. The technique we describe is simple and can handle multiple samples quickly, making it more attractive than RT-PCR for the longitudinal analysis of immune function in HIV+ individuals undergoing therapeutic treatments. Preliminary data collected over a 12-month period on two healthy controls has revealed variations in IL-2 and IFN-γ production (< 10%). However, all results were within the ranges of control individuals presented here, suggesting that, as with other immunological parameters (e.g. CD4 counts), there are diurnal variations; more work will be required to determine precisely the normal fluctuations of these parameters.
As combination therapies reduce viral load to undetectable levels for prolonged periods in patients, it will be important to understand whether there is concomitant recovery of the immune system. Autran and co-workers have recently shown positive effects on the immune system of AIDS patients associated with response to triple combination therapy [31]. If the processes which result in low IL-2 production from activated whole blood cultures from HIV+ individuals are reversible, then the strong correlation observed between IL-2 versus viral load (Fig. 4) suggests that suppression of viral replication may result in restored T cell IL-2 production. Flow cytometric analysis of intracellular cytokines could make a useful contribution to assess the dynamics and extent of such a response.
Acknowledgments
We would like to thank the individuals who participated in the study. We are also very grateful to Skip Maino and Joyce Ruitenberg at Becton Dickinson Immunocytometry Systems for their support with the intracellular cytokine reagents and staining technique, and to Professor Jim Booth for the viral load data. M.W. was funded by Glaxo Wellcome; J.B.M. was funded by the MRC ADP (grant no. G9313953), and M.G. was supported by Celgene.
References
- 1.Clerici M, Stocks NI, Zajac RA, et al. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989;84:1892–9. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haynes BF, Pantaleo G, Fauci AS. Toward an understanding of the correlates of protective immunity to HIV infection. Sci. 1996;271:324–8. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 3.Gougeon ML, Lecoeur H, Dulioust A, et al. Programmed cell death in peripheral lymphocytes from HIV-infected persons. J Immunol. 1996;156:3509–20. [PubMed] [Google Scholar]
- 4.Clerici M, Hakim FT, Venzon DJ, et al. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin Invest. 1993;91:759–65. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barcellini W, Rizzardi GP, Borghi MO, Fain C, Lazzarin A, Meroni PL. Th1 and Th2 cytokine production by peripheral-blood mononuclear-cells from HIV-infected patients. AIDS. 1994;8:757–62. doi: 10.1097/00002030-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Fan J, Bass HZ, Fahey JL. Elevated IFN-gamma and decreased IL-2 gene expression are associated with HIV infection. J Immunol. 1993;151:5031–40. [PubMed] [Google Scholar]
- 7.Hagiwara E, Sacks T, Leitman-Klinman SF, Klinman DM. Effect of HIV infection on the frequency of cytokine-secreting cells in human peripheral blood. AIDS Res Hum Retroviruses. 1996;12:127–33. doi: 10.1089/aid.1996.12.127. [DOI] [PubMed] [Google Scholar]
- 8.Caruso A, Canaris AD, Licenziati S, et al. CD4(+) and CD8(+) lymphocytes of patients with AIDS synthesize increased amounts of interferon-gamma. J Acquir Immune Def Syndr Hum Retrovirol. 1995;10:462–70. doi: 10.1097/00042560-199512000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Graziosi C, Gantt KR, Vaccarezza M, et al. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc Natl Acad Sci USA. 1996;93:4386–91. doi: 10.1073/pnas.93.9.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pantaleo G, Demarest JF, Soudeyns H, et al. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature. 1994;370:463–7. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- 11.Meyaard L, Hovenkamp E, Keet IP, et al. Single cell analysis of IL-4 and IFN-gamma production by T cells from HIV-infected individuals: decreased IFN-gamma in the presence of preserved IL-4 production. J Immunol. 1996;157:2712–8. [PubMed] [Google Scholar]
- 12.Garcia-Sanz JA, Lenig D. Translational control of interleukin 2 messenger RNA as a molecular mechanism of T cell anergy. J Exp Med. 1996;184:159–64. doi: 10.1084/jem.184.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klausner RD, Donaldson JG, Lippincott-Schwartz J, Brefeldin A. Insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picker LJ, Singh MK, Zdraveski Z, et al. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–19. [PubMed] [Google Scholar]
- 15.Anderson SJ, Coleclough C. Regulation of CD4 and CD8 expression on mouse T cells: active removal from the surface by two mechanisms. J Immunol. 1993;151:5123. [PubMed] [Google Scholar]
- 16.Chun T-W, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 17.Mahalingam M, Peakman M, Davies ET, Pozniak A, McManus TJ, Vergani D. T cell activation and disease severity in HIV infection. Clin Exp Immunol. 1993;93:337–43. doi: 10.1111/j.1365-2249.1993.tb08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kestens L, Vanham G, Vereecken C, et al. Selective increase of activation antigens HLA-DR and CD38 on CD4+ CD45RO+ T lymphocytes during HIV-1 infection. Clin Exp Immunol. 1994;95:436–41. doi: 10.1111/j.1365-2249.1994.tb07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahalingam M, Pozniak A, McManus TJ, Vergani D, Peakman M. Cell cycling in HIV infection: analysis of in vivo activated lymphocytes. Clin Exp Immunol. 1995;102:481–6. doi: 10.1111/j.1365-2249.1995.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caruso A, Licenziati S, Canaris AD, et al. T cells from individuals in advanced stages of HIV-1 infection do not proliferate but express activation antigens in response to HIV-1-specific antigens. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:61–69. doi: 10.1097/00042560-199705010-00010. [DOI] [PubMed] [Google Scholar]
- 21.Wiertz EJ, Mukherjee S, Ploegh HL. Viruses use stealth technology to escape from the host immune system. Mol Med Today. 1997;3:116–23. doi: 10.1016/S1357-4310(96)10059-9. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz O, Alizon M, Heard JM, Danos O. Impairment of T cell receptor-dependent stimulation in CD4+ lymphocytes after contact with membrane-bound HIV-1 envelope glycoprotein. Virol. 1994;198:360–5. doi: 10.1006/viro.1994.1042. [DOI] [PubMed] [Google Scholar]
- 23.Banda NK, Bernier J, Kurahara DK, et al. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J Exp Med. 1992;176:1099–106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cottrez F, Manca F, Dalgleish AG, Arenzana-Seisdedos F, Capron A, Groux H. Priming of human CD4+ antigen-specific T cells to undergo apoptosis by HIV-infected monocytes. A two-step mechanism involving the gp120 molecule. J Clin Invest. 1997;99:257–66. doi: 10.1172/JCI119154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo J, Chen H, Kraus T, et al. Altered cytokine production and accessory cell function after HIV-1 infection. J Immunol. 1996;157:1313–20. [PubMed] [Google Scholar]
- 26.Chirmule N, McCloskey TW, Hu R, Kalyanaraman VS, Pahwa S. HIV gp120 inhibits T cell activation by interfering with expression of costimulatory molecules CD40 ligand and CD80 (B71) J Immunol. 1995;155:917–24. [PubMed] [Google Scholar]
- 27.Borthwick NJ, Bofill M, Gombert WM, et al. Lymphocyte activation in HIV-1 infection. II. Functional defects of CD28− T cells. AIDS. 1994;8:431–41. doi: 10.1097/00002030-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Levine BL, Mosca JD, Riley JL, et al. Antiviral effect and ex vivo CD4+ T cell proliferation in HIV-positive patients as a result of CD28 costimulation. Sci. 1996;272:1939–43. doi: 10.1126/science.272.5270.1939. [DOI] [PubMed] [Google Scholar]
- 29.Caruso A, Licenziati S, Canaris AD, et al. Characterization of T-cell subsets involved in the production of IFN-gamma in asymptomatic HIV-infected patients. AIDS Res Hum Retroviruses. 1996;12:135–41. doi: 10.1089/aid.1996.12.135. [DOI] [PubMed] [Google Scholar]
- 30.Clerici M, Shearer GM. The Th1-Th2 hypothesis of HIV-infection—new insights. Immunol Today. 1994;15:575–81. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 31.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Sci. 1997;277:112–6. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]


