Abstract
Free light chains (FLC) are a natural product of B lymphocytes and, as such, represent a quantifiable biomarker of cellular proliferation. Accurate measurement of the concentrations of these components in serum and urine provides a unique means of ascertaining B cell immunoglobulin synthesis during physiologic and, especially, pathologic states, where such information has important diagnostic and therapeutic implications. Previously, use of such quantitative assays has been limited due to the lack of potent serologic reagents specific for these components. We have immunized mice with κ- and λ-type monoclonal human light chains (Bence Jones proteins (BJP)) and have obtained monoclonal antibodies (MoAbs) that differentiate between unbound and bound light chains. These highly specific MoAbs were used to measure by ELISA the concentrations of FLC in the serum of 22 normal individuals and in urine from 16 of these subjects. The mean serum κ and λ FLC concentrations were found to be 16.6 ± 6.1 μg/ml and 33.8 ± 14.8 μg/ml, respectively. In contrast, the values for urinary κ and λ FLC were 2.96 ± 1.84 μg/ml and 1.07 ± 0.69 μg/ml, respectively. In each case studied, the serum κ:λ ratio was consistently less than that of urine (mean values, serum ≈ 1:2; urine ≈ 3:1). That the rate of synthesis of λ-type FLC exceeded that of κ was evidenced in assays of culture fluid supernatants of unstimulated normal peripheral blood mononuclear cells (PBMC), where the mean κ:λ ratio was determined to be 1:1.4. Metabolic studies in which mice were injected with pools of κ- and λ-type BJP prepared in ratios of 1:1, 1:2 and 1:4 demonstrated that, regardless of the proportion, κ FLC were preferentially excreted. Our studies provide the first evidence that λ FLC are secreted by normal PBMC at a greater rate than are κ FLC, as evidenced in biosynthetic studies and by measurement of their serum concentrations. Further, we posit that quaternary structural differences between the two light-chain isotypes may account for the predominance of κversusλ components in urine.
Keywords: immunoglobulin, Bence Jones protein, ELISA, monoclonal antibody
INTRODUCTION
Free light chains (FLC), i.e. light chains that are not covalently linked to heavy chains (as found in complete immunoglobulin molecules), have been shown to have important regulatory and biological functions [1–9]. Additionally, these components serve as unique biomarkers of neoplastic and reactive B cell-related disorders [10–14]. Measurement of κ- and λ-type FLC in serum, urine, or other body fluids has, to date, yielded disparate results, presumably due to the lack of specificity or potency of the antisera used for immunoassay. Such analyses have also been limited by the paucity of serologic reagents that can distinguish between free and heavy chain-bound light chains and the variable results obtained with commercially available anti-FLC MoAbs. In the course of preparing anti-human light-chain MoAbs that recognize particular epitopes expressed on the variable (V) or constant (C) region of κ or λ light polypeptide chains [15, 16], we have identified two that recognize only free κ- or free λ-chains, respectively. We have used these highly specific reagents to measure κ and λ FLC concentrations in serum and urine obtained from normal adults. These assays have demonstrated a striking difference in the mean κ:λ FLC ratio in serum: ≈ 1:2 compared with urine ≈ 3:1. Further, the predominance of λ (versusκ) FLC was evidenced in specimens of saliva and cerebrospinal fluid, as well as in culture fluid supernatants of unstimulated normal peripheral blood mononuclear cells (PBMC). In contrast, metabolic experiments in which mice were injected with human Bence Jones proteins (BJP) demonstrated that κ-chains were preferentially excreted over λ proteins, and that this phenomenon accounted for the predominance of κ FLC in urine.
MATERIALS AND METHODS
Serum and urine specimens
Serum was obtained from 22 normal subjects (ages 22–40 years), 16 of whom also provided urine samples. Prior to assay, all specimens were stored at −80°C. Polyclonal IgG, IgA and IgM protein standards were purchased from Hoechst-Behring (Protein-Standard-Serum LC-A, lot no. 041713, and LC-V, lot no. 041818G; Behringwerke AG, Marburg, Germany).
Proteins
Monoclonal κ- and λ-type BJP and IgG, IgA and IgM proteins were obtained from patients with multiple myeloma or Waldenström's macroglobulinaemia. These components were isolated from urine and serum, respectively, by zone electrophoresis and purified by gel filtration as previously described [17]. The light- and heavy-chain isotypes were established by immunofixation electrophoresis (Paragon Electrophoresis System; Beckman, Brea, CA), and characterization of the light-chain variable-region (VL) was performed serologically using anti-VL subgroup-specific MoAbs [16]. Results were confirmed by amino acid sequence analysis. Normal polyclonal IgG, IgA and IgM proteins were purchased from Sigma (St Louis, MO). Concentrations of proteins in solution were measured by a modification of the Folin Ciocalteu method [17].
Antibodies
The production and characterization of anti-human light-chain murine MoAbs have been described previously [15, 16]. Briefly, 6–8-week-old female BALB/c mice were immunized repeatedly with heat-precipitated BJP. Three days after the final immunization, donor splenocytes were fused with mouse SP2/0 myeloma cells and plated into 96-well culture plates. Culture supernatants were screened using a liquid-phase antigen-capturing ELISA and a reference panel of biotinylated BJP, myeloma proteins, and Waldenström's macroglobulins representative of the major Vκ- and Vλ-subgroup isotypes (VκI, VκII, VκIII, VκIV and VλI, VλII, VλIII, VλIV, VλVI). Selected hybridomas were subcloned, propagated, and injected intraperitoneally into pristane-primed mice. The MoAbs were purified from ascitic fluid by 40% ammonium sulphate precipitation followed by ion exchange chromatography. Peroxidase-labelled goat F(ab′)2 anti-human κ and λ light chain-specific antisera were purchased from Tago Inc. (Burlingame, CA). Affinity-purified goat antibodies specific for human IgG and IgM were obtained from Zymed Labs Inc. (South San Francisco, CA).
Competitive inhibition ELISAs
Ninety-six-well microtitre plates were coated with purified anti-human MoAbs. After blocking and washing, the wells were filled in duplicate with 100 μl of 0.1-, 0.5-, 1.0-, 5.0- and 10.0-μg/ml solutions of representative κ- and λ-type BJP and polyclonal IgG, IgA and IgM proteins purified from normal pooled serum (Sigma). The wells were washed and then filled with a 2-μg/ml solution of either κ or λ biotinylated BJP as previously described [16]. After incubation and washing, an avidin–biotin complex was added (Vectastain; Vector Labs, Burlingame, CA), followed by a 2.2′-azino-bis [3-ethylbenzthiazoline-6 sulphonic acid] (ABTS) substrate solution. The plates were read in an ELISA plate reader (Bio-Tek Instruments, Winoski, VT) at 415 nm.
Quantitative ELISAs
Assays forκandλFLC.
Polystyrene 96-well microtitre plates were coated with 100 μl of a 3-μg/ml solution of the κ or λ anti-FLC-specific MoAb diluted in 0.05 m bicarbonate buffer pH 9.6 (Sigma), and incubated overnight at 4°C. After washing twice with distilled water, 150 μl of a 1% solution of bovine serum albumin (BSA) in PBS were added to saturate non-specific binding sites. The wells were washed × 3 with PBS containing 0.05% Tween 20, followed by addition (in duplicate) of 100-μl volumes of serial dilutions of serum, urine samples, culture fluid supernatants, or 1.0–1000-ng/ml solutions (designated ‘standard protein solutions’) consisting of equimolar mixtures of 20 different κ or λ BJP representative of the major Vκ and Vλ subgroups dissolved in diluent buffer (PBS containing 0.5% BSA and 0.05% Tween 20). After 2 h of incubation at room temperature and subsequent washing (× 5), the wells were filled with 100 μl of peroxidase-conjugated goat F(ab′)2 anti-human κ or λ light chain-specific antibodies diluted 1:4000 in diluent buffer. The plates were incubated for 1 h at room temperature, the wells were washed seven times, and then 100 μl of ABTS substrate were added. The enzymatic reaction was terminated after 10 min by the addition of 100 μl of 2% oxalic acid. The plates were read at 415 nm.
Assays for κ- and λ-type IgG, IgA and IgM proteins
Microtitre plates were coated with 100 μl of a 5-μg/ml solution of affinity-purified goat anti-human IgG, IgA or IgM. After blocking and washing, the wells were filled with diluted culture supernatants or 1.0–1000-ng/ml solutions of purified monoclonal κ and λ type IgG, IgA or IgM protein standards. After incubation and washing, peroxidase-labelled goat F(ab′)2 anti-human κ or λ light chain was added and the plates processed as described above.
Cell separation and culture
PBMC, isolated by centrifugation through a Ficoll–Hypaque gradient, were cultured at 5 × 105 cells/ml in F12 medium containing 5% fetal bovine serum (FBS), 100 μg/ml penicillin and 100 μg/ml streptomycin. Each well of a 24-well culture plate was filled with 2 ml of culture fluid and the plates incubated at 37°C in 5% CO2. Supernatants from each well were harvested after 1, 2, 4, 7, 10 and 13 days of culture. For quantification by ELISA of free and bound κ- and λ-chains, the supernatants were diluted 1:3 and 1:9, respectively.
Metabolic studies
Equimolar amounts of 10 κ and 10 λ BJP representative of the four major Vκ (κI, κII, κIII, κIV) and five Vλ (λI, λII, λIII, λIV, λVI) subgroups were dissolved in PBS and mixed together to obtain solutions containing light chains at a κ:λ ratio of either 1:1, 1:2 or 1:4. The composite κ and λ mixtures were injected into tail veins of mice housed in special humidified metabolic cages where urine could be collected and studied free from faecal contamination. Blood was obtained from the retroorbital plexus of anaesthetized mice 20 h post-injection and the serum separated before analysis. All urine excreted for the first 20 h was collected for assay. Mouse urine and serum specimens were diluted 1:10 and 1:30, respectively, for measurement of human κ and λ FLC concentrations.
RESULTS
Specificity of anti-human light-chain MoAbs
Among our MoAbs prepared against human κ or λ type BJP, we found that, with rare exception, these reagents did not discriminate between monoclonal FLC, i.e. BJP, and light chains bound covalently to γ, α or μ heavy chains in the form of monoclonal IgG, IgA or IgM molecules, respectively. When such antibodies were tested in a fluid-phase, antigen-binding ELISA against BJP and intact immunoglobulins, a comparable degree of reactivity was evidenced, as exemplified by results obtained with two such reagents, Tκ-6E4 and Tλ-3F4 (Table 1). In contrast, another pair of MoAbs, designated Fκ-C8 and Fλ-G9, exclusively recognized κ and λ FLC, respectively. These two reacted neither with a large number of monoclonal κ or λ type IgG, IgA and IgM components, nor with polyclonal IgG, IgA or IgM molecules (Table 1). Further, the anti-FLC MoAbs did not recognize VL-related determinants when they were tested with BJP representative of the major Vκ and Vλ subgroups (data not shown), and were thus deemed to be CL-specific.
Table 1.
Reactivity by ELISA of anti-free and anti-total κ and λ light-chain MoAbs*

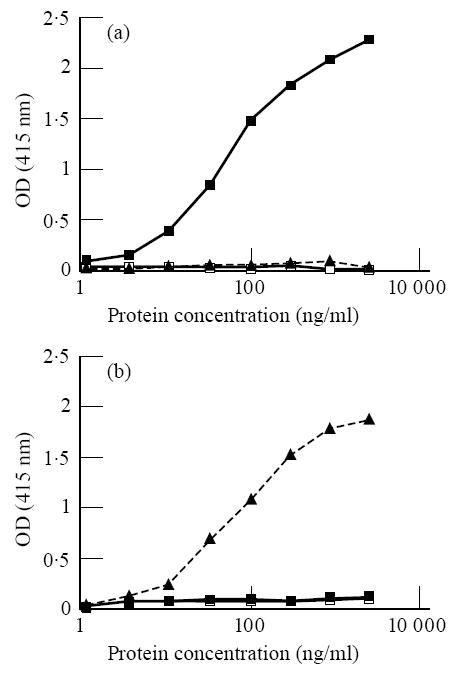
The specificities of the anti-free κ and λ MoAbs Fκ-C8 and Fλ-G9, respectively, were substantiated further in competitive inhibition assays using κ and λ type BJP and polyclonal IgG, IgA and IgM populations. As shown in Fig. 1a, κ light chains effectively blocked the binding of Fκ-C8, whereas the IgG, IgA and IgM proteins and λ-chains were virtually ineffective as inhibitors. Concordant results were obtained with the FλG9 reagent—namely, binding was abrogated by a λ BJP but not by the intact polyclonal immunoglobulins or a κ type BJP (Fig. 1b). In contrast, when the same experiments were performed with the anti-total κ (Tκ-6E4) and anti-total λ (Tλ-3F4) MoAbs, both intact immunoglobulins and BJP were effective inhibitors (data not shown).
Fig. 1.

Specificity of anti-light-chain MoAbs for free light chains. Inhibition by κ Bence Jones protein (BJP) of anti-free-κ MoAb Fκ-C8 (a) and by λ BJP of anti-free-λ MoAb Fλ-G9 (b) using ELISA and polyclonal IgG, IgM and IgA proteins and κ and λ type BJP. OD, Optical density. ▪, BJP κ; ▴, BJP λ; ▵, IgA; ○, IgM; □, IgG.
The specificity of our anti-free κ and λ light chain MoAbs was further evidenced through analysis of a normal serum specimen in which the proteins were separated by size-exclusion chromatography. The reactivity of these reagents was confined exclusively to molecules contained in the portion of the chromatogram that corresponded to monomeric and dimeric forms of light chains (i.e. 22 000–45 000 D). No reactivity was found in eluates containing intact IgG (or IgM) molecules.
Quantification of serum and urinary FLC in normal individuals
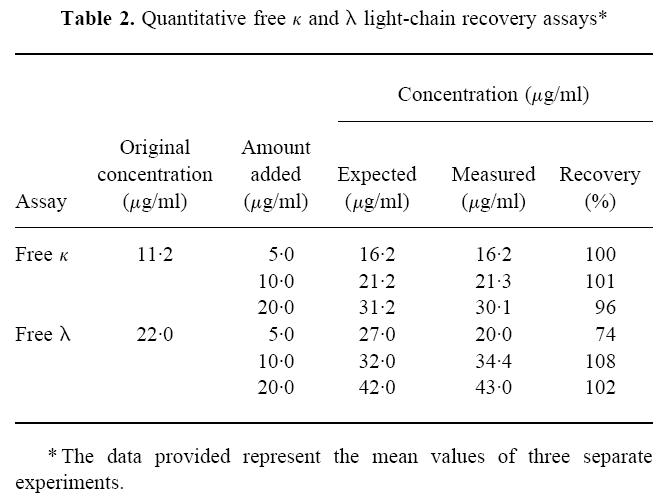
To measure by ELISA serum or urinary concentrations of FLC, we first prepared standard curves for each light-chain isotype using the 1–1000-ng/ml solutions of the κ and λ reference BJP. These data were obtained by measuring the reactivity of the anti-free κ or λ MoAbs with reference mixtures of BJP over a 4-log protein concentration (Fig. 2). The quantitative capability of the FLC assay was determined by adding known amounts of individual κ or λ BJP or mixtures of the same to the standard protein solutions. In each case, the volumetric effect of dilution was considered in the calculations of the protein concentration. The data given in Table 2 represent the mean value of three separate experiments, and with one exception there was close agreement between the measured and expected concentrations of κ or λ FLC in the various samples assayed. Additionally, investigation of the effect of repeated washing of the wells after addition of protein solutions revealed no measurable differences when wells were washed three, five or seven times. However, background values were considerably less with multiple washes.
Fig. 2.
Quantitative free light chain (FLC) assays. Standard curve for κ (a) and λ (b). Results obtained using reference mixtures of κ Bence Jones proteins (BJP), λ BJP, and polyclonal IgG. OD, Optical density. ▪, κ FLC; ▴, λ FLC; □, IgG.
Table 2.
Quantitative free κ and λ light-chain recovery assays*
For quantitative serum and urine FLC assays, specimens were appropriately diluted (i.e. serum 1:50–1:800; urine 1:10–1:160) in order to establish a mean value from at least two points falling within the linear portion of the standard curve. As indicated in Table 3, the mean concentrations and s.d. of κ and λ FLC in serum samples obtained from 22 normal subjects were 16.6 ± 6.1 μg/ml (range 8.0–29.6 μg/ml) and 33.8 ± 14.8 μg/ml (range 8.8–66.4 μg/ml), respectively. The corresponding values in urine specimens from 16 of this group were 2.96 ± 1.84 μg/ml (range 0.22–5.85 μg/ml) and 1.07 ± 0.69 μg/ml (range 0.15–2.91 μg/ml), respectively. In all 16 cases, the urinary κ:λ FLC ratios were consistently higher than those found in serum. For the entire group of 22, the mean serum κ:λ ratio was ≈ 1:2 (range ≈ 1:1–1:4) versus a mean urine κ:λ ratio of ≈ 3:1 (range ≈ 1.4:1–4.4:1). The intra- and interassay coefficients of variation for free κ measurements were 3.7% and 14.1%, respectively; for free λ, the values were 7.5% and 8.4%.
Table 3.
Concentration of free κ and λ type light chains in the serum and urine of normal subjects

Quantification of κ and λ FLC in other body fluids
The concentrations of κ and λ FLC were measured in cerebrospinal fluid obtained from seven patients with acute myelocytic leukaemia who were in complete remission. These values ranged from 23.0 to 76.5 ng/ml and from 49.2 to 188.3 ng/ml, respectively. Except in one case, the amount of free λ-chains exceeded that of κ; the mean κ:λ FLC ratio in the seven specimens was ≈ 1:2. Analyses of saliva obtained from three normal individuals revealed that free κ and λ concentrations ranged from 705 to 1550 ng/ml and from 843 to 1805 ng/ml (mean κ:λ ratio ≈ 1:2), respectively, and were comparable to their serum FLC values (data not shown).
Synthesis of κ and λ FLC by PBMC
PBMC were obtained from four normal subjects and grown over a 13-day period. The concentrations of κ and λ FLC and of IgM and IgG molecules in culture fluid supernatants were measured by quantitative ELISA using our specific anti-free and anti-total κ and λ MoAbs, respectively (Table 4). Despite considerable variation in immunoglobulin synthesis among the samples, the κ:λ FLC ratios were comparable throughout the time of culture, and the mean κ:λ value at day 7 (≈ 1:1.5) was similar to that found in normal serum. In contrast, the ratios of IgMκ to IgMλ and IgGκ to IgGλ molecules in the culture fluids were 1.5:1 and 1.4:1, respectively.
Table 4.
Quantification of secreted immunoglobulins in 7-day cultures of unstimulated peripheral blood mononuclear cells (PBMC) from four normal subjects*
Catabolism of κ and λ FLC
To determine if the predominance of λ FLC (versusκ) found in serum of normal subjects reflected a variation in the catabolic or excretory rate of each light-chain isotype, we injected mice intravenously with mixtures of κ and λ FLC containing BJP representative of the four major Vκ and five Vλ subgroups. Three different pools were formulated that had κ:λ ratios of either 1:1, 1:2 or 1:4. Specimens of blood were obtained 20 h post-injection and the concentrations of the human FLC determined by ELISA. With each of the three preparations injected, the κ:λ serum ratio at 20 h ranged from 1:1.3 to 1:1.9. In contrast, urine specimens collected over the 20-h period after injection had values of ≈ 1:1–2.3:1.
DISCUSSION
The development of MoAbs that specifically recognize free κ or λ type light chains (in contrast to those reagents that cannot distinguish between light-chain molecules that are either bound to heavy chains or exist in the unbound state) has made it possible to measure directly the concentrations of these components in serum, urine and other body fluids. Using our anti-κ and anti-λ FLC-specific MoAbs to quantify by ELISA the concentrations of these components in the serum of normal individuals, we found notable differences in the κ:λ ratios. In serum, the mean FLC κ:λ value was ≈ 1:2, whereas in urine it was ≈ 3:1.
The quantitative data we obtained for κ and λ FLC in the serum and urine of normal individuals differed somewhat (especially in the case of λ) from those reported by other investigators. In part, these discrepancies may be due to technical factors related to the reagents used in the various types of analyses, e.g. radioimmunoassays [18–20], competitive enzyme immunoassays [14, 21, 22], and immunoturbidimetric assays [13] (see also [23] and [24]). The most notable dissimilarities occurred in the calculation of the serum λ-chain concentrations, that invariably resulted in a κ:λ ratio of 1:1 or above. In contrast to the range of 8.8–66.4 μg/ml we found with our anti-free λ-chain MoAb, Nelson et al. [14] used a commercial reagent and reported values of 0.4–4.2 μg/ml; for κ, they determined the concentration to be 1.6–15.2 μg/ml. Comparably low serum λ FLC values were also found by Sølling [19], who separated serum FLC from intact immunoglobulins by gel filtration and then used immunoglobulin-absorbed polyclonal anti-light-chain antisera for immunoassay. In the case of urine, our determination of the ratio of κ to λ FLC in normal individuals was in agreement with that reported previously by other investigators using polyclonal [19] or monoclonal [21, 22] anti-FLC reagents.
The finding that the concentration of λ FLC in serum exceeded that of κ implies that λ-chains are secreted at a higher rate. Indeed, we found a predominance of λ FLC (versusκ) in culture fluid supernatants of normal PBMC, in contrast to IgG and IgM molecules, where κ type molecules were more prevalent. Whether these findings can be attributed to a higher rate of λ- versusκ-chain synthesis or, alternatively, a higher rate of κversusλ light-chain incorporation into intact immunoglobulin molecules has not been established.
As evidenced in our metabolic studies, the striking reversal in the κ:λ ratio of FLC in urine compared with serum was due to the propensity of κ-chains to be excreted more rapidly than λ proteins. In experiments designed to measure FLC clearance by the kidney, mice were injected with pools of κ and λ type human BJP that were formulated to contain κ:λ ratios ranging from 1:1 to 1:4. In each case, regardless of the ratio, the proportion of κ to λ FLC in serum specimens collected 20 h post-injection was consistently < 0.7, whereas in urine collected over this same time period, it was at least 1.0 or greater. The preferential excretion of κ over λ FLC has been attributed to quaternary structural differences exhibited by the two light-chain isotypes [25]. The fact that κ-chains exist as monomers, non-covalent dimers, or both (depending on the dimerization constant of the monomeric subunits) contrasts with λ-chains that occur predominately in the form of covalently linked dimers. Thus, the lower molecular mass typical of κ components would facilitate their renal excretion [26]. Whether or not the selective removal of κ FLC in the normal state has physiologic import remains to be established.
The measurement of free or unbound light chains can be used as a unique biomarker of the humoral immune system. In multiple myeloma and related plasma cell dyscrasias, the overproduction of κ or λ type monoclonal FLC, i.e. BJP, has diagnostic as well as pathologic relevance [11–14,23]. Further, compared with the healthy state, the synthesis of polyclonal FLC is markedly increased in conditions associated with B cell activation as found in certain inflammatory or autoimmune diseases, e.g. systemic lupus erythematosus [27–29], rheumatoid arthritis [30], or multiple sclerosis [31, 32], as well as in cancer [30], diabetes mellitus [33], and AIDS [34]. Additionally, the functional importance of FLC has been evidenced through studies that have demonstrated the capability of these proteins to bind antigens [35] and to serve as immune regulators [3–5]. Due to the seminal role of FLC in disease as well as in the normal state, the ability to detect and quantify accurately these components has obvious clinical relevance and would, most importantly, provide a means to gain further insight into their metabolic functions and properties.
Acknowledgments
This work was supported in part by USPHS Research Grant CA10056 from the National Cancer Institute. A.S. is an American Cancer Society Clinical Research Professor. The authors thank Julie Ottinger and Lolita Davis for preparation of the manuscript.
References
- 1.Hannam-Harris AC, Gordon J, Smith JL. Immunoglobulin synthesis by neoplastic B lymphocytes: free light chain synthesis as a marker of B cell differentiation. J Immunol. 1980;125:2177–81. [PubMed] [Google Scholar]
- 2.Hannam-Harris AC, Smith JL. Free immunoglobulin light chain synthesis by human foetal liver and cord blood lymphocytes. Immunol. 1981;43:417–23. [PMC free article] [PubMed] [Google Scholar]
- 3.Jorgensen T, Bogen B, Hannestad K. T-helper cells recognize an idiotype located on peptide 88–114/117 of the light chain variable domain of an isologous myeloma protein (315) J Exp Med. 1983;158:2183–8. doi: 10.1084/jem.158.6.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNamara M, Kohler H. Regulatory idiotypes. Induction of idiotype-recognizing helper T cells by free light and heavy chains. J Exp Med. 1984;159:623–8. doi: 10.1084/jem.159.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkly LC, Wortis HH. T cell regulation of light chain expression: preferential enhancement of Igκ production by T cells in the response to DNP. J Immunol. 1985;135:1577–81. [PubMed] [Google Scholar]
- 6.Holmberg I, Lundkvist L, Ivars F, Coutinho A. Absence of immunoglobulin heavy chain expression results in altered κ/λ light chain ratios. J Mol Cell Immunol. 1985;2:51–56. [PubMed] [Google Scholar]
- 7.Hopper JE, Papagiannes E. Evidence by radioimmunoassay that mitogen-activated human blood mononuclear cells secrete significant amounts of light chain Ig unassociated with heavy chain. Cell Immunol. 1986;101:122–31. doi: 10.1016/0008-8749(86)90191-7. [DOI] [PubMed] [Google Scholar]
- 8.Ambrosino DM, Kanchana MV, Delaney NR, Finberg RW. Human B cells secrete predominantly λL chains in the absence of H chain expression. J Immunol. 1991;146:599–602. [PubMed] [Google Scholar]
- 9.Hopper JE, O'Brien J, Papagiannes E. Restriction of blood and marrow CLL-B cells to free L-chain Ig secretion: implication for normal B-cell function and control. Am J Hematol. 1988;29:125–33. doi: 10.1002/ajh.2830290302. [DOI] [PubMed] [Google Scholar]
- 10.Gordon J, Howlett AR, Smith JL. Free light chain synthesis by neoplastic cells in chronic lymphocytic leukaemia and non-Hodgkin's lymphoma. Immunol. 1978;34:397–404. [PMC free article] [PubMed] [Google Scholar]
- 11.Cole PW, Durie BGM, Salmon SE. Immunoquantitation of free light chain immunoglobulins: application in multiple myeloma. J Immunol Methods. 1978;19:341–9. doi: 10.1016/0022-1759(78)90018-2. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson FK, Spellberg M, Smith JL. Monoclonal immunoglobulin light chain in urine of patients with B lymphocytic disease: its source and use as a diagnostic aid. Br J Cancer. 1983;47:607–12. doi: 10.1038/bjc.1983.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tillyer CR, Iqbal J, Raymond J, et al. Immunoturbidimetric assay for estimating free light chains of immunoglobulins in urine and serum. J Clin Pathol. 1991;44:466–71. doi: 10.1136/jcp.44.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson M, Brown RD, Gibson J, Joshua DE. Measurement of free kappa and lambda chains in serum and the significance of their ratio in patients with multiple myeloma. Br J Haematol. 1992;81:223–30. doi: 10.1111/j.1365-2141.1992.tb08211.x. [DOI] [PubMed] [Google Scholar]
- 15.Abe M, Goto T, Wolfenbarger D, et al. Novel immunization protocol and ELISA screening methods used to obtain and characterize monoclonal antibodies specific for human light chain variable-region subgroups. Hybridoma. 1993;12:457–83. doi: 10.1089/hyb.1993.12.475. [DOI] [PubMed] [Google Scholar]
- 16.Abe M, Goto T, Kennel SJ, et al. Production and immunodiagnostic applications of antihuman light-chain monoclonal antibodies. Am J Clin Pathol. 1993;100:67–74. doi: 10.1093/ajcp/100.1.67. [DOI] [PubMed] [Google Scholar]
- 17.Solomon A. Light chains of human immunoglobulins. Methods Enzymol. 1985;116:101–21. doi: 10.1016/s0076-6879(85)16008-8. [DOI] [PubMed] [Google Scholar]
- 18.Sølling K. Free light chains of immunoglobulins. Studies by radioimmunoassay of normal values, polymerism, mechanisms of renal handling and clinical significance. Scand J Clin Lab Invest. 1981;157(Suppl.):1–83. [PubMed] [Google Scholar]
- 19.Sølling K. Free light chains in normal serum and urine determined by radioimmunoassay. Scand J Clin Lab Invest. 1975;35:407–12. [PubMed] [Google Scholar]
- 20.Robinson EL, Gowland E, Ward ID, Scarffe JH. Radioimmunoassay of free light chains of immunoglobulins in urine. Clin Chem. 1982;28:2254–8. [PubMed] [Google Scholar]
- 21.Brouwer J, van Otting-de Ruit M, van Busking-de Lely H. Estimation of free light chains of immunoglobulins by enzyme immunoassay. Clin Chim Acta. 1985;150:267–74. doi: 10.1016/0009-8981(85)90254-2. [DOI] [PubMed] [Google Scholar]
- 22.Axiak SM, Krishnamoorthy L, Guinian J, Raison RL. Quantitation of free κ light chains in serum and urine using a monoclonal antibody based inhibition enzyme-linked immunoassay. J Immunol Methods. 1987;99:141–7. doi: 10.1016/0022-1759(87)90043-3. [DOI] [PubMed] [Google Scholar]
- 23.Levinson SS, Keren DF. Free light chains of immunoglobulins: clinical laboratory analysis. Clin Chem. 1994;40:1869–78. [PubMed] [Google Scholar]
- 24.Tillyer CR. The estimation of free light chains of immunoglobulins in biological fluids. Int J Clin Lab Res. 1992;22:152–8. doi: 10.1007/BF02591415. [DOI] [PubMed] [Google Scholar]
- 25.Stevens FJ, Westholm FA, Solomon A, Schiffer M. Self-association of human immunoglobulin κI light chains: role of the third hypervariable region. Proc Natl Acad Sci USA. 1980;77:1144–8. doi: 10.1073/pnas.77.2.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson PA, Bergg×rd I. Urinary immunoglobulin components in normal, tubular, and glomerular proteinuria: quantities and characteristics of free light chains, IgG, IgA, and Fcγ fragment. Eur J Clin Invest. 1971;1:255–64. doi: 10.1111/eci.1971.1.4.255. [DOI] [PubMed] [Google Scholar]
- 27.Epstein WV, Tan M. Increase of L-chain proteins in the sera of patients with systemic lupus erythematosus and the synovial fluids of patients with peripheral rheumatoid arthritis. Arthritis Rheum. 1966;9:713–9. doi: 10.1002/art.1780090508. [DOI] [PubMed] [Google Scholar]
- 28.Hopper JE, Sequeira W, Martellotto J, et al. Clinical relapse in systemic lupus erythematosus: correlation with antecedent elevation of urinary free light-chain immunoglobulin. J Clin Immunol. 1989;9:338–50. doi: 10.1007/BF00918666. [DOI] [PubMed] [Google Scholar]
- 29.Tsai C-Y, Wu T-H, Sun K-H, et al. Increased excretion of soluble interleukin 2 receptors and free light chain immunoglobulins in the urine of patients with active lupus nephritis. Ann Rheum Dis. 1992;51:168–72. doi: 10.1136/ard.51.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sølling K, Sølling J, Rømer FK. Free light chains of immunoglobulins in serum from patients with rheumatoid arthritis, sarcoidosis, chronic infections and pulmonary cancer. Acta Med Scand. 1981;209:473–7. doi: 10.1111/j.0954-6820.1981.tb11632.x. [DOI] [PubMed] [Google Scholar]
- 31.DeCarli C, Menegus MA, Rudick RA. Free light chains in multiple sclerosis and infections of the CNS. Neurol. 1987;37:1334–8. doi: 10.1212/wnl.37.8.1334. [DOI] [PubMed] [Google Scholar]
- 32.Mehta PD, Cook SD, Troiano RA, Coyle PK. Increased free light chains in the urine from patients with multiple sclerosis. Neurol. 1991;42:540–4. doi: 10.1212/wnl.41.4.540. [DOI] [PubMed] [Google Scholar]
- 33.Groop L, Makipernaa A, Stenman S, et al. Urinary excretion of kappa light chains in patients with diabetes mellitus. Kidney Int. 1990;37:1120–5. doi: 10.1038/ki.1990.94. [DOI] [PubMed] [Google Scholar]
- 34.Elovaara I, Seppala I, Kinnunen E, Laaksovirta H. Increased occurrence of free immunoglobulin light chains in cerebrospinal fluid and serum in human immunodeficiency virus-1 infection. J Neuroimmunol. 1991;35:65–77. doi: 10.1016/0165-5728(91)90162-z. [DOI] [PubMed] [Google Scholar]
- 35.Ely KR, Heron JN, Edmunsdon AB. Three-dimensional structure of a hybrid light chain dimer: protein engineering of a binding cavity. Mol Immunol. 1990;27:101–14. doi: 10.1016/0161-5890(90)90105-9. [DOI] [PubMed] [Google Scholar]


