Abstract
The coexistence of anti-La (SS-B) and anti-Ro (SS-A) autoantibodies in pSS is probably explained by intermolecular spreading of autoimmunity toward different components of the La/Ro ribonucleoprotein (RNP). In order to evaluate the role of the HLA class II phenotype in controlling diversification of this autoantibody response, 80 patients with pSS were typed by polymerase chain reaction sequence-specific oligonucleotide (PCR-SSO) at the HLA class II loci DRB1, DQA1 and DQB1. Serum samples were examined for anti-La and anti-Ro by counterimmunoelectrophoresis and by ELISA using purified recombinant La and 60-kD Ro proteins. Patient sera were classified according to the extent of diversification of the anti-La, anti-Ro response including the presence or absence of precipitating anti-La antibodies. Immunogenic characteristics of these stratified groups were then studied. All patients with pSS, with or without autoantibodies to Ro and La, were found to have at least one of the HLA-DRB1 types DR2, DR3 or DR5. The HLA DR3-DQA1*0501-DQB1*02 (DR3-DQ2) haplotype was primarily associated with a diversified La/Ro RNP response containing precipitating autoantibodies to La (P < 0.001); whereas the haplotype HLA DR2-DQA1*0102-DQB1*0602 (DR2-DQ1) was associated with a less diversified La/Ro RNP response containing non-precipitating (restricted epitope) anti-La autoantibodies (P < 0.001). Anti-La-positive patients lacking both HLA-DR2 and HLA-DR3 all expressed the HLA-DQA1*0501 allele, which was present at increasing frequency with greater diversification of the anti-La/Ro autoantibody response. The association of distinct HLA haplotypes with different degrees of autoantibody diversification in patients with pSS suggests a model of HLA-restricted presentation of La/Ro peptide determinants to autoreactive helper T cells. We propose that non-precipitating anti-La responses are driven by limited intermolecular help from DR2-DQ1-restricted T helper cells recognizing Ro determinants. On the other hand, we speculate that the more diversified, precipitating anti-La responses obtain more efficient cognate T help from DR3-DQ2-restricted T helper cells recognizing La determinants, where HLA-DQA1*0501 may be a critical determinant for antigen presentation.
Keywords: Sjögren's syndrome, HLA, anti-La (SS-B), anti-Ro (SS-A), autoantibodies
INTRODUCTION
Autoantibodies to Ro (SS-A) and La (SS-B) are found in the serum of patients with pSS and systemic lupus erythematosus (SLE). Anti-Ro antibodies may be found in isolation, yet anti-La antibodies are virtually always found in conjunction with anti-Ro [1]. Concurrence of autoantibody responses to the Ro and La nuclear autoantigens is likely to be accounted for by T cell-dependent spreading of autoimmunity between components of the La/Ro ribonucleoprotein (RNP) after initiation of immunity to a single component of this structure. Such spreading in autoimmunity has been reported in mice immunized with either the Ro or La polypeptides separately [2] and following immunization with an autologous subdominant La determinant [3]. If autoantibody spreading most often arises from a T helper response to a single peptide in either Ro or La, then a single HLA class II association might be evident, whereas mixed T cells seeing unique peptide determinants present in both autoantigens may result in distinct HLA associations. In support of the latter possibility, two HLA haplotypes, DR3-DQ2 and DR2-DQ1, have frequently been reported to be associated with autoantibody responses to both Ro and La autoantigens [4–6]. Moreover, recent studies have shown that the DR3-DQ2 haplotype might be a marker for more active immune responses in Finnish patients with pSS [7]. These findings reported in clinical studies are consistent with animal models of autoimmunity showing that the efficiency of natural spreading of autoimmune responses may vary according to the genetic background of immunized animals [3].
We previously reported a subset of ‘precipitin-negative’ patients who possess circulating antibodies to La polypeptide detectable by ELISA, but not detected by counterimmunoelectrophoresis (CIE). Autoantibodies in these patients showed restricted epitope recognition of the La protein when compared with ‘precipitin-positive’ anti-La sera [8]. The limited autoantibody diversification in the La precipitin-negative patients with pSS was associated with significantly lower serum rheumatoid factor and IgG levels than anti-La precipitin-positive patients. In addition, the precipitin-negative group also tended to have lower levels of antibodies directed against denatured 60-kD Ro [9]. Furthermore, the limited diversification of the response to the La/Ro RNP in the precipitin-negative anti-La group represented a stable serological subset over time. In order to explain these observations, we postulated that spreading of autoimmunity between determinants of the La/Ro RNP, and thus the formation of precipitating or non-precipitating anti-La antibodies, was likely to be dependent in part upon the presentation of unique peptide determinants to helper T cells by specific HLA class II alleles. This study was therefore designed to evaluate the influence of the HLA class II phenotype in controlling diversification of the autoantibody response to the La/Ro RNP in pSS, by comparing the distribution of HLA types within normal controls and patient subgroups containing different patterns of autoantibody spreading.
We found the HLA-DR2-DQA1*0102-DQB1*0602 (DR2-DQ1) haplotype to be strongly associated with the presence of anti-Ro antibodies, often in conjunction with non-precipitating anti-La antibodies. Notably, the risk of anti-Ro autoantibody spreading to produce precipitating anti-La antibodies was markedly increased in individuals with the DR3-DQA1*0501-DQB1*02 (DR3-DQ2) haplotype, suggesting the presence of distinct determinants for T cell recognition on the La/Ro RNP. We propose a model of differential antigen presentation of La and Ro determinants to explain these observations. An additional observation from this study was that patients with neither the DR2-DQ1 nor DR3-DQ2 haplotype expressed HLA-DR5-DQA1*0501-DQB1*0301. Since the DQA1*0501 allele is known to be in linkage disequilibrium with both HLA-DR3 and HLA-DR5, it may represent a primary risk factor in the development of La/Ro autoimmunity, consistent with recent findings [10].
MATERIALS AND METHODS
Patients and controls
Blood was collected from 80 patients from our institution fulfilling at least four of the six European consensus criteria for the diagnosis of pSS [11]. All patients were examined for other clinical features of pSS, notably salivary gland enlargement, Raynaud's phenomenon, arthralgia and joint stiffness. Control sera were collected from 25 healthy donors. The control group for HLA typing comprised 164 healthy South Australian donors to the Australian Bone Marrow Registry.
Serological measurements
CIE was performed using rabbit thymus extract (Pel-Freez; Rogers, AR) and extracts of K562 (a human myeloid cell line [12]). Full-length cDNA encoding the 60-kD Ro molecule was cloned from a human T cell line (HUT 78) cDNA library [13]. Recombinant soluble human 60-kD Ro and La [14] were produced in bacteria as in-frame six-histidine fusion proteins in the pQE vector (Qiagen, Chatsworth, CA) and purified by Ni2+ affinity chromatography as described previously [9]. Anti-La and anti-Ro antibody levels were measured by ELISA using the recombinant human autoantigens [13]. Checkerboard assay was used to determine the optimal coating concentration of antigen (1 μg/ml) and a screening dilution (1:500) which gave a value below the saturated portion of the serum dilution curve. The Ro ELISA was considered to measure reactivity to predominantly denatured 60-kD Ro. Results were expressed in optical density (OD)410 nm units as the mean of duplicates. Serum IgG and rheumatoid factor concentrations were measured by nephelometry (ICSII; Beckman, Brea, CA). Ro60-transfected HEp-2 cells (HEp2000) were purchased from Immunoconcepts Inc. (Sacramento, CA).
DNA preparation
DNA was prepared from blood samples by the salt precipitation method [15].
HLA class II typing
High resolution DRB1, DRB3, DRB5, DQA1 and DQB1 typings were performed by polymerase chain reaction sequence-specific oligonucleotide (PCR-SSO) using 11th and 12th International Histocompatibility Workshop protocols [16, 17] and an enhanced chemiluminescent (ECL) system (Amersham, Aylesbury, UK).
Statistical analysis
Quantitative differences in serum autoantibodies and IgG between precipitin-positive and precipitin-negative anti-La were analysed by the Mann–Whitney U-test. HLA associations were analysed by odds (or cross-product) ratios (OR) which are approximations to the relative risk in the case of retrospective data. Simple, single associations were tested by the 2 × 2 table odds ratio (with 0.5 added to each cell count when there were zero counts in any cell). Significance was assessed by χ2 values. Phenotypic HLA associations were analysed by base-line logistic regression, separately for DRB1, DQA1 and DQB1, with each group of pSS patients compared in turn with the controls, with variables included for each allele. Regression was performed in a forward step-wise manner to select the most parsimonious model. The (antilog) of the regression coefficients are the odds ratios. The probability of the regression coefficients was taken as the significance (probability) values of the odds ratios. The significant HLA associations are tabulated.
RESULTS
A spectrum of diversification and amplification of the autoimmune response to the La/Ro RNP in pSS
Previously we have shown that the specificity of non-precipitating anti-La antibodies is highly restricted, whereas precipitating anti-La antibodies are highly polyclonal [8]. Accordingly, the 80 patients with pSS were stratified into four categories based on the extent of diversification of their autoimmune response to the La/Ro RNP: (i) seronegative for anti-Ro and anti-La antibodies (n = 11); (ii) anti-Ro antibodies without any detectable anti-La (n = 10); (iii) anti-Ro and non-precipitating anti-La antibodies (n = 15); (iv) anti-Ro and precipitating anti-La antibodies (n = 44). These subsets define points within a spectrum of diversification and amplification of the autoimmune response to the La/Ro RNP. All sera containing anti-Ro precipitins on CIE were also positive by indirect immunofluorescence on Ro60-transfected HEp-2 cells, consistent with a B cell response to conformational epitopes on 60-kD Ro [13]. Autoantibodies were not detected in sera from the 25 normal controls, nor in previously examined blood bank donors [13]. No significant differences were found between the four subgroups of pSS patients with respect to age, female:male ratio, age at onset, salivary gland enlargement, Raynaud's phenomenon, arthralgia, or joint stiffness (data not shown). However, when compared with precipitin-positive anti-La patients, the precipitin-negative anti-La subgroup had significantly lower anti-La ELISA values (mean 0.84 ± 0.47 versus1.58 ± 0.38, P < 0.001); lower anti-Ro60 ELISA values (mean 0.74 ± 0.55 versus1.22 ± 0.57, P < 0.05); lower rheumatoid factor (mean 96 U/ml versus366 U/ml, P < 0.001); and lower serum IgG levels (mean 17 versus28, P < 0.001).
HLA-DR2, HLA-DR3 and HLA-DR5 are risk factors for pSS
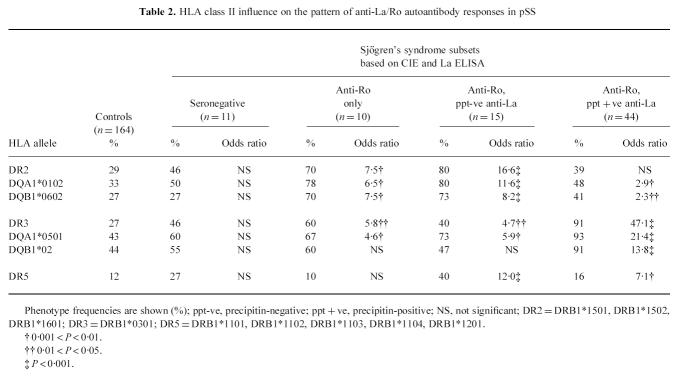
We examined the complete data set for associations with each DRB1, DQB1 and DQA1 phenotype, and found the only significant HLA class II associations of pSS to be with haplotypes linked with DR2 (DR15 P < 0.0001, DR16 P = 0.036, DQA1*0102 P < 0.0001, and DQB1*0602 P < 0.0001); DR3 (DR3 P < 0.0001, DQA1*0501 P < 0.0001, DQB1*0201 P < 0.0001); and DR5 (DR11 P = 0.002, DR12 P = 0.076). Even though DR16 and DR12 were of marginal significance, these alleles are relatively rare in our population and would require larger sample sizes to detect strong significance. The DQB1 analysis fits the statistical model poorly, indicating that the DQB1 associations were likely to be secondary or due to linkage disequilibrium. Furthermore, linkage disequilibrium between the HLA-DRB1 and -DQA1 loci was too strong to allow reliable detection of the strongest effect in this analysis; such that the HLA class II associations with pSS could be adequately described in terms of DR2/3/5 or alternatively DQA1*0102/0501.
Compared with 97 of the 164 controls, all patients with pSS (both seropositive and seronegative) expressed at least one of the alleles DR2, DR3 or DR5 (OR 111), indicating that the development of pSS is strongly associated with genes present in the HLA-DR2, -DR3 or -DR5 haplotypes (Table 1). Notably, DQA1*0501 or DQA1*0102 were present in 99% of patients with pSS compared with 67% of controls. Due to the small number of autoantibody-seronegative patients, it was not possible to distinguish whether the HLA-mediated risk was with the disease or with the development of autoantibodies associated with disease. Since the function of HLA class II molecules is to present antigen to T helper cells, we therefore determined whether the expression of distinct HLA class II haplotypes influenced diversification and amplification of the autoantibody response in patients with pSS.
Table 1.
Patients with pSS all express either HLA-DR2, HLA-DR3 or HLA-DR5
Diversification of La/Ro autoimmunity is influenced by distinct HLA class II alleles
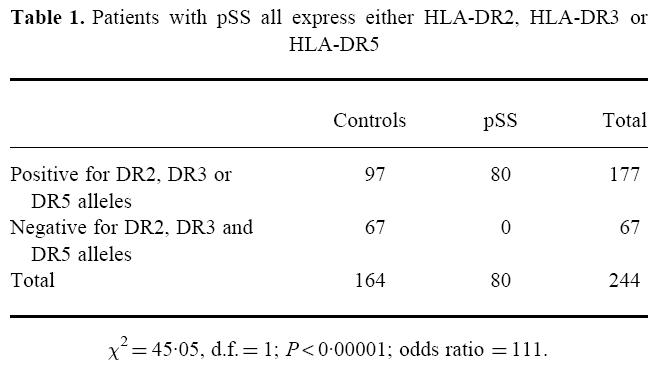
Table 2 shows the phenotypic frequency of HLA class II alleles in patients stratified according to the degree of autoantibody diversity. Notably 12/15 (80%) of the anti-Ro, precipitin-negative anti-La group expressed HLA-DR2, and 40/44 (91%) of the anti-Ro, precipitin-positive anti-La group expressed HLA-DR3. The DR2-DQA1*0102-DQB1*0602 (DR2-DQ1) haplotype was strongly associated with autoantibodies reactive with either Ro alone (DR2, OR = 7.5) or Ro in conjunction with non-precipitating anti-La antibodies (DR2, OR = 16.6). Similarly, the DR3-DQA1*0501-DQB1*02 (DR3-DQ2) haplotype was associated with autoantibodies to Ro alone (DR3, OR = 5.8) and with anti-Ro in conjunction with non-precipitating anti-La (DR3, OR = 4.7). Significantly however, the relative risk of precipitating anti-La antibodies was approximately an order of magnitude greater in the presence of the DR3-DQ2 haplotype (DR3, OR = 47.1). Thus, although the DR3-DQ2 haplotype predisposes patients to both anti-Ro and anti-La autoantibodies, the influence on the anti-La immune response is most striking. Examination of each subgroup by χ2analysis showed no significant increase in the proportion of HLA-DR2/DR3 heterozygotes above that expected given the individual frequencies of DR2 and DR3 in the patient cohort (data not shown). Therefore, we concluded that the DR2 and DR3 haplotypes confer independent susceptibility in pSS.
Table 2.
HLA class II influence on the pattern of anti-La/Ro autoantibody responses in pSS
Only five of the 80 pSS patients lacked both DR2 and DR3—one seronegative, one anti-Ro precipitin-negative anti-La, and three anti-Ro precipitin-positive anti-La patients. Each of these patients expressed DR5 (three DR11 and two DR12 subtypes), which was strongly associated with anti-Ro in association with both non-precipitating (OR 12.0) and precipitating (OR 7.1) anti-La antibodies. All of the DR5-positive/DR2-negative/DR3-negative patients expressed DQA1*0501, which is known to be in linkage disequilibrium with both DR3 and DR5. This finding suggested that DQA1*0501 may be a critical determinant in T cell recognition of the La/Ro RNP. In support of this notion, the frequency of DQA1*0501 increased in patient subgroups with increasing diversification of the autoantibody response to the La/Ro RNP (Table 2).
A role for DQA1*0501 in controlling anti-La/Ro autoimmune diversification
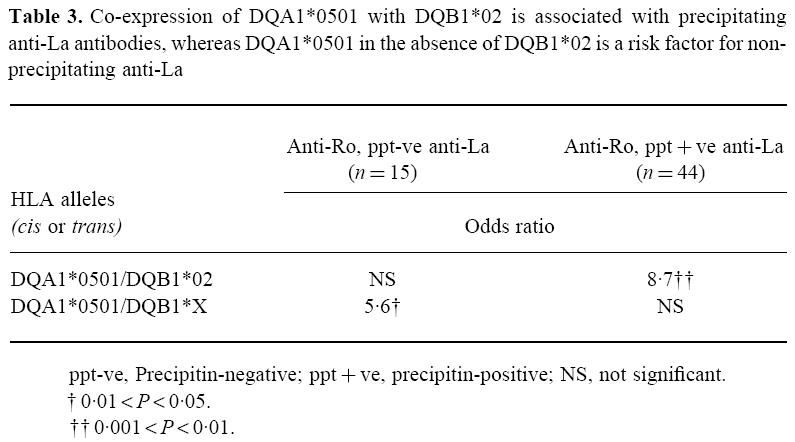
The progressive increase in the HLA-DQA1*0501 phenotype frequency with increasing autoantibody diversification suggested that this allele may be of primary importance in the expression of La/Ro autoimmunity in our pSS population. Although DQA1*0501 is most commonly co-expressed with the linked DQB1 allele DQB1*02, it sometimes occurs in the absence of this DQB1 allele. Therefore we examined the effect of co-expression of DQA1*0501 and DQB1*02 upon diversification of autoantibody responses to Ro and La. We observed that when DQA1*0501 and DQB1*02 were co-expressed then anti-La antibodies were significantly more likely to be diversified and precipitating (OR 8.7). Expression of HLA-DQA1*0501 in the absence of DQB1*02, however, was associated with non-precipitating anti-La (OR 5.6, Table 3).
Table 3.
Co-expression of DQA1*0501 with DQB1*02 is associated with precipitating anti-La antibodies, whereas DQA1*0501 in the absence of DQB1*02 is a risk factor for non-precipitating anti-La
DISCUSSION
Evidence from murine models of autoimmunity suggests that linked autoantibody responses to Ro and La are most likely to occur by T cell-dependent spreading of autoimmunity between components of the La/Ro RNP after initiation of immunity to a single component of this structure [2, 3]. Furthermore, the extent of intermolecular spreading of autoimmunity differs between various strains of mice, suggesting that genetic factors are important in modulating the patterns of autoimmune responsiveness [18]. A spectrum of autoantibody diversification is also recognized in the immune response to the La/Ro RNP in patients with pSS. Autoantibody responses can vary from undetectable to high-titre responses involving anti-Ro and anti-La precipitins. Patients with intermediate levels of autoantibody diversification remain stable in their expression of either anti-Ro without anti-La, or anti-Ro with low-titre non-precipitating anti-La antibodies [8, 9]. We therefore postulated that serological subtypes of La/Ro autoimmunity observed in pSS were likely to be genetically determined. More specifically, we predicted that separate HLA class II associations might reflect T cell recognition of unique peptides derived from either or both of the La/Ro polypeptides within the La/Ro RNP. Comparison of HLA class II phenotypes between the pSS subgroups and the normal control population clearly demonstrated that alleles of the HLA-DR2-DQA1*0102-DQB1*0602 (DR2-DQ1) and HLA-DR3-DQA1*0501-DQB1*02 (DR3-DQ2) haplotype were strongly associated with autoantibodies to Ro alone, with or without a restricted anti-La antibody response (non-precipitating). By contrast, the tendency to develop a more amplified polyclonal autoimmune phenotype with precipitating anti-La antibodies was markedly increased in patients carrying elements of the DR3-DQA1*0501-DQB1*02 (DR3-DQ2) haplotype.
A simple model to explain these findings is proposed in Fig. 1. The model proposes the normal existence of separate populations of circulating, surface immunoglobulin-positive B cells with autospecificity for La and Ro polypeptides. Following uptake and processing of La/Ro RNPs through capture by the Ro-specific B cell receptor, anti-Ro antibody responses in autoimmune patients are driven by cognate interactions between Ro-specific B cells and helper T cells. Initiation of anti-Ro autoimmunity can occur when autoreactive B cells present HLA-DR2- or DQ1-restricted Ro peptides to Ro-specific T helper cells (Fig. 1a, upper panel). We propose that the DR2-DQ1 haplotype is deficient at activating La-specific T cells. However, in patients with DR2-DQ1, La-specific B cells can also take up La/Ro RNP via their surface immunoglobulin, and may then present HLA-DR2- or DQ1-restricted Ro peptides to Ro-specific T helper cells, thereby gaining intermolecular help for anti-La antibody production (Fig. 1a, lower panel). This intermolecular help is limited, as reflected by the production of only non-precipitating anti-La antibodies, because only a subset of La molecules is associated with Ro RNP and thus restricted numbers of La-specific B cells are able to simultaneously capture La/Ro RNPs by surface immunoglobulin. These La-specific B cells might compete poorly with Ro-specific B cells for T cell help, resulting in the production of low-titre, low-affinity, pauciclonal anti-La antibodies lacking the ability to form latticed precipitates.
Fig. 1.

Model showing HLA-restricted control of anti-La/Ro autoantibody diversification. (a) The model proposes that anti-La/Ro autoimmunity can be initiated when anti-Ro T cells are activated and provide helper signals for Ro-specific B cells presenting HLA-DR2- or -DQ1-restricted processed Ro peptides (upper panel). Ro-specific B cells selectively bind La/Ro RNPs through their membrane immunoglobulin, which then undergoes receptor-mediated endocytosis leading to antigen processing and presentation of captured proteins. La-specific B cells also take up La/Ro RNP via their mIg receptor and also present HLA-DR2- or -DQ1-restricted Ro peptides to Ro-specific T helper cells, thereby gaining intermolecular help for anti-La antibody production (lower panel). The latter intermolecular help is limited because there is a pool of free La which restricts the number of anti-La B cells able to take up La and Ro together. This results in the production of low-titre, low-affinity, pauciclonal anti-La antibodies lacking the ability to form latticed precipitates. (b) Anti-Ro autoimmunity can also be initiated by T helper cells recognizing Ro peptides presented by DR3 or DQ2 (lower panel), but it is proposed that these HLA molecules also present La peptides (upper panel). Thus, high-affinity precipitating anti-La antibody responses are driven by La-specific T helper cells following presentation of HLA-DR3-DQ2-restricted processed La peptides by a broad range of La-specific B cells.
In contrast, we propose that patients with the DR3-DQ2 haplotype can present peptides derived from both Ro and La antigens to specific helper T cells. Thus, high-titre, high-affinity precipitating anti-La antibody responses are driven by La-specific T helper cells following presentation of HLA-DR3-DQ2-restricted La peptides by polyclonal La-specific B cells (Fig. 1b, upper panel). In addition, a strong anti-Ro antibody response is driven by polyclonal B cell presentation of Ro peptides to Ro-specific T cells also restricted by the HLA-DR3-DQ2 haplotype (Fig. 1b, lower panel).
The model proposed here is clearly speculative and other alternatives are obviously possible. For instance, the data are entirely consistent with effects of non-structural HLA class II or other MHC genes in linkage disequilibrium with specific class II alleles. Such influences could either act alone or concurrently with HLA-encoded risk. Candidate genetic factors might include polymorphisms at gene promoters, genes associated with antigen presentation, TNF genes or as yet uncharacterized genes within the MHC. Furthermore, a generalized or non-specific effect for the DR3-DQ2 phenotype on antibody production cannot be ruled out, since patients with precipitating anti-La antibodies tended to have higher rheumatoid factor levels and total IgG levels than precipitin-negative patients. Nonetheless, recent findings in animal models of autoimmunity have revealed that defined peptide determinants within nuclear self antigens can induce complex patterns of autoimmunity towards RNP complexes [19, 20], including the La/Ro RNP [3]. In other words, initiating immunity to a single determinant, presumably presented by a single HLA molecule, can lead to a diversified autoantibody response to Ro and La autoantigens. This observation suggests how HLA control of the response to just one or two peptides within a self antigen might control subsequent diversification and amplification of autoimmunity.
A feature of our study is the careful inclusion of those patients with only pSS in a racially homogeneous population of Caucasians. This may be why some of the findings are not exactly the same as those of other studies examining particular ethnic groups, or studies including patients with SLE. Because of the strong linkage disequilibrium between alleles of the DRB1, DQA1 and DQB1 loci within the MHC locus on chromosome 6, this type of study has limited ability to determine whether disease associations are primarily with DR or DQ alleles. Both HLA-DR5 and HLA-DR3 have been reported to be associated with pSS in different populations [10,21–23], and as both these alleles are in linkage disequilibrium with DQA1*0501, this DQA1 allele has previously been implicated as the common HLA risk factor for pSS. There are several lines of evidence in support of the notion that DQA1*0501 may be a critical determinant for anti-La responses. First, the four anti-La-positive patients who lacked both DR2 and DR3 all expressed the DQA1*0501 allele. Second, increasing diversification of the autoantibody response to the La/Ro RNP was associated with increasing frequency of HLA-DQA1*0501 (controls = 43%; seronegatives = 60%; anti-Ro alone = 67%; anti-Ro and precipitin-negative anti-La = 73%; anti-Ro and precipitin-positive anti-La = 93%). Finally, we observed that anti-La antibodies were likely to be precipitating when DQA1*0501 and DQB1*02 were co-expressed, whereas expression of HLA-DQA1*0501 in the absence of DQB1*02 (DR5 haplotypes) was predominantly associated with non-precipitating anti-La. These data imply that La peptide determinants may be efficiently presented to T cells by DQ molecules composed of the DQA1*0501 and DQB1*02 chains, and less efficiently presented by DQ molecules bearing DQA1*0501 in conjunction with other DQβ chains.
We have presented evidence that in a clinical population, diversification and amplification of the autoimmune response to the La/Ro RNP is strongly influenced by distinct HLA class II alleles. We suggest a mechanism by which different HLA class II molecules might preferentially engender Ro-specific and La-specific T helper responses, thereby controlling diversification of the autoantibody response. This hypothesis can be further tested by examining the specificity and HLA restriction of anti-La/Ro T cells in patients with primary Sjögren's syndrome.
Acknowledgments
We thank the Arthritis Foundation of South Australia Lupus/Scleroderma group for informing primary Sjögren's syndrome patients about this work. Additional thanks to Dr Catherine Keech for providing technical assistance, and Mr Greg Bennett for his assistance in coordination of the HLA typing. This work was supported by grants from the NH & MRC Australia, Arthritis Foundation of Australia and the Flinders Medical Centre Research Foundation, South Australia.
References
- 1.Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- 2.Topfer F, Gordon T, McCluskey J. Intra- and inter-molecular spreading of autoimmunity involving the nuclear self-antigens La (SS-B) and Ro (SS-A) Proc Natl Acad Sci USA. 1995;92:8875–9. doi: 10.1073/pnas.92.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds P, Gordon TP, Purcell AW, Jackson DC, McCluskey J. Hierarchical self tolerance to T cell determinants within the ubiquitous nuclear self antigen La (SS-B) permits induction of systemic autoimmunity in normal mice. J Exp Med. 1996;184:1–14. doi: 10.1084/jem.184.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahearn JM, Provost TT, Dorsch CA, Stevens MB, Bias WB, Arnett FC. The interrelationships of HLA-DR, MB and MT phenotypes, autoantibody expression and clinical features in systemic lupus erythematosus. Arthritis Rheum. 1982;25:1031–40. doi: 10.1002/art.1780250901. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton RG, Harley JB, Bias WB, Roebber M, Reichlin M, Hochberg MC, Arnett FC. Two Ro (SS-A) autoantibody responses in systemic lupus erythematosus. Correlation of HLA-DR/DQ specificities with quantitative expression of Ro (SS-A) autoantibody. Arthritis Rheum. 1988;31:496–505. doi: 10.1002/art.1780310406. [DOI] [PubMed] [Google Scholar]
- 6.Lulli P, Sebastiani GD, Trabace S, et al. HLA antigens in Italian patients with systemic lupus erythematosus: evidence for the association of DQw2 with the autoantibody response to extractable nuclear antigens. Clin Exp Rheumatol. 1991;9:475–9. [PubMed] [Google Scholar]
- 7.Kerttula TO, Collin P, Polvi A, Korpela M, Partanen J, Mäki M. Distinct immunologic features of Finnish Sjögren's syndrome patients with HLA alleles DRB1*0301, DQA1*0501, and DQB1*0201. Alterations in circulating T cell receptor γ/δ subsets. Arthritis Rheum. 1996;39:1733–9. doi: 10.1002/art.1780391017. [DOI] [PubMed] [Google Scholar]
- 8.Gordon T, Mavrangelos C, McCluskey J. Restricted epitope recognition by precipitin-negative anti-La/SS-B-positive sera. Arthritis Rheum. 1992;35:663–6. doi: 10.1002/art.1780350609. [DOI] [PubMed] [Google Scholar]
- 9.Beer RG, Rischmueller M, Coates T, Purcell AW, Keech CL, McCluskey J, Gordon TP. Nonprecipitating anti-La (SS-B) autoantibodies in primary Sjögren's syndrome. Clin Immunol Immunopathol. 1996;79:314–8. doi: 10.1006/clin.1996.0084. [DOI] [PubMed] [Google Scholar]
- 10.Roitberg-Tambur A, Friedmann A, Safirman C, et al. Molecular analysis of HLA class II genes in primary Sjögren's syndrome. A study of Israeli Jewish and Greek non-Jewish patients. Human Immunol. 1993;36:235–42. doi: 10.1016/0198-8859(93)90130-s. [DOI] [PubMed] [Google Scholar]
- 11.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Preliminary criteria for the classification of Sjögren's syndrome. Arthritis Rheum. 1993;36:340–7. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura RM, Peebles CL, Rubin RL, Molden DP, Tan EM. 2. Chicago: American Soc Clin Pathol Press; 1985. Autoantibodies to nuclear antigens (ANA) [Google Scholar]
- 13.Keech CL, McCluskey J, Gordon TP. Transfection and overexpression of the human 60-kDa Ro/SS-A autoantigen in HEp-2 cells. Clin Immunol Immunopathol. 1994;73:46–51. doi: 10.1006/clin.1994.1181. [DOI] [PubMed] [Google Scholar]
- 14.Provost TT, Watson R. Cutaneous manifestations of Sjögren's syndrome. Rheum Dis Clin North Am. 1992;18:609–16. [PubMed] [Google Scholar]
- 15.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucl Acid Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura A, Sasazuki T. Eleventh International Histocompatibility Workshop reference protocol for the HLA DNA typing technique. In: Tsuji K, Aizawa M, Sasazuki T, editors. HLA 1991: Proceedings of the Eleventh International Histocompatibility Workshop and conference. Vol. 1. Oxford Science Publications; 1992. p. 397. [Google Scholar]
- 17.Fernandez-Vina M, Bignon J. Primers and probes for HLA class II typing. Twelfth International Histocompatibility Workshop Technical Handbook (Version 01/95)
- 18.Keech CL, Gordon TP, McCluskey J. The immune response to 52-kilodalton Ro and 60-kilodalton Ro is linked in experimental autoimmunity. J Immunol. 1996;157:3694–9. [PubMed] [Google Scholar]
- 19.Bockenstedt LK, Gee RJ, Mamula MJ. Self-peptides in the initiation of lupus autoimmunity. J Immunol. 1995;154:3516–24. [PubMed] [Google Scholar]
- 20.James JA, Gross T, Scofield RH, Harley JB. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B′-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J Exp Med. 1995;181:453–61. doi: 10.1084/jem.181.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinzova E, Ivanyi D, Sula K, Horejs J, Dostal C, Drizhal I. HLA-Dw3 in Sjögren's syndrome. Tissue Antigens. 1977;37:10–15. doi: 10.1111/j.1399-0039.1977.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 22.Chused TM, Kassan SS, Opelz G, Moutsopoulus HM, Terasaki PI. Sjögren's syndrome associated with Dw3. N Engl J Med. 1977;296:895–7. doi: 10.1056/NEJM197704212961602. [DOI] [PubMed] [Google Scholar]
- 23.Papasteriades C, Skopouli FN, Drosos AA, Andonopoulos AP, Moutsopoulos HM. HLA alloantigen associations in Greek patients with Sjögren's syndrome. J Autoimmun. 1988;1:85–91. doi: 10.1016/0896-8411(88)90079-0. [DOI] [PubMed] [Google Scholar]