Abstract
Increasing evidence has implicated TNF-α as a pivotal molecule involved in the systemic inflammatory manifestations of ENL, an acute inflammatory complication that may occur in the chronic course of leprosy. In the present study, the mechanism of action of pentoxifylline (PTX) as an alternative therapy for management of leprosy reactions has been evaluated. The effect of PTX on TNF-α production was examined in leprosy patients at the protein level and at the transcriptional level as well. Treatment of ENL patients with PTX (1200 mg daily) ameliorated the systemic symptoms and favoured the evolution of reactional leprosy lesions. Serum TNF-α was assayed before and during treatment with PTX in 15 patients. The increased TNF-α levels seen in the circulation during the reaction were dramatically reduced within 3–7 days of therapy. No significant effect on serum IL-6 was noted. In vitro TNF-α production was assayed upon culture stimulation with Mycobacterium leprae. A reduction of inducible TNF-α in peripheral blood mononuclear cells (PBMC) was seen after 1–2 weeks of in vivo administration of PTX. Furthermore, no effect of the drug on IL-10 secretion was detected in these cultures. A kinetic analysis of the expression of TNF-α and IL-6 mRNA at the site of leprosy lesion was performed in six reactional patients by semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR). The amount of TNF-α mRNA was increased in the tissue during ENL compared with before the reaction, and decreased thereafter following treatment for reaction (either PTX or thalidomide). These data suggest that PTX inhibits TNF-α production in ENL patients both in vivo and in vitro, and it may be useful in the treatment of leprosy patients undergoing ENL.
Keywords: pentoxifylline, tumour necrosis factor-alpha, erythema nodosum leprosum, thalidomide, RT-PCR
INTRODUCTION
All information accumulated in the past few years has implicated cytokines in the pathogenic mechanisms of several human diseases. Since then, TNF-α has been considered a key molecule in the intricate net of interaction between blood leucocytes and tissue cells. TNF-α is thought to be a proximal factor of the inflammatory response, and it has been considered an important mediator in a wide variety of acute and chronic inflammatory disease states [1–3].
Our first demonstration that leprosy patients undergoing reactional inflammatory episodes (ENL) present increased TNF-α and IL-1β levels in their sera [4] has been confirmed by other groups [5, 6]. ENL develops more frequently towards the lepromatous pole of leprosy and it is characterized by symptoms resembling septic syndrome accompanied by both local and systemic inflammation. Increased evidence has so far implicated TNF-α as a pivotal molecule involved in the inflammatory manifestations of ENL and in mediating tissue damage in leprosy. It has been demonstrated that cells from these patients release enhanced amounts of TNF-α when stimulated in vitro, compared with unreactional lepromatous patients or normal individuals [7, 8]. In addition, increased expression of TNF-α mRNA and TNF-α protein has been identified in reactional leprosy lesions [9].
IL-6, also a proinflammatory cytokine, has also been found in the serum of leprosy patients with reaction [10], probably implicating this cytokine in the complex set of responses seen in these reactional leprosy patients, such as enhanced acute-phase response. Accordingly, it has been reported that levels of C-reactive protein (CRP) and serum amyloid A (SAA) are markedly elevated in ENL patients [11].
On the other hand, increased production of IL-10 has been recently implicated in the functional impairment of the immune response. IL-10 has a profound inhibitory role by decreasing macrophage function. It inhibits antigen presentation and cytokine synthesis by monocytes [12–14]. Furthermore, it has been suggested that IL-10, released mainly from Mycobacterium leprae-infected macrophages [15], could be involved in mediating the anergic state seen in the lepromatous form of leprosy [15, 16].
Because of the inflammatory symptoms and of neurological complications that are very often associated with these reactional episodes in leprosy, they have to be considered as a clinical emergency, and treatment with thalidomide and/or steroids needs to be promptly initiated. It was first shown by our group that thalidomide, the drug of choice for treatment of ENL, inhibits TNF-α production either in vivo or in vitro [10, 17]. The effect of thalidomide was specific, and it suppressed lipopolysaccharide (LPS)- and mycobacteria-induced monocyte-derived TNF-α production in vitro, at the level of both TNF mRNA expression and protein secretion. The drug seemed to enhance TNF-α mRNA degradation [18] and, in vivo, it was also demonstrated to down-modulate the expression of activation molecules (intercellular adhesion molecule-1 (ICAM-1), HLA-DR) in the reactional tissue when the reactional episode subsided [17]. Nevertheless, the predominant side-effects of corticosteroids and the teratogenic activity of thalidomide have limited their use in reactional patients, mainly females of child-bearing age. The use of other anti-inflammatory drugs for treatment of reactional leprosy patients is therefore warranted.
The methylxanthine PTX is a drug of known haemorrheological activity, used clinically for treatment of intermittent claudication and other conditions involving defective regional microcirculation [19]. Following the demonstration that PTX suppress induction of TNF-α in endotoxin-treated murine macrophages [20], and in healthy human volunteers given endotoxin [21], the drug has been used as a therapeutic tool in many other immunological based disorders in which TNF seems to play a major role, with a clinically beneficial effect in some cases [22, 23].
We have previously reported our preliminary clinical observations on the use of PTX as an alternative therapy for management of leprosy reactions [24]. Although PTX is not as rapidly acting as thalidomide, treatment of ENL patients with the former drug alleviated the clinical manifestations of reaction in most of the patients tested. In the present study, the effect of PTX on TNF-α production has been examined in these patients, both in vivo and in vitro, at the protein level and also at the transcriptional level. Here, cytokine gene expression has been evaluated in leprosy biopsies before and after treatment with PTX, compared with thalidomide, by semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR).
PATIENTS AND METHODS
Patient population
Leprosy patients from the Leprosy Out-Patient Unit, Oswaldo Cruz Foundation (Rio de Janeiro, Brazil) were diagnosed according to the Ridley–Jopling classification [25]. A total of 18 multibacillary patients (14 males and four females), 15–64 years old (mean ± s.d. 34.9 ± 15 years), who were undergoing acute inflammatory reactional episodes, was included in the study. Patients were classified as lepromatous leprosy (LL, n = 13) and borderline lepromatous (BL, n = 5). All were lepromin-negative, having a bacterial index (BI) ranging from 2.0 to 5.5 + (mean BI ± s.d. 4.2 ± 1.1 +), and had been treated with multidrug therapy (MDT) as recommended by WHO [10]. The clinical features of each patient are summarized in Table 1. Seven ENL patients were newly diagnosed leprosy patients who had not received any kind of treatment prior to reaction (patients 3, 6, 10, 14–16, 18). In these individuals, MDT was initiated while patients were still on treatment for reaction (either PTX or thalidomide). Four patients (patients 5, 11–13, 17) developed ENL when they had already completed the 2 years of chemotherapy and were under surveillance (Surv). For the other patients (patients 1, 2, 4, 7–9), MDT was continued throughout the study. For treatment of the reactional episode, patients (n = 16) were prescribed with PTX 400 mg (Hoechst do Brasil, S.A., Sao Paulo, Brazil), three times a day, for a 2-month period. A simultaneous analysis was performed in two lepromatous patients who developed ENL (patients 17 and 18, Table 1) and were treated with thalidomide (300 mg/day) until total remission of the inflammatory symptoms.
Table 1.
Clinical features of ENL patients enrolled into the study
Skin biopsies
After informed consent, biopsies (6 mm punch) were obtained from the patients at the time of leprosy diagnosis and at the onset of the reactional episode. Biopsies were processed for histological and PCR analysis. For RT-PCR, fresh biopsy samples were collected at diagnosis (unreactional leprosy lesion, two patients), at the onset of the reactional episode (ENL lesion, six patients), and after 3 and/or 7 days of PTX (reactional lesion, four patients) or thalidomide (two patients) therapy.
Serum samples
Serum was collected for determination of circulating cytokine levels during ENL (day 0), and at different periods (3–7, 10–14, 30 and 60 days) after PTX administration. Serum was collected aseptically, processed under sterile conditions, stored in 0.5-ml aliquots and kept frozen until use (−20°C).
Culture stimulation
Armadillo-derived M. leprae antigen, provided by Dr R. J. W. Rees (IMMLEP Bank, The National Institute of Medical Research, Mill Hill, UK), was used at 10 μg/ml. In some cultures, LPS (from Salmonella minnesota R595; Sigma Chemical Co., St Louis, MO) 1 μg/ml was used as a positive control for TNF-α induction. For in vitro use, PTX (Sigma) was dissolved in PBS pH 7.0 and used in the cell cultures at final concentrations of 25 and 50 μg/ml. Concentrations of contaminating endotoxin in stock solutions, culture medium, and antigens were estimated by the Limulus amoebocyte lysate assay (LAL; Whittaker M.A. Bioproducts, Walkersville, MD). Reagents contained < 10 pg/ml endotoxin.
Cell preparation and culture
Heparinized venous blood was collected for in vitro tests and peripheral blood mononuclear cells (PBMC) were isolated by Ficoll–Hypaque (Pharmacia Fine Chemicals, Piscataway, NJ) density centrifugation. Cells were suspended in RPMI 1640 medium (Gibco Labs, Gaithersburg, MD), supplemented with 10% human AB serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mml-glutamine (Gibco, complete medium), and cultured (1 × 106 cells) onto a 24-well plate (Costar Corp., Cambridge, MA) for in vitro cytokine stimulation (PBMC cultures). For monocyte cultures, PBMC were allowed to adhere for 2 h at 37°C. After this period, non-adherent cells were removed by rinsing four times with warmed RPMI medium and cultures replaced with 1 ml complete medium. The purity of monocytes was > 95% as determined by morphological examination and non-specific esterase staining. Both the PBMC and the attached monocyte-enriched population were then further incubated in the presence or absence of TNF-α-inducing agonists (either M. leprae or LPS) for 18–20 h, when culture supernatants were harvested, centrifuged, and kept frozen until use (−20°C).
Cytokine determination
Concentration of TNF-α, IL-6 and IL-10 in supernatants and serum samples was determined using commercial specific ELISA kits (IL-6, R&D Systems, Inc., Minneapolis, MN; IL-10, Endogen Inc., Boston, MA; TNF-α, Innogenetics Inc., Antwerp, Belgium) processed according to the manufacturers' specifications. Serial samples from a given patient were assayed at the same time. Cytokine levels are expressed as pg of protein/ml. The detection limit of the assay was 5 pg/ml for TNF-α, 3 pg/ml for IL-10, and 0.4 pg/ml for IL-6.
RNA isolation and cDNA synthesis
Frozen biopsies were recovered, when dermis and epidermis were separated, and the tissues homogenized in a Politron PT-3000 in 3 ml of Trizol (Gibco). Total RNA was extracted according to the manufacturer's instructions. Spectrophotometric analysis at 260 nm was used to measure the purity and concentration of RNA. One microgram of total RNA obtained from the dermis was reverse-transcribed into cDNA. The RT reaction mixture was performed by mixing 1 μg RNA with 1 μg of an oligo-dT primer (United States Biochemical Corp., Cleveland, OH), in a volume of 8 μl, and incubated for 10 min at 65°C [26]. After cooling on ice, a solution containing 5× RT buffer, 40 U RNAsin (Promega Biotec, Madison, WI), 10 mm DTT (Boehringer Mannheim Biochemicals, Indianapolis, IN), 100 μg/ml acetylated bovine serum albumin (BSA; Promega Biotec), 250 μm of each dNTP (Perkin Elmer Cetus, Emeryville, CA), and SuperScript II RNAse H− reverse transcriptase (Gibco) at 200 U, in a final volume of 20 μl, was added to the tubes, which were incubated for 1 h at 42°C. Finally, tubes were heated to 95°C for 10 min, and 80 μl of dH2O were added to the mixture. Samples were stored at −20°C until further use. This reaction was always performed simultaneously for parallel samples from one experiment.
PCR conditions
Cytokine-specific oligonucleotide primer pairs TNF-α, IL-6 and β-actin were purchased (Stratagene Cloning System, La Jolla, CA) or synthesized (Macromolecular Resources, Fort Collins, CO). The primers were RNA-specific in that both the 5′ and 3′ primers span the junctions of two exons, thus precluding amplification of genomic DNA. PCR primer sequences (5′ and 3′) for the cytokines and the control β-actin were as follows: TNF-α, CGGGACGTGGAGCTGGCCGAGGAG, CACCAGCTGGTTATCTCTCAGCTC; IL-6, ATGAACTCCTTCTCCACAAGCGC, GAAGAGCCCTCAGGCTGGACTG; β-actin, TGACGGGGGTCACCCACACTGTGCCCATCTA, CTAGAAGCATTTGCGGTGGGACGGATGGAGGG. cDNA (5 μl) was added to 20 μl of a solution consisting of 1 U Taq DNA polymerase (Perkin Elmer), 200 μm dNTPs, 1 mm primers, and PCR buffer (1.5 mm MgCl2, 50 mm KCl, 10 mm Tris–HCl pH 8.3, 0.01% gelatin, and 0.1% Triton X-100). The reaction mixture was overlaid with a drop of mineral oil, and PCR was performed in a DNA thermocycler 480 (Perkin Elmer) for 35 cycles of denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 1.5 min. PCR product (5 μl) was subjected to electrophoresis on 2% agarose gels and visualized by staining with ethidium bromide. Specificity of the amplified bands was validated by their predicted size. PCR-assisted mRNA amplification was performed simultaneously for all samples of each patient included in the study.
Hybridization of PCR product
To confirm the specificity of PCR amplification, and for semiquantitative analysis, PCR products were transferred to Hybond-N nylon membranes (Amersham Corp., Arlington Heights, IL) and hybridized with a radioactive oligonucleotide probe complementary to sequences internal to those recognized by the specific primers. Sequences of the probes were: β-actin, CTGCTGACCGAGGCCCCCCTGAACCCC; TNF-α, TGAGGGTTTGCTA CATGGGCTA; IL-6, TCTTGTTACATGTCTCCTTTCTCAG. The probe was labelled at the 5′ end with T4 polynucleotide kinase (Boehringer) and 32P-γATP (6000 Ci/mm; Amersham). Blots were hybridized with probe overnight, washed twice for 15 min with 2× SSC and 0.5% SDS, followed by 0.5× SSC and 0.1% SDS, at 42°C, and then exposed to x-ray film (ECL Hyperfilm; Amersham). Densitometer analysis was performed by scanning the images from autoradiograms (Quick Scan; Helena Labs, Beverly, MA). The relative amount of PCR product present in an individual sample was expressed as percentage relative to the most intense band, assigned the value of 100. To make sure that the samples contained identical quantities of cDNA, β-actin was used in order to normalize the exact levels of input cDNA present among the different samples tested.
Validity of PCR amplification for semiquantitative comparison of cytokine mRNA expression
A number of controls were employed to permit semiquantitative comparison of cytokine mRNA expression in the biopsy samples studied. Sensitivity of PCR was equivalent for each cytokine based on titration of plasmid cDNA (serial 10-fold dilutions). There was a log-linear correlation between the number of starting copies of plasmid and the yield of PCR products across the range of plasmid concentrations investigated. Linearity of PCR amplification was also demonstrated by varying the number of PCR cycles. Repeat PCR analysis of the same samples yielded reproducible results. These studies indicate that our PCR conditions were not within the plateau phase of amplification. In addition, precautions were taken to avoid cross-contamination of samples, including use of aliquotted reagents, pipettes dedicated for assembling PCR reactions, and use of aerosol-resistant pipette tips.
Statistical analysis
The variation observed in cytokine levels in patients' samples during PTX treatment was assayed through the Friedman anova test (Statsoft Inc., 1995; STATISTICA for Windows (Computer Program Manual) Tulsa, OK) [27]. For the Friedman anova by ranks test, the interpretation of results is similar to that of repeated measures anova, to which it is a non-parametric alternative. All data are given as mean ± s.e.m. For statistical analysis of paired samples, the Wilcoxon signed-rank test was used. The significance level adopted was P < 0.05.
RESULTS
As previously reported [24], reactional leprosy patients treated with PTX (1200 mg daily) experienced, within the first week of treatment, some degree of relief to total regression of their systemic symptoms, which included fever, malaise, headache, and insomnia. Patients reported an increased sense of well-being, with no adverse side-effects. In addition, a decrease in the inflammatory aspects of ENL skin lesions was noted after 7–14 days. Our data showed that oral PTX is safe and well tolerated in patients with leprosy.
Effect of oral PTX on circulating cytokines
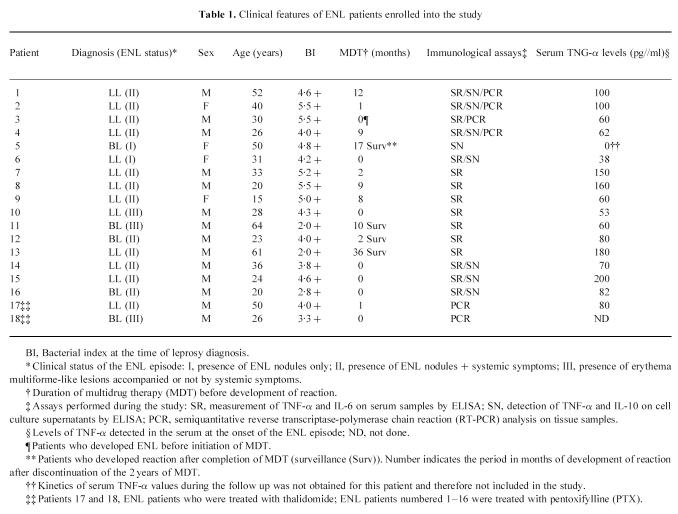
Serum TNF-α levels were assayed before and during treatment with PTX in 15 patients. Elevated amounts of TNF-α were found in the serum of reactional patients who presented systemic symptoms (Table 1) [7]. As shown in Fig. 1, LL patients had increased TNF-α levels (mean ± s.e.m. 101 ± 14.8 pg/ml) in the circulation during the reaction (ENL, day 0), and treatment with PTX dramatically reduced TNF-α values already within 3–7 days of treatment (Fig. 1a,b). The reduction in mean TNF-α noted after 7 days (24.4 ± 5.4 pg/ml (mean ± s.e.m.)) was statistically significant (Fig. 1d, P = 0.006, Friedman anova test). In four patients (Fig. 1b), although systemic clinical manifestations were impaired with PTX, baseline serum levels rose again in the course (after 2–3 weeks) of PTX therapy. In these patients, the further appearance of ENL nodules, noted during PTX, in the absence of systemic symptoms [24], did not comprise a major clinical complaint. No significant difference was observed in the increase in mean TNF-α values noted after 14 (P = 0.102) and 30 days of treatment (P = 0.414) compared with 7 days of PTX (Fig. 1d). Nevertheless, when compared with the values seen at the onset of reaction, the reduction in TNF-α was still significant (P < 0.02 and 0.05, respectively). Moreover, mean TNF-α at the end of the study (total of 8 weeks under PTX) was 13.3 ± 7.6 pg/ml, still a significant decrease (86.8% of baseline levels) in TNF-α compared with values obtained at the onset of the study (Fig. 1d, P = 0.004). In addition, on an individual basis, the decrease in systemic TNF-α paralleled the improvement in patients' clinical symptomatology (not shown). In two patients, who seemed to be refractory to PTX, serum TNF-α was found to be even higher after 1 week of PTX (Fig. 1c), when treatment with thalidomide and/or steroid was initiated and improvement of their clinical condition was noted 4 days later. Therefore, TNF-α values from these two patients were not included in the previous analysis.
Fig. 1.
Levels of TNF-α were assayed in the serum of 15 reactional patients who had been treated with 1200 mg pentoxifylline (PTX) daily for a total of 2 months. Serum was collected at the onset of the reaction episode and during treatment with PTX. (a) TNF-α values were promptly reduced within the first week of treatment (n = 9). (b) In four patients, although systemic symptoms were impaired by PTX, baseline serum TNF levels rose again in the course of PTX therapy. (c) There are two patients who were refractory to PTX. Closed symbols represent the period when treatment with thalidomide and/or steroids was initiated in order to control the inflammatory manifestations of reaction. Cut-off value for positive response = 45 pg/ml, which is 3 s.d. above mean values found in (*) healthy donors (n = 10, mean ± s.d.). (d) Serum TNF-α levels of reactional leprosy patients. Data are mean ± s.e.m. of 13 patients. *P < 0.05; **P < 0.01; ***P < 0.005, significant differences compared with levels obtained at the onset of the reactional episode (day 0). Variations in TNF levels between 7 and 14 or 7 and 30 days of PTX therapy were not significant (P > 0.05, Friedman anova test). Inset: levels of IL-6 in the serum of these same patients assayed before and during treatment with PTX. Data are mean ± s.e.m. No significant differences in mean IL-6 were noted (P > 0.05, Friedman anova test).
We were then interested to determine whether PTX affected the levels of IL-6 detected in the serum of leprosy patients with reaction. Similarly to what is observed for TNF-α, levels of IL-6 (382 ± 187 pg/ml) were significantly (P < 0.002, Wilcoxon rank test) enhanced in the serum of leprosy patients with reaction (n = 13) compared with levels obtained from the same patients before the reaction (61 ± 32 pg/ml). However, although in all patients but one, levels of IL-6 were diminished following PTX (not shown), there were substantial variations in IL-6 values between subjects (range 64–1890 pg/ml at day 0). Hence, no significant differences (P > 0.05, Friedman anova test) in mean IL-6 were detected at any time point tested during treatment with PTX (Fig. 1d, inset).
Effect of PTX on induced cytokine production
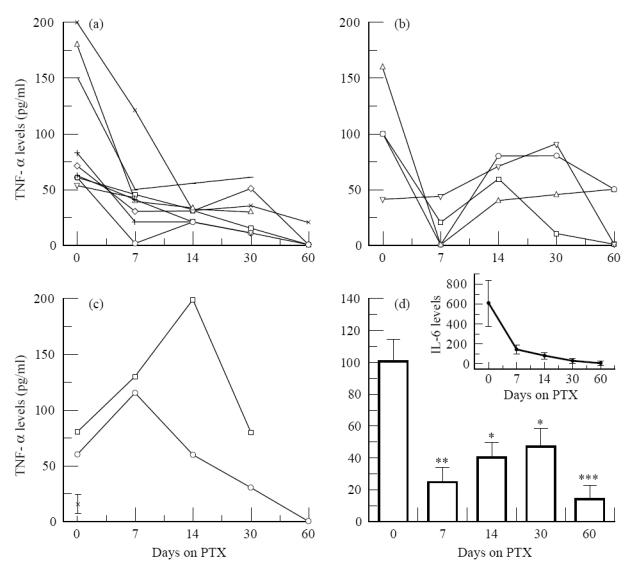
It has been previously reported that PTX inhibits LPS-induced TNF-α production by cultured monocytes in vitro [28]. So, at first, we intended to determine whether the drug was able to interfere with M. leprae-induced TNF-α production in vitro compared with its effects when LPS was used as the stimulus. PTX used at a concentration of 50 μg/ml (178 μm) induced a range of 77–88% inhibition of in vitro TNF-α release following M. leprae stimulation of PBMC/monocyte cultures (Table 2). The same effect was noted when the cells were stimulated with LPS in the presence of the drug. However, no inhibitory action of in vitro PTX was observed on either IL-6 or IL-10 secretion in those cultures (Table 2). The next step was then to determine whether PTX administered orally to leprosy patients induced a similar phenomenon in in vitro TNF-α secretion. Likewise, the production of TNF-α by PBMC and isolated monocytes cultured overnight following stimulation with M. leprae was assayed for this group. Since the most pronounced effect of PTX on serum TNF-α was observed during the first week of treatment, most immunological in vitro assays were then performed at this period. In vitro TNF-α production by PBMC and purified monocytes was assayed in eight patients who had been receiving oral PTX. Interestingly, a reduction in inducible TNF-α was seen in PBMC cultures stimulated with M. leprae from six patients, after 1–2 weeks of PTX therapy. The percentage reduction of baseline mean TNF-α values was 61.9% for PBMC and 41.2% for monocytes after 14 days of treatment. The decay in mean TNF-α released in vitro by PBMC observed after 14 days of PTX was statistically significant (P < 0.014, Friedman anova test, Fig. 2a). Nevertheless, it was observed that in monocyte cultures, although on an individual basis there was a tendency for decreased TNF-α secretion, the differences in mean TNF-α values were not statistically significant after 7 or 14 days of PTX compared with day 0 (P = 1.0 and 0.414, respectively).
Table 2.
Effect of in vitro pentoxifylline (PTX) on Mycobacterium leprae- or lipopolysaccharide (LPS)-induced cytokine production by cultured mononuclear cells
Fig. 2.
Effect of pentoxifylline (PTX) therapy on in vitro ( a) TNF-α and (b) IL-10 release from (▪) peripheral blood mononuclear cells (PBMC) and (•) monocytes stimulated with Mycobacterium leprae. Data are mean ± s.e.m. of eight individual experiments. *Significant difference (P < 0.05) compared with levels obtained at the onset of the reactional episode (day 0).
Since IL-10 is a well known cytokine involved in down-modulating macrophage functions, we then investigated the possibility that PTX could be additionally leading to the inhibition of TNF-α production secondary to an increased induction of IL-10 release in these cultures. In the same way, there was a trend toward increased in vitro IL-10 production in M. leprae-stimulated PBMC cultures obtained from patients after 7 days of PTX therapy (Fig. 2b, P = 0.058, Friedman anova test). Additionally, the differences in mean IL-10 values between 0 (ENL) and 14 days of PTX were no longer significant (P = 1.0).
Cytokine mRNA expression at the lesion site
Modulation of cytokine gene expression in the tissue by PTX and thalidomide was also analysed. Since PTX and thalidomide have been shown to inhibit TNF-α production in vivo and in vitro, a kinetic analysis of the expression of TNF-α mRNA at the site of leprosy lesion was performed in six ENL patients by semiquantitative RT-PCR (four patients treated with PTX and two patients treated with thalidomide). Histologically, ENL lesions contain dense cellular infiltrates extending from the lower dermis into the subcutaneous fat, composed predominantly of mononuclear cells and neutrophils. Although the epidermis of the reactional tissue also shows profound histological modifications, at this stage cytokine gene expression at the dermis was assessed.
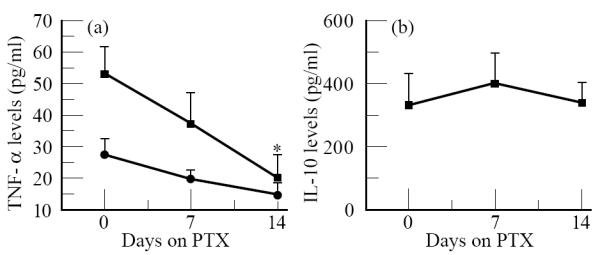
In two patients (patients 1 and 2), biopsies were collected before, at the onset of the ENL episode, and after 1 week of treatment with PTX. The amount of TNF-α mRNA was increased in the tissue during ENL compared with before the reaction, and decreased thereafter following PTX treatment (Fig. 3a). For the other two patients (patients 3 and 4), biopsies were collected during the reactional episode and at 3 and/or 7 days of PTX therapy (Fig. 3b). The high amount of TNF-α mRNA present in the lesion during ENL decreased at different rates following 1 week of PTX. The decay in the relative amount of TNF-α message varied from three- to a 50-fold decrease in three patients tested (patients 1–3). In the other patient (patient 4), a slight variation in the amount of TNF-α mRNA was noted at the lesion site following PTX therapy. Biopsies taken 3 days after PTX also showed a reduction in TNF-α message expression (Fig. 3b, patient 3).
Fig. 3.
TNF-α mRNA expression in leprosy lesions evaluated by semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR). (a) Skin biopsies were taken from two patients before (lanes 1 and 4), at the onset of the reactional episode (ENL, lanes 2 and 5), and after 7 days of pentoxifilline (PTX) (lanes 3 and 6). mRNA from different samples was reverse transcribed and the cDNA derived from lesions was normalized to yield equivalent β-actin products. The relative amounts of TNF-α mRNA were compared among different samples from each individual patient and assessed as percentage of the most intense band for each experiment. (b) Biopsies were taken from two other patients at the onset of the ENL episode (lanes 1 and 4), and after 3 (lane 2) or 7 (lanes 3 and 5) days of PTX treatment. Biopsy samples were processed as described above for RT-PCR and semiquantitative analysis. (c) TNF-α mRNA expression during thalidomide treatment evaluated by semiquantitative RT-PCR as described. Skin biopsies were taken from two patients at the onset of the reactional episode (ENL, lanes 1 and 3), and following 3 (lane 2) or 7 (lane 4) days of treatment with thalidomide.
As shown in Fig. 3c, biopsies taken from two patients (patients 17 and 18) treated with thalidomide were assayed for TNF-α mRNA expression, at the onset of the reactional episode (ENL) and during treatment with thalidomide. Similarly to what has been observed for PTX, a decreased expression of TNF-α message at the lesion site was noted after both 3 and 7 days of thalidomide therapy compared with before treatment.
Furthermore, we were interested to determine whether the expression of IL-6 mRNA at the lesion site was affected following treatment for reaction (Fig. 4). The in vivo expression of IL-6 gene was down-regulated in vivo during therapy with PTX (Fig. 4a) or thalidomide (Fig. 4b). The relative amount of IL-6 message was reduced by five- to 50-fold after 3 or 7 days of treatment.
Fig. 4.

IL-6 mRNA expression in leprosy lesions evaluated by semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR). One representative experiment for each group of patients is shown. Skin biopsies were taken from leprosy patients at the onset of the ENL episode (lanes 1 and 4), and after 3 (lane 2) or 7 days (lanes 3 and 5) of treatment with (a) pentoxifilline (PTX) or (b) thalidomide. mRNA from tissue samples was reverse transcribed and the cDNA normalized to yield equivalent β-actin products. The relative amount of IL-6 mRNA was compared among different samples from each individual patient and assessed as percentage of the most intense band.
DISCUSSION
In the present study, we were able to demonstrate that oral PTX inhibits in vivo and in vitro TNF-α production in leprosy patients with ENL. The high levels of TNF-α seen in the serum of reactional patients decreased following PTX treatment in 13 out of the 15 patients assayed. These findings are in agreement with our own previous studies [4, 7], as well as those from others [5, 6] which have confirmed that TNF-α is overproduced during the reactional states and is a key mediator of systemic symptoms and of tissue damage in leprosy in both reversal reaction (RR) and ENL [9, 10, 29]. All available data have shown that reactional leprosy patients present high circulating TNF-α levels [4–7], and that cells from these patients secrete elevated amounts of TNF-α following in vitro stimulation as well [7, 8, 30].
More interesting is the fact that here modulation of TNF-α mRNA expression at the lesion site (reactional tissue) was also achieved following patients' treatment with PTX. In addition, treatment of ENL patients with thalidomide also inhibited TNF-α gene expression at the reactional lesion. To provide meaningful comparisons between different samples, we normalized the cDNAs to the β-actin PCR product, a marker for all cells. Although we carefully controlled for levels of input cDNA, the RT-PCR analysis used is purely semiquantitative and only allows comparisons of the relative amounts of cytokine mRNA present in the lesions of the same patient.
This is the first time that inhibition of TNF-α gene expression in the tissue by PTX and thalidomide has been demonstrated. Previous studies showed that PTX therapy inhibits TNF-α mRNA expression in the PBMC of AIDS patients [ring ENL, compared with before the reaction, and decreased following treatment for reaction. This effect correlated with the increased TNF-α values seen in these patients' sera during the reactional episode, which were reduced after PTX. These data further suggest a direct relationship between increased in vivo production and release of TNF-α and the clinical symptoms presented by these patients during the reaction. In a similar way, in a very recent publication, Khanolkar-Young et al. [9] have demonstrated an increased expression of TNF-α mRNA (by in situ hybridization) and TNF-α protein in macrophages infiltrating the skin and peripheral nerves in reversal reaction. In our hands, immunohistochemical analysis of skin biopsies with anti-TNF MoAbs revealed higher numbers of TNF-α-positive cells in lepromatous reactional lesions, either RR or ENL (Sarno et al. unpublished data).
The ability of M. leprae and its fractions to induce the release of TNF-α by mononuclear cells in vitro was recently demonstrated by our group and others [7, 8, 30]. Our present data show that PTX added in vitro to the cultures significantly inhibited TNF-α release by M. leprae-stimulated PBMC and monocytes, similarly to its effect on LPS-stimulated cultures. In addition, M. leprae-induced TNF-α production in vitro by PBMC was also down-modulated following oral administration of PTX. Unexpectedly, whereas there was a trend toward TNF-α inhibition, no significant effect of PTX therapy on in vitro TNF-α release by cultured monocytes was detected. The reason for the differential effect of the drug on PBMC and monocytes in vivo is not clear. Note that purified monocytes produced less TNF-α than PBMC (Table 2 and Fig. 2a) containing an equivalent number of monocytes. This suggests a possible positive cooperation (cell–cell interaction) between monocytes and lymphocytes in the production of TNF-α, which could lead to a differential effect of the drug on these cells. PTX has been shown to modulate TNF-α production via inhibition of TNF-α mRNA transcription [20, 28] and to decrease the activity of the transcription factor NF-κB [33]. The presumed mechanism of action of PTX is secondary to the inhibition of phosphodiesterase resulting in an intracellular increase of cAMP [28, 34]. Hence, it is possible that different signals are then triggered through these different cell types by one and the same drug.
The fact that PTX has been largely used for treatment of vascular disorders suggests that other non-immune cells have a major role through the release of inflammatory factors such as TNF and IL-6. This fact raises the possibility that PTX might exert its effect also on cell types other than monocytes/macrophages, such as epithelial cells, keratinocytes, endothelial cells, and fibroblasts [35], all of them recently described as TNF-producing cells.
Increased serum levels of IL-1β and IL-6 in the sera of leprosy patients with reaction have also been previously reported [4, 10]. Here, we have further confirmed and extended these observations. Although there was a trend toward lower IL-6 values following PTX therapy, the increased levels of IL-6 detected in the sera of reactional leprosy patients were not significantly affected by oral treatment with PTX. Likewise, and consistent with findings in previous studies [18, 36], PTX when added to cell cultures did not affect the release of IL-6 following M. leprae or LPS in vitro stimulation. On the other hand, IL-6 mRNA expression in the reactional lesion was reduced following in vivo treatment for reaction (either PTX or thalidomide). Since we could not see a direct effect of both drugs on the in vitro release of IL-6 [17, 18], these data, together with the observed tendency of PTX to inhibit serum IL-6, indicate that, in vivo, inhibition of TNF-α by these drugs does have an impact on the synthesis of other inflammatory cytokines. As TNF-α is known to modulate synthesis of other inflammatory molecules [1], inhibition of TNF in vivo could ultimately lead to a reduction in other inflammatory mediators, including IL-6.
An additional mechanism by which PTX would lead to the inhibition of TNF-α release could be through the induction of an increased production of IL-10. IL-10 has been shown to inhibit cytokine synthesis by monocytes, namely TNF-α, IL-1, IL-6, IL-8, IL-12 [13, 37, 38]. Interestingly, following patients' oral administration, PTX resulted in a trend toward significantly increased IL-10 secretion by stimulated PBMC after 7 days of therapy. However, no effect of the drug when added in vitro to cell cultures has been noted. Again, we cannot exclude that this in vivo effect is the result of the natural resolution of the inflammatory response. Curiously, it was recently reported that PTX inhibited IL-10 production by human T cells infected with HIV [39].
The diverse biological effects of TNF-α make it a prime suspect in either the initiation and/or amplification of tissue injury in leprosy reaction. We have postulated that TNF-α is involved in mediating nerve damage in leprosy [29]. Limiting the development of reactions and deformities in leprosy patients constitutes a major effort in controlling the disease. Thalidomide and/or steroids have been used in these patients in order to control the clinical manifestations of reaction. Because of their side-effects, the use of alternative drugs for treatment of ENL which down-regulate TNF production seems a logical approach to management of reaction.
In conclusion, we demonstrate here that PTX decreased systemic TNF-α levels in these reactional patients, suppressed induction of TNF-α protein by mononuclear cells in vitro, and down-regulated TNF-α and IL-6 mRNA expression at the lesion site. This study constituted an open controlled pilot trial on the use of PTX for treatment of leprosy patients with ENL reaction [24]. Although the effect of PTX in ENL might be of no longer lasting benefit compared with thalidomide or steroids, this pilot study suggests that PTX may be useful as an alternative therapy for ENL patients, mainly females of child-bearing age, and in patients whose treatment with steroids is not well tolerated. Controlled clinical trials are needed to confirm these expectations.
Acknowledgments
We thank R. B. Oliveira, R. C. L. Martins and A. P. V. Castro for technical assistance. Thanks are also due to A. S. Almeida for the graphic work and E. R. Leite for efficient secretarial help.
References
- 1.Fiers W. Biologic therapy with TNF. Preclinical studies. In: De Vita VT Jr, Hellman S, Rosenberg SA, editors. Biology therapy of cancer. 2. Philadelphia JB: Lippincott Co.; 1995. pp. 295–327. [Google Scholar]
- 2.Sharief MK, Hentges R. Association between tumor necrosis factor-α and disease progression in patients with multiple sclerosis. N Engl J Med. 1991;325:467–72. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- 3.Tetta C, Camussi G, Modena V, Di Vittoria C, Baglioni C. Tumor necrosis factor in serum and synovial fluid of patients with active and severe rheumatoid arthritis. Ann Rheum Dis. 1990;49:665–7. doi: 10.1136/ard.49.9.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarno EN, Grau GE, Vieira LMM, Nery JA. Serum levels of tumour necrosis factor-alpha and interleukin-1β during leprosy reactional states. Clin Exp Immunol. 1991;84:103–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Parida SK, Grau GE, Zahfer SA, Mukherjee R. Serum tumor necrosis factor and interleukin 1 in leprosy and during lepra reactions. Clin Immunol Immunopathol. 1992;62:23–27. doi: 10.1016/0090-1229(92)90088-6. [DOI] [PubMed] [Google Scholar]
- 6.Foss NB, Oliveira EB, Silva CL. Correlation between TNF production, increase of plasma C-reactive protein level and suppression of T lymphocyte response to concanavalin A during erythema nodosum leprosum. Int J Lepr. 1993;61:218–26. [PubMed] [Google Scholar]
- 7.Sampaio EP, Moreira AL, Sarno EN, Malta AM, Kaplan G. Prolonged treatment with recombinant interferon γ induces erythema nodosum leprosum in lepromatous leprosy patients. J Exp Med. 1992;175:1729–37. doi: 10.1084/jem.175.6.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampaio EP, Duppre NC, Moreira AL, Nery JAC, Sarno EN. Development of giant reaction in response to PPD skin test in lepromatous leprosy patients. Int J Lepr. 1993;61:205–13. [PubMed] [Google Scholar]
- 9.Khanolkar-Young S, Rayment N, Brickell PM, Katz DR, Vinayakumar S, Colston MJ, Lockwood DNJ. Tumour necrosis factor-alpha (TNFα) synthesis is associated with the skin and peripheral nerve pathology of leprosy reversal reactions. Clin Exp Immunol. 1995;99:196–202. doi: 10.1111/j.1365-2249.1995.tb05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampaio EP, Kaplan G, Miranda A, Nery JAC, Miguel CP, Viana SM, Sarno EN. The influence of thalidomide on the clinical and immunological manifestation of erythema nodosum leprosum. J Infect Dis. 1993;168:408–14. doi: 10.1093/infdis/168.2.408. [DOI] [PubMed] [Google Scholar]
- 11.Hussain R, Lucas SB, Kifayet A. Clinical and histological discrepancies in diagnosis of ENL reactions classified by assessment of acute phase proteins SAA and CRP. Int J Lepr. 1995;63:222–30. [PubMed] [Google Scholar]
- 12.Howard M, O'Garra A. Biological properties of interleukin 10. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 13.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wanidworanun C, Strober W. Predominant role of tumor necrosis factor-α in human monocyte IL-10 synthesis. J Immunol. 1993;151:6853–61. [PubMed] [Google Scholar]
- 15.Sieling PA, Abraams JS, Yamamura M, Salgame P, Bloom BR, Rea TH, Modlin RL. Immunosuppressive roles for IL-10 and IL-4 in human infection. In vitro modulation of T cell responses in leprosy. J Immunol. 1993;150:550–10. [PubMed] [Google Scholar]
- 16.Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL. Defining protective responses to pathogens: cytokine profile in leprosy lesions. Sci. 1991;254:277–9. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 17.Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor α production by stimulated human monocytes. J Exp Med. 1991;173:699–703. doi: 10.1084/jem.173.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Ehlers S, Smith KA, Kaplan G. Thalidomide exerts its inhibitory action on TNFα by enhancing mRNA degradation. J Exp Med. 1993;177:1675–80. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samlaska CP, Winfield EA. Pentoxifylline. J Am Acad Dermatol. 1994;30:60–21. doi: 10.1016/s0190-9622(94)70069-9. [DOI] [PubMed] [Google Scholar]
- 20.Doherty GM, Jensen C, Alexander R, Buresh CM, Norton JA. Pentoxifylline suppression of tumor necrosis factor gene transcription. Surg. 1991;110:192–8. [PubMed] [Google Scholar]
- 21.Zabel P, Schonharting MM, Wolter DT, Schade UF. Oxpentifylline in endotoxaemia. Lancet. 1989;23:1474–7. doi: 10.1016/s0140-6736(89)92929-2. [DOI] [PubMed] [Google Scholar]
- 22.Dezube BJ, Sherman ML, Fridovich-Keil JL, Allen-Ryan J, Pardee AB. Down-regulation of tumor necrosis factor expression by pentoxifylline in cancer patients: a pilot study. Cancer Immunol Immunother. 1993;36:57–60. doi: 10.1007/BF01789132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gramnger W, Thalhammer F, Lockr G. Pentoxifylline in cerebral malaria. J Infect Dis. 1991;164:829. doi: 10.1093/infdis/164.4.829. [DOI] [PubMed] [Google Scholar]
- 24.Sarno EN, Nery JAC, Garcia CC, Sampaio EP. Is pentoxifylline a viable alternative in the treatment of ENL? Int J Lepr. 1995;63:570–1. [PubMed] [Google Scholar]
- 25.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr. 1966;34:255–73. [PubMed] [Google Scholar]
- 26.Ehlers S, Smith KA. Differentiation of T cell lymphokine gene expression: the in vitro acquisition of T cell memory. J Exp Med. 1991;173:25–36. doi: 10.1084/jem.173.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman M. The use of ranks to avoid the assumption of normality implicit in the analysis of variance. J Am Assoc. 1937;32:675–701. [Google Scholar]
- 28.Strieter RM, Remick DG, Ward PA, Spengler RN, Lynch JP, Larrick J, Kunkel S. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Bioch Biophys Res Commun. 1988;155:1230–6. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- 29.Sarno EN, Sampaio EP. Role of inflammatory cytokines in tissue injury in leprosy. Int J Lepr. 1996;64:S69–S74. [PubMed] [Google Scholar]
- 30.Barnes PF, Chatterjee D, Brennan PJ, Rea TH, Modlin RL. Tumor necrosis factor production in patients with leprosy. Infect Immun. 1992;60:1441–6. doi: 10.1128/iai.60.4.1441-1446.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dezube BJ, Pardee AB, Chapman B, et al. Pentoxifylline decreases tumor necrosis factor expression and serum triglycerides in people with Aids. J Acquir Immune Defic Syndr. 1993;6:787–94. [PubMed] [Google Scholar]
- 32.Dezube BJ, Fridovich-Keil JL, Lange RF, Pardee AB. Pentoxifylline and wellbeing in patients with cancer. Lancet. 1990;335:662. doi: 10.1016/0140-6736(90)90450-j. [DOI] [PubMed] [Google Scholar]
- 33.Biswas DK, Dezube BJ, Ahlers CM, Pardee AB. Pentoxifylline inhibits HIV-1 LTR-driven gene expression by blocking NF-κB action. J Acq Immune Defic Syndr. 1993;6:778–86. [PubMed] [Google Scholar]
- 34.Enres S, Fulle H-J, Sinha B, Stoll D, Dinarello CA, Gerzer R, Weber PC. Cyclic nucleotides differentially regulate the synthesis of tumour necrosis factor-α and interleukin-1β by human mononuclear cells. Immunol. 1991;72:56–60. [PMC free article] [PubMed] [Google Scholar]
- 35.Duncan MR, Hasan A, Berman B. Pentoxifylline, pentifylline, and interferons decrease type I and III procollagen mRNA levels in dermal fibroblasts: evidence for mediation by nuclear factor 1 down-regulation. J Invest Dermatol. 1995;104:282–6. doi: 10.1111/1523-1747.ep12612819. [DOI] [PubMed] [Google Scholar]
- 36.Zabel P, Schade FU, Schlaak M. Inhibition of endogenous TNF formation by pentoxifylline. Immunobiol. 1993;187:447–63. doi: 10.1016/S0171-2985(11)80356-6. [DOI] [PubMed] [Google Scholar]
- 37.Fiorentino DF, Zlotnik A, Mosmann TR, Howard MH, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 38.D'Andrea A, Aste-Amezaga M, Valianten NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon γ-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–8. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navarro J, Punzón MC, Pizarro A, Fernández-Cruz E, Fresno M, Muñoz-Fernández MA. Pentoxifylline inhibits acute HIV-1 replication in human T cells by a mechanism not involving inhibition of tumour necrosis factor synthesis or nuclear factor-kB activation. AIDS. 1996;10:469–75. doi: 10.1097/00002030-199605000-00004. [DOI] [PubMed] [Google Scholar]