Abstract
Caprine arthritis encephalitis (CAE) is a lentiviral infection of goats characterized by mononuclear cell infiltration of various tissues, most prominently the joints, mammary glands and, in young animals, the brain. We have investigated the early stages of arthritis induced by intracarpal and intravenous infection with molecularly cloned CAE virus. Analysis of the synovial membranes by immunohistological methods showed that the proportion of CD8+T cells peaked around day 12 post-infection. CD4+ T cells increased to a lesser degree. The relative proportion of B cells rose steadily post-infection. At 33 days post-infection, plasma cells accounted for over one third of all inflammatory cells in the inflamed synovium. Histopathologically, the arthritic lesions in the synovial membranes closely resembled those in membranes of animals with a 2-year history of chronic arthritis. Our observations indicate that this type of short-term experimental infection is particularly suitable for studying the pathogenesis of goat lentiviral infection. In addition, our observations support the view that a predominantly humoral (type 2) immune response may contribute to the pathogenesis of CAE.
Keywords: caprine arthritis encephalitis virus, immunopathogenesis, rheumatoid arthritis, T cells, B cells, macrophages
INTRODUCTION
Caprine arthritis encephalitis virus (CAEV), a lentivirus of the Retroviridae family, is related closely to Maedi Visna virus [1] and, more distantly, to HIV. The main feature of goat lentivirus infection is the slow progressive course of the disease. The virus is transmitted via colostrum and milk as well as horizontally. Infected animals develop mainly chronic progressive arthritis, with the initial clinical manifestations occurring as late as several months post-infection [2].
Clinically, arthritis is characterized by proliferative synovitis, fibrosis and mineralization of soft tissue [3]. Increased carpal/metacarpal ratios reflect the marked joint swelling [3]. Histologically, chronic arthritis is characterized by massive infiltration of mononuclear cells in the synovial membrane [3]. In particular, CAEV-induced chronic arthritis has been shown to be associated with a marked accumulation of CD45+ CD5− B cells [3]. The concentration of antiviral antibody detected in the synovial fluid of chronically arthritic goats was two to five times higher than in serum [4]. These antibodies are mainly directed against the envelope glycoprotein gp135 and the transmembrane glycoprotein gp38 [5, 6]. It remains to be investigated whether immune complexes [7] play a role in the development of CAE.
Besides B cells, T lymphocytes were observed in the lymphoid-like follicles of goat synovial membranes [3]. Thus, the histopathological appearance of goat lentiviral arthritis is similar to that of rheumatoid arthritis (RA) in humans [8], which has been proposed but not proven to be caused by retroviruses [9, 10]. A viral aetiology of RA may be difficult to prove because such agents may merely serve as triggers of inflammation. Hence, they may no longer be detectable at a later stage when a diagnosis of RA is established [11]. The observation that inflammation progresses unchecked in caprine arthritis is of considerable interest, because the replication of CAEV is severely restricted after the earliest stage of experimental infection [12, 13]. Both the histopathological similarity of CAE to RA and the clear viral aetiology of CAE suggest that CAE may be a useful model for studying certain aspects of the pathogenesis of RA, particularly in the latter's early stages when biopsies are not normally taken. In this study, we have determined the phenotypes of the inflammatory cells and the kinetics of their appearance in the synovial membrane following experimental intra-articular and intravenous infection of goats with CAEV.
MATERIALS AND METHODS
Infection of goats and preparation of synovial membranes
Saanen goats were infected intravenously and intracarpally with 5 × 104 TCID50 of molecularly cloned CAEV strain CO [14] (kindly supplied by Dr R. Vigne, Marseilles, France) grown in cultured goat synovial membrane cells. Virus was titrated by serial dilution on cultured goat synovial membrane cells and cultures showing cytopathic effects were rated positive. The left carpal joint was injected with virus-containing cell culture supernatant and the right joint with supernatant of uninfected cultured goat synovial membrane cells to serve as a control for possible effects due to injection of cell culture medium. The tissues of at least two animals each were analysed at days 6, 12 and 33 post-infection, and those of two naturally infected animals which had shown clinical arthritis for at least 2 years were included in this study to represent the chronic stage of arthritis (Table 1). After euthanasia by i.v. injection of sodium pentobarbital, samples of the synovial membrane were fixed in 4% paraformaldehyde in PBS pH 7.5, for 24 h at room temperature, followed by embedding in paraffin using routine methods. Additionally, fresh tissue was frozen in ornithine carbamoyltransferase medium on dry ice and stored at −80°C. For immunolabelling, 4-μm sections were cut at −20°C using a Cryostat (Leica, Leitz, Solms, Germany) and placed on Superfrost Plus glass slides (Menzel Gläser, Braunschweig, Germany). The sections were fixed in 100% acetone at room temperature for 10 min and stored at 4°C for up to 1 month.
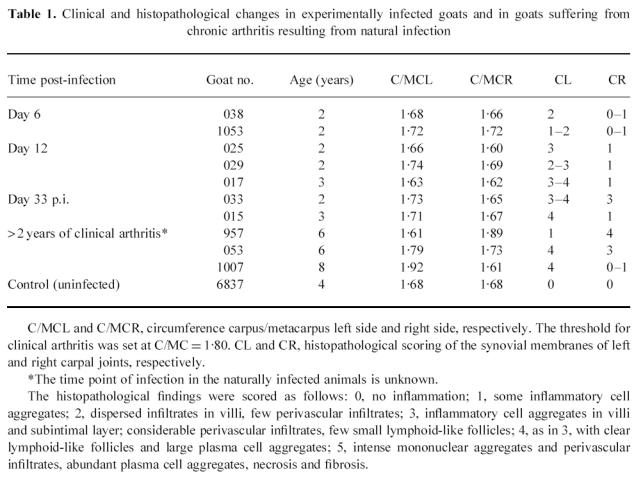
Table 1.
Clinical and histopathological changes in experimentally infected goats and in goats suffering from chronic arthritis resulting from natural infection
Antibodies
Expression of CD4 and CD8 was determined using the MoAbs GC50A [15] and 7C-2, respectively (European Collection of Cell Cultures, Centre for Applied Microbiology and Research, Porton Down, UK). The MoAb F10-197 (kindly donated by W. Hein, Institute of Immunology, Basel, Switzerland) was used to identify the T19 γδ T cell subset [16]. DH59B [3] recognizes macrophages, monocytes and granulocytes. MHC class II DR molecules were detected using antibody VPM54 [17]. CD45+ CD5− B lymphocytes were determined using double staining with GS5A, a marker for CD45R, and GR60A, a marker for CD5 [3]. Expression of IL-2 receptor (IL-2R) was detected using antibody CACT116 A [3]. Control sections were incubated with an irrelevant isotype-matched antibody.
Indirect alkaline phosphatase labelling
Plasma cell staining was performed using an indirect alkaline phosphatase procedure [18]. After incubation with 0.2% trypsin (Difco Labs, Detroit, MI) and 14 mm CaCl2 on a shaker at 37°C for 30 min, non-specific binding of the tissue was blocked with 10 mg/ml human IgG (Globuman Berna; Schweizerisches Serum- und Impfinstitut, Bern, Switzerland). A rabbit anti-goat IgG serum prepared at our Institute was applied to the tissue at a dilution of 1:200. A second alkaline phosphatase-marked mouse anti-rabbit MoAb (Sigma A2556, St Louis, MO) was used at a dilution of 1:1000 in Tris buffer (0.25 m NaCl, 20 mm Tris pH 7.5, 0.25% Tween 20). Incubation was for 60 min at room temperature on a shaker. Between each incubation step the slides were washed twice for 5 min in Tris buffer. The tissue was then stained with fast red (2 mg/ml; Fast Red TR-salt, Chroma-Gesellschaft, Kongen, Germany), 1 mg/ml naphtol ASMX-phosphate (Sigma), 0.5 mg/ml Levamisole (Sigma) in Tris-buffered saline (TBS), pH 8.0. Colour development was stopped by rinsing with tap water and the tissue was counterstained with Mayer's haematoxylin and mounted in glycerol-gelatin GG-1 (Sigma).
Indirect biotin–avidin immunoperoxidase labelling
Cryostat tissue sections were stained using a modification of the indirect biotin–avidin complex immunoperoxidase procedure [3]. After acetone fixation and air drying, the sections were rehydrated in TBS containing 50 mm Tris, 225 mm NaCl and 0.025% Tween 20 pH 7.6. Non-specific binding was blocked with human γ-globulin. The primary antibody was applied for 60 min at 37°C. Subsequently, the sections were incubated for 30 min at 37°C with a biotinylated goat anti-mouse antibody (Jackson ImmunoResearch, West Grove, PA) diluted 1:700 in TBS containing 1.5% goat serum. Endogenous peroxidase was quenched by TBS containing 0.3% H2O2 and 0.1% azide. The tissue was incubated with avidin–biotin peroxidase complex (Vector Labs, Burlingame, CA) diluted 1:100 for 30 min at 37°C before addition of the substrate diaminobenzidine tetrahydrochloride (Sigma). Between each incubation step the slides were washed twice in TBS for 5 min. For double-staining the slides were washed in TBS for 30 min and the procedure described above was repeated using avidin–biotin alkaline phosphatase complex and the appropriate substrate.
Evaluation
In serial sections the labelled cells were counted in areas equivalent to 1 mm2 in three inflamed regions chosen randomly using an ocular micrometer grid. Intimal, subintimal and subsynovial regions were compared [2]. Histopathological lesions were scored on haematoxylin and eosin (H–E)-stained tissue sections. Statistical analysis was carried out by anova using logarithmic numbers of cells and adjustment for multiple comparisons (SAS Institute Inc., Cary, NC).
RESULTS
Histological evidence of synovitis was observed in all tissue samples of synovial membranes obtained from carpal joints that had been injected with virus-containing cell culture supernatants. In contrast, no such changes were observed in synovial membranes of mock-infected contralateral joints and in those of carpal joints of uninfected animals. Depending on the stage a more or less prominent mononuclear cell infiltration was observed (Fig. 1). The histopathological lesions were scored according to the criteria outlined in the footnote to Table 1.
Fig. 1.
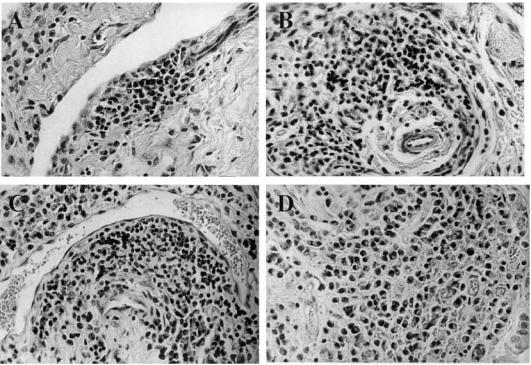
Histopathology of the subintimal region of the carpal synovium of caprine arthritis encephalitis virus (CAEV)-infected goats (H–E staining; mag. × 200). (A) Focal mononuclear cell infiltration at day 6 post-infection. (B) Perivascular cell infiltration at day 12 post-infection. (C) Extensive perivascular cuff at day 33 post-infection. (D) Diffuse mononuclear cell infiltration in the synovial membrane of a goat suffering from clinical arthritis for over 2 years.
Histological appearance of synovial membranes at day 6 post-infection
The most consistent finding at day 6 was the hyperplasia of the intimal cell layer of the synovial membrane. Few mononuclear cells were observed throughout the subintima.
At this stage, the numbers of both B cells and plasma cells had increased compared with the synovial membrane of the uninfected control (P = 0.034 and P = 0.001, respectively). Small aggregates of plasma cells were observed. B cells were localized in foci associated with CD5+ lymphocytes throughout the intimal and subintimal layers. The number of CD4+ and CD8+ lymphocytes exceeded that found in the synovial membrane of mock-infected joints (P = 0.0153 and P = 0.005, respectively). The T lymphocytes were located in inflammatory foci with or without association with blood vessels. Macrophages were scattered throughout the tissue but no statistically significant increase in numbers was observed. Cells with a morphological appearance of macrophage-like type A synoviocytes as well as fibroblast-like type B synoviocytes stained for MHC class II. Additional MHC class II-stained cells were macrophages, fibroblasts, vascular endothelial cells as well as lymphocytes. Only a few scattered lymphocytes stained for IL-2R. Compared with synovial membranes from uninfected animals, the number of γδ cells was increased at day 6 post-infection (P = 0.016).
Histological appearance of synovial membranes at day 12 post-infection
Angiogenesis accompanied by an increased number of mononuclear cells mainly in the intimal and subintimal layers was a prominent feature within the hypertrophied villi. Inflammatory cells were either scattered, organized in foci, or located perivascularly. Few small lymphoid-like follicles were noted. Most inflammatory cells were located in the intimal and subintimal layers, but few were seen also in the subsynovial area. CD4+ and CD8+ cells were located in perivascular cuffs which contained only few scattered γδ T lymphocytes and a low number of B cells. Occasionally, CD4+ and CD8+ T cells were found at the periphery of cell aggregates containing B cells, probably representing early stage lymphoid-like follicles. DH59B antibody stained cells with a morphological appearance of type A synoviocytes as well as macrophages. The former were located in the intimal layer whereas the latter were found in perivascular cuffs and scattered throughout areas rich in lymphocytes. MHC class II+ cells were observed throughout the inflammatory areas and their number was higher than that found in uninfected animals (P = 0.004). A slight but statistically not significant increase in IL-2R staining cells was noted compared with day 6 post-infection.
Histological appearance of synovial membranes at day 33 post-infection
Lymphoid-like follicles, early fibrosis and smooth muscle hyperplasia of small arterial walls were observed. The histological changes were similar to those in naturally infected goats with chronic arthritis. Mononuclear inflammation extended preferentially to the subintimal layer, with plasma cells being the predominant cell type (Fig. 2A–D). B cells and macrophages were found to have increased steadily, whereas CD4+ and CD8+ T cells had decreased in numbers.
Fig. 2.
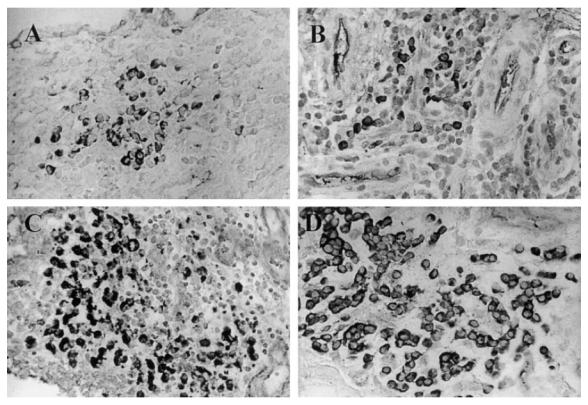
Plasma cell infiltration of carpal synovium of caprine arthritis encephalitis virus (CAEV)-infected goats. Plasma cells were stained for IgG as described in Materials and Methods. Mag. × 200. (A) Day 6 post-infection. (B) Day 12 post-infection. (C) Day 33 post-infection. (D) Plasma cells in the synovial membrane of a goat suffering from clinical arthritis for over 2 years.
Three areas were differentiated at 33 days post-infection: (i) perivascular areas containing CD4+ and CD8+T lymphocytes, a much lower number of B lymphocytes and only few, if any, plasma cells. MHC class II expression was high in these regions. Only few γδ T cells were found scattered throughout the inflamed areas of the tissue; (ii) round-shaped cell aggregates in the subintimal layer resembling lymphoid-like follicles. MHC class II-expressing B cells were located mainly in the centre of the lymphoid-like follicles and were surrounded by CD5+, CD4+and CD8+ lymphocytes; (iii) cell aggregates consisting predominantly of plasma cells with only few other cell types. At 33 days post-infection B cell aggregates surrounded by a broad plasma cell mantle were observed in the subintima. MHC class II-expressing cells increased steadily post-infection and their numbers differed significantly from those of the control at day 33 post-infection (P = 0.004). We noted a slight (P = 0.050) increase of IL-2R+ cells at day 33 post-infection, but their absolute numbers remained small throughout the course of infection.
Histological appearance of synovial membranes in the chronic stage of arthritis
Massive infiltration of inflammatory cells was observed in the synovial membrane. Degeneration and necrosis of the synovial membrane were noted in only one goat that had been infected for over 5 years. Abundant plasma cells were present in the synovial membranes derived from chronically infected goats. Both macrophages and B cells were numerous in the inflamed areas. Few CD4+ T cells were detected and, compared with day 33 in experimentally infected animals, there was only a slight increase in CD8+ T cells. These T cells were either associated with blood vessels near the apex of hypertrophied villi and distributed as small cell groups, or, less frequently, arranged as a band at the rim of the synovial membranes. In these chronic cases, lymphoid-like follicles were more abundant than perivascular cuffs. MHC class II-stained cells were frequent, whereas cells expressing IL-2R had decreased in numbers, compared with day 33 post-experimental infection.
As summarized in Fig. 3, the pattern of inflammatory cells changed during the course of infection. CD4+ and γδ T lymphocytes decreased after 12 days, whereas numbers of B lymphocytes and macrophages increased. The number of CD8+ T cells reached a maximum at day 12 post-infection and stayed relatively high thereafter. Staining for IL-2R remained low throughout the experiment. The most conspicuous change was seen in plasma cells, which in the synovial membranes of chronically infected goats amounted to ≈ 70% of all inflammatory cells.
Fig. 3.
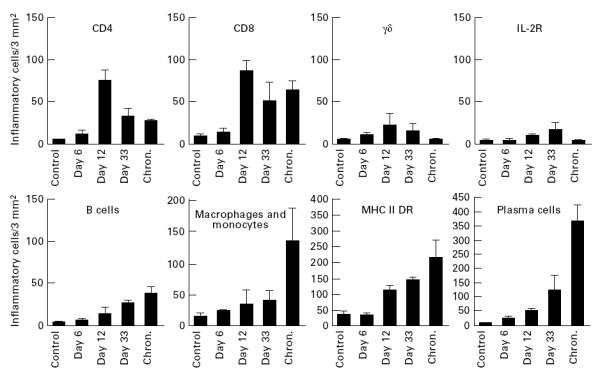
Kinetics of cell infiltration in the synovial membrane of the carpal joints of goats. The synovial membranes were stained and results were analysed using anova, as described in Materials and Methods (Evaluation). Bars indicate s.e.m. For statistical evaluation refer to the text.
DISCUSSION
Typically, clinical arthritis in goats appears months to years after natural infection [2]. We were interested in the events occurring early in infected joints, as key pathogenic changes conceivably take place soon after the homing of the virus to the joints. In this study, using an infection model similar to that originally proposed by Adams et al. [12], we analysed the cell types and kinetics of their appearance in the synovial membrane of goats infected intracarpally and intravenously with molecularly cloned CAEV.
Initial evidence of arthritis, such as fluctuation of the bursa and joint swelling, was noted at 12 days post-infection. As early as 6 days post-infection, we detected an accumulation of inflammatory cells in the intimal and subintimal layers of the synovial membrane of intracarpally infected joints. In contrast, in the contralateral, mock-infected joint, such changes were not seen before day 33 post-infection. Interestingly, in joints not injected at all, virus appeared even later. Thus, it appears that even though this unspecific irritation caused by mock-infection does not induce an inflammatory response per se, it facilitates the haematogenic homing of the virus to the contralateral joint ([13] and unpublished observation). The striking similarity between the histopathological features observed at 33 days post-infection and those observed in chronically infected animals strongly supports the relevance of this short-term infection model of virus-induced arthritis [9, 10]. During the initial stages of infection abundant viral RNA was detected by in situ hybridization in the synovial membrane [13]. This indicates that the presence of viral antigen in the joint is required to trigger those mechanisms leading to chronic arthritis. However, virus expression rapidly decreased, and at day 33 post-infection viral RNA-positive cells were either rare or absent [13], which suggests that inflammation may persist and even progress despite the apparent absence of pronounced viral replication.
During the course of our experiment, synovitis of increasing severity was noticed in all synovial tissue samples of experimentally CAEV-infected goats. As from day 12 post-infection, this was accompanied by a strong angiogenic activity which, in chronic inflammation, is frequently involved in repair processes [19]. Tumour necrosis factor-alpha (TNF-α), transforming growth factor-beta (TGF-β) and E-selectin, inter alia, were shown to provide an appropriate environment for neovascularization [19–22], similar to that previously described for infections with Maedi Visna virus [23], simian immunodeficiency virus (SIV) [24] and HIV [25], as well as for RA [26]. It remains to be investigated which cell types are predominantly involved in the production and secretion of angiogenic mediators. Our previous observations suggest that macrophages are the main source of TNF-α in CAEV-induced arthritis [27].
Our immunohistochemical analysis showed that at the onset of CAEV infection CD8+and CD4+ T lymphocytes accumulated in the synovial membrane. The CD8+ lymphocytes might represent cytotoxic, virus-specific T cells [28]. CD4+ and CD8+ T lymphocytes were found in perivascular areas containing fewer B lymphocytes and only a few plasma cells. The pronounced MHC class II expression in such regions suggests a local immune activation. Interestingly, IL-2R expression remained low throughout the development of arthritis. The marked increase in B cells, and especially in plasma cells, noted in our experiment suggests that, as infection progresses, an antibody-centred (or type 2) immune response may gain predominance. This has previously been shown to occur in chronically CAEV-infected goats [3], and numerous plasma cells are also noted in RA [8, 11]. The antibodies demonstrated in the synovial fluid of arthritic goats [4] are conceivably produced by the abundant plasma cells present in the synovium.
In conclusion, our experiments revealed a steady increase in the number of plasma cells in the inflamed joints during the early stages of CAEV infection. The titres of antibodies against gp135 and gp38 as well as the numbers of plasma cells in the synovial membrane appear to correlate with the severity of arthritis [5, 6]. Collectively, these observations support the view that a predominantly humoral immune response may well contribute to the formation of arthritic lesions in goat lentiviral arthritis.
Acknowledgments
We thank Claude Gaillard for help with the statistical analysis, Barbara Blacklaws for the VPM 54 antibody, and Ruth Parham for linguistic improvements of the manuscript. This work was supported by SNF grants 3100-09733.93/1 (E.P.) and 3139-041859.94 (G.B.).
References
- 1.Peterhans E, Pohl B, Zanoni R, Lazary S. Caprine arthritis-encephalitis. In: Smolen JS, Kalden JR, Maini RN, editors. Rheumatoid arthritis. Berlin: Springer Verlag; 1992. pp. 216–30. [Google Scholar]
- 2.Wilkerson MJ, Davis WC, Cheevers WP. Peripheral blood and synovial fluid mononuclear cell phenotypes in lentivirus induced arthritis. J Rheumatol. 1995;22:8–15. [PubMed] [Google Scholar]
- 3.Wilkerson MJ, Davis WC, Baszler TV, Cheevers WP. Immunopathology of chronic lentivirus-induced arthritis. Am J Pathol. 1995;146:1433–43. [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson GC, Adams DS, McGuire TC. Pronounced production of polyclonal immunoglobulin G1 in the synovial fluid of goats with caprine arthritis-encephalitis virus infection. Infect Immun. 1983;41:805–15. doi: 10.1128/iai.41.2.805-815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knowles D, Cheevers WP, McGuire T, Stem T, Gorham J. Severity of arthritis is predicted by antibody response to gp135 in chronic infection with caprine arthritis-encephalitis virus. J Virol. 1990;64:2396–8. doi: 10.1128/jvi.64.5.2396-2398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertoni B, Zahno ML, Zanoni R, et al. Antibody reactivity to the immunodominant epitopes of the caprine arthritis encephalitis virus gp38 transmembrane protein associates with the development of arthritis. J Virol. 1994;68:7139–47. doi: 10.1128/jvi.68.11.7139-7147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harkiss GD, Green C, Anderson A, Watt NJ. Immunoglobulin deposits in synovial membrane and cartilage and phenotype analysis of chondrocyte antigens in sheep infected with the visna retrovirus. Rheumatol Int. 1995;15:15–22. doi: 10.1007/BF00286764. [DOI] [PubMed] [Google Scholar]
- 8.Lydyard PM, Edwards JCW. The pathophysiology of rheumatoid arthritis. Clin Exp Rheumatol. 1994;12:55–58. [PubMed] [Google Scholar]
- 9.Wilder RL. Hypothesis for retroviral causation of rheumatoid arthritis. Curr Opin Rheumatol. 1994;6:295–9. doi: 10.1097/00002281-199405000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Harris E. Rheumatoid arthritis, pathophysiology and implications for therapy. N Eng J Med. 1990;322:1277–89. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- 11.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 12.Adams DS, Crawford TB, Klevjer-Anderson P. A pathogenetic study of the early connective tissue damage of viral arthritis-encephalitis. Am J Pathol. 1980;99:257–78. [PMC free article] [PubMed] [Google Scholar]
- 13.Lechner F, Vogt HR, Seow HF, Bertoni G, Cheevers WP, von Bodungen U, Zurbriggen A, Peterhans E. Expression of cytokine mRNA in lentivirus-induced arthritis. Am J Pathol. 1997;151:1053–65. [PMC free article] [PubMed] [Google Scholar]
- 14.Saltarelli M, Querat G, Konings DAM, Vigne R, Clements JE. Nucleotide sequence and transcriptional analysis of molecular clones of CAEV which generate infectious virus. Virol. 1990;179:347–64. doi: 10.1016/0042-6822(90)90303-9. [DOI] [PubMed] [Google Scholar]
- 15.Perry LL, Wilkerson MJ, Hullinger GA, Cheevers WP. Depressed CD4+ T lymphocyte proliferative response and enhanced antibody response to viral antigen in chronic lentivirus-induced arthritis. J Infect Dis. 1995;171:328–34. doi: 10.1093/infdis/171.2.328. [DOI] [PubMed] [Google Scholar]
- 16.Hein WR, Mackay CR. Prominence of γδ T cells in the ruminant immune system. Immunol Today. 1991;12:30–34. doi: 10.1016/0167-5699(91)90109-7. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy-Stoskopf S, Zink C, Narayan O. Pathogenesis of ovine lentivirus-induced arthritis: phenotypic evaluation of T lymphocytes in synovial fluid, synovium and peripheral circulation. Clin Immunol Immunopathol. 1989;52:323–30. doi: 10.1016/0090-1229(89)90183-9. [DOI] [PubMed] [Google Scholar]
- 18.Plenat F, Martinet Y, Martinet N, Vignaud JM. Immunohistochemical methods for studying mononuclear phagocytes in tissue sections. J Immunol Methods. 1994;174:133–54. doi: 10.1016/0022-1759(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 19.Pandey A, Shao H, Marks RM, Polverini PJ, Dixit VM. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-alpha-induced angiogenesis. Sci. 1995;268:567–9. doi: 10.1126/science.7536959. [DOI] [PubMed] [Google Scholar]
- 20.Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci USA. 1986;83:7297–301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fajardo LF, Kwan HH, Kowalski J, Prionas SD, Allison AC. Dual role of tumor necrosis factor-alpha in angiogenesis. Am J Pathol. 1992;140:539–44. [PMC free article] [PubMed] [Google Scholar]
- 22.Koch AE, Halloran MM, Haskell CJ, Sha MR, Polverini PJ. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature. 1995;376:517–9. doi: 10.1038/376517a0. [DOI] [PubMed] [Google Scholar]
- 23.Anderson AA, Harkiss GD, Watt NJ. Quantitative analysis of immunohistological changes in the synovial membrane of sheep infected with maedi-visna virus. Clin Immunol Immunopathol. 1994;72:21–29. doi: 10.1006/clin.1994.1102. [DOI] [PubMed] [Google Scholar]
- 24.Roberts ED, Martin LN. Arthritis in rhesus monkeys experimentally infected with simian immunodeficiency virus (SIV/DELTA) Lab Invest. 1991;65:637–43. [PubMed] [Google Scholar]
- 25.Dalton ADA, Harcourt-Webster JN, Keat ACS. Synovium in AIDS: a postmortem study. Br Med J. 1990;300:1239–40. doi: 10.1136/bmj.300.6734.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch AE, Polverini PJ, Leibovich SJ. Stimulation of neovascularisation by rheumatoid synovial tissue macrophages. Arthritis Rheum. 1986;29:471–9. doi: 10.1002/art.1780290403. [DOI] [PubMed] [Google Scholar]
- 27.Lechner F, Vogt HR, Seow HF, v Bodungen U, Bertoni G, Zurbriggen A, Peterhans E. Expression of TNF-alpha in arthritis caused by caprine arthritis encephalitis virus. Vet Immunol Immunopathol. 1996;54:281–9. doi: 10.1016/s0165-2427(96)05701-7. [DOI] [PubMed] [Google Scholar]
- 28.Doherty PC. The keys to cell-mediated immunity. JAMA. 1995;274:1067–8. doi: 10.1001/jama.274.13.1067. [DOI] [PubMed] [Google Scholar]


