Abstract
When certain strains of mice bearing H-2Ak are immunized with the interphotoreceptor retinoid-binding protein (IRBP), EAU is induced. Thus far uveitogenic determinant(s) has not been determined in the H-2Ak mouse system. In addition it is hard to prepare purified IRBP. In the present study, to circumvent these problems we attempted to identify uveitogenic peptides derived from bovine IRBP in H-2Ak haplotype mice. Six peptides which had been selected according to the H-2Ak binding motif (Dxxxxxxxx[A, R, T]) were synthesized. We report here that all the peptides are immunogenic but only one peptide, K2, which consisted of IRBP201–216 residues, induces EAU in various mice carrying H-2Ak. Amino acid substitution of K2 revealed that the core region interacted with both H-2Ak and T cell antigen receptor (TCR). The amino acid sequence of the core region derived from bovine IRBP was identical to the corresponding region of mouse IRBP. In addition, K2 appeared to be a natural peptide antigen processed from bovine IRBP. Altogether, we concluded that K2 is one of the natural autoantigens involved in induction of EAU in H-2Ak mice.
Keywords: peptide antigen, IRBP, EAU, MHC restriction
INTRODUCTION
EAU is an organ-specific, T cell-mediated autoimmune disease that may serve a model for several human sight-threatening ocular disorders (endogenous uveoretinitis), Behçet's disease, sarcoidosis, Vogt–Koyanagi–Harada syndrome, birdshot retinochoroidopathy, and sympathetic ophthalmia. Histopathology in the animal model resembles the human disease and is characterized by posterior retinal and choroidal lesions, photoreceptor damage, vasculitis, vitritis, granuloma formation, and varying degrees of anterior chamber infiltration [1–3].
EAU can be induced in several animal models by immunization with retinal antigens emulsified in Freund's complete adjuvant (FCA) or by the adoptive transfer of retinal antigen-specific T lymphocytes into syngeneic recipients [4–6]. Recently, it was reported that EAU was induced in mice which had been administered with interphotoreceptor retinoid-binding protein (IRBP) as a uveitogen [7]. IRBP is a 140-kD glycolipoprotein residing in the interphotoreceptor matrix between the neural retina and the retinal pigment epithelium [8]. The IRBP consists of a four-fold repeat structure with 30–40% amino acid sequence homology shared between the repeats [9]. When the amino acid sequence of IRBP is compared between bovine and humans, approximately 80% sequence homology is detected [8, 9].
It has been reported that mice of the haplotypes H-2r, H-2k, and H-2b develop EAU after immunization with IRBP, whereas those of other haplotypes are resistant [10]. Thus, susceptibility to mouse EAU is at least in part controlled by the MHC. However, among susceptible haplotypes hierarchy of the susceptibility was present (H-2r > H-2k > H-2b). In addition, using intra-H-2 recombinant strains it has also been shown that the control element of susceptibility to EAU is confined to the H-2A subregion [10]. At any rate, the mouse EAU model appears to be important for the investigation of uveitic diseases, because various mutant and transgenic mice can be used to analyse EAU genetically as well as immunologically. In an early study, it was demonstrated that recombinant protein including the first repeat of bovine IRBP induced EAU in mice carrying the H-2r haplotype [11]. Then it was shown that the human IRBP peptide 161–180 (SGIPYIISYLHPGNTILHVD) in the first repeat was uveitogenic in B10.RIII (H-2r) mice. Thus, it appeared that this peptide contained a major pathogenic site immunodominant for the H-2r haplotype [12]. Although the same sequence peptide was shown to be immunogenic in the other two haplotypes (H-2k, H-2b), this peptide induced no pathological legions in these mice.
In the present study we attempt to identify the pathogenic peptide deduced from bovine IRBP in mice carrying H-2k, paying particular attention on H-2Ak molecules as described above. It is an essential requisite for CD4 + T cell responses to be induced that the peptide antigen can bind to MHC class II molecules. Allen et al. [13] reported that peptides possessing aspartic acid (D) at position 1 and arginine (R) at position 8 bound to H-2Ak. We also defined another H-2Ak binding motif (aspartic acid (D) at position 1 and alanine (A) at position 9) [14, 15]. Especially, aspartic acid (D) at position 1 was shown to be most critical in binding to H-2Ak. In the present study, on the basis of the H-2Ak binding motif (Dxxxxxxxx[A, R, T]), aspartic acid (D) at position 1 and either alanine (A), arginine (R) or threonine (T) at position 9, we selected six peptide fragments from whole sequence of IRBP. Then, we synthesized these peptides and examined immunogenicity and pathogenicity in mice carrying H-2Ak. We report here that one of the peptides indeed induced EAU in H-2Ak mice.
MATERIALS AND METHODS
Animals
B10.BR (Ak, Ek) and B10.A (Ak, Ek) female mice 5–8 weeks old were obtained from Shizuoka Laboratory Animal Corporation (Hamamatu, Japan). B10.A(4R) (Ak, E−) was maintained in a specific pathogen-free (SPF) condition of our animal facility at Hokkaido University.
Peptides and adjuvants
All peptides were synthesized using automatic peptide synthesizer PSSM-8 (Shimazu Co, Kyoto, Japan). FCA and Mycobacterium tuberculosis H37Ra were purchased from Difco Labs (Detroit, MI). Bordetella pertussis inactive bacterial suspension was purchased from Wako Pure Chemical Industries (Tokyo, Japan).
Immunization
Mice were immunized in the footpads and base of the tail with 20 nmol of each peptide or 100 μg IRBP (kindly provided by Dr I. Gery, NEI, NIH) in emulsion with FCA for the T cell proliferative responses. To induce EAU, mice were immunized in the footpad and base of the tail with 50 nmol of each peptide in 100 μl emulsion with FCA (1:1 v/v) with addition of 2.5 mg/ml of M. tuberculosis H37Ra. Concurrently, B. pertussis inactive bacterial suspension (1010) in a volume of 100 μl was injected intraperitoneally as an additional adjuvant.
T cell proliferative responses
Ten days after immunization, T cell-enriched fractions from the draining lymph nodes were prepared by passing the dispersed cells over nylon wool columns. These cells (4 × 105/well) were cultured with 30 Gy-irradiated syngeneic spleen cells as antigen-presenting cell (APC) and various concentrations of peptides in a 96-well flat-bottomed microtitre plate for 72 h. Evaluation of T cell proliferation was determined by 3H-thymidine incorporation in duplicate and the data are presented as the mean incorporation with the background value (medium alone) subtracted (Δcpm) [14, 15].
Assessment of EAU
Four weeks after immunization, eyes were enucleated and fixed for 1 h in 4% phosphate-buffered glutaraldehyde and transferred into 10% phosphate-buffered formaldehyde until processing. Fixed tissues were embedded in paraffin, and 4–6-μm sections, cut through the pupillary–optic nerve plane, were stained by standard haematoxylin and eosin. Incidence and severity of EAU were graded on a scale of 0–4 as shown previously [7]. In brief, focal non-granulomatous, monocytic infiltration in the choroid, ciliary body and retina were scored as 0.5. Retinal perivascular infiltration and monocytic infiltration in the vitreous were scored as 1. Granuloma formation on the uvea and retina, the presence of occluded retinal vasculitis, along with photoreceptor folds, serous detachment and loss of photoreceptor were scored as 2. In addition, the formation of Dalen–Fuchs nodules (granuloma at the level of the retinal pigmented epithelium) and the development of subretinal neovascularization were scored as 3 and 4 according to the number and the size of the lesions.
RESULTS
T cell proliferative responses to peptides synthesized according to the H-2Ak binding motif
According to an H-2Ak binding motif (Dxxxxxxxx[A,R,T]) which had been determined in our previous studies and those of other laboratories [13–15], six kinds of 16mer-peptides (K1 to K6) were selected from the sequence of bovine IRBP and synthesized (Fig. 1). In previous studies it was shown that 13–16mer length peptides were necessary for eliciting T cell proliferative responses [16] and inducing EAU in mice [12]. Immunogenicity of these peptides was first examined by the T cell proliferative responses. All the six peptides generated T cell proliferative responses in B10.BR mice in an immunogen-specific manner (Fig. 2). T cells primed with K1, K2, K3, K4, K5 or K6 showed no cross-reactivity to other peptide antigens. Furthermore, K2 and K6 peptides were the most potent in eliciting T cell responses among the six peptides examined. The next most potent peptide was K5 and the other peptides showed relatively weak immunogenicity compared with K2, K5 or K6 peptides. These results indicate that the peptides selected according to the H-2Ak binding motif were able to bind MHC class II (H-2Ak or H-2Ek) and subsequently induced T cell responses.
Fig. 1.

Alignment of amino acid sequences of peptides used in this study. Peptides deduced from bovine interphotoreceptor retinoid-binding protein (IRBP) were selected according to the presence of H-2Ak binding motif. P1 and P9 anchors are indicated in bold. Numbers indicate positions on IRBP.
Fig. 2.
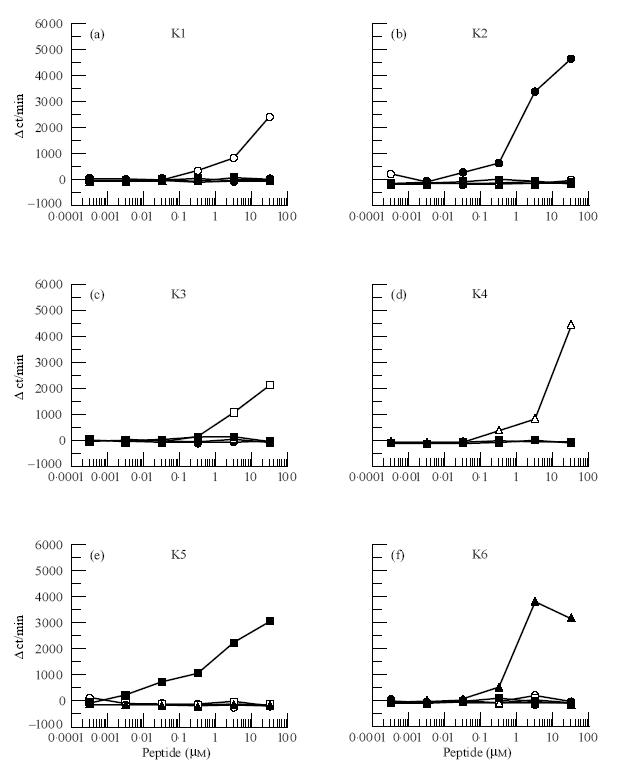
T cell proliferative responses of the peptide-primed T cells. B10.BR mice were immunized with the peptides K1 (a) (○), K2 (b) (•), K3 (c) (□), K4 (d) (▵), K5 (e) (▪) or K6 (f) (▴), shown in Fig 1. Responses of each primed T cells were measured as described in Materials and Methods.
Determination of uveitogenicity of the synthetic peptides
To examine uveitogenicity of the peptides, 50 nmol (80 μg) of each peptide were administrated to B10.BR mice. Silver et al. [12] reported that 50 μg of IRBP-derived peptides were sufficient to induce EAU in mice. We found that among six peptides only K2 (bovine IRBP 201–216) induced significant EAU in H-2Ak mice. Other peptides showed no uveitogenicity at all (Table 1).
Table 1.
Incidence and severity of EAU induced by synthetic peptides in B10.BR mice

Next, to determine whether H-2Ak molecules are really responsible for the T cell responses, H-2E-deficient B10.A(4R)(Ak, E−) mice were immunized with K2. T cells from the B10.A(4R) mice exhibited almost same level of proliferative responses in an immunogen-specific manner as seen in those of B10.BR mice (Fig. 3). This finding may permit us to conclude that the T cell responses are H-2Ak-restricted. We then examined the uveitogenicity of K2 using B10.A and B10.A(4R) (H-2Ak) mice and compared it with that in B10.BR mice. The incidence of EAU in B10.A or B10.A(4R) mice was 8/10 or 6/10, respectively (Table 2). Although a slight difference was seen in the incidence and severity among these three strains, these findings suggest that pathogenicity of K2 is dependent on H-2Ak molecules.
Fig. 3.
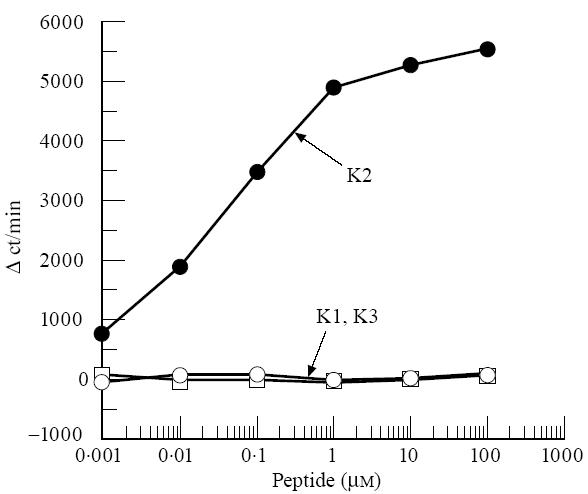
T cell responses of K2-primed T cells to various peptide antigens in B10.A(4R) mice. B10.A(4R) mice were immunized with K2. T cell proliferative responses were measured as described in Fig. 2.
Table 2.
Incidence and severity of EAU induced by K2 peptide in various H-2Ak-carrying mice
Pathological features induced by the uveitogenic peptide K2
Figure 4 illustrates the ocular histopathology resulting from active immunization with various peptides. Figure 4a shows a representative ocular tissue from B10.BR mice which were immunized with K1 peptide. No inflammatory lesion is detected. Peptides K3, K4, K5 and K6 also induced no EAU in B10.BR mice. In contrast, K2 induced substantial retinal vasculitis (open arrow) and choroidal inflammation (narrow arrow) in B10.BR mice (Fig. 4b). Thus, inflammation was localized at choroid and retina of the eye. This histopatholigical change was graded 2 of EAU. Figure 4c shows another tissue lesion in which more severe EAU was induced. Vitritis (small arrows), retinal vasculitis (open arrow), retinal granuloma (bold arrow) are seen. Furthermore, marked structural changes, retinal folds and retinal detachment (asterisk) are noted in this figure. This feature was defined as histopathological grade 4.
Fig. 4.
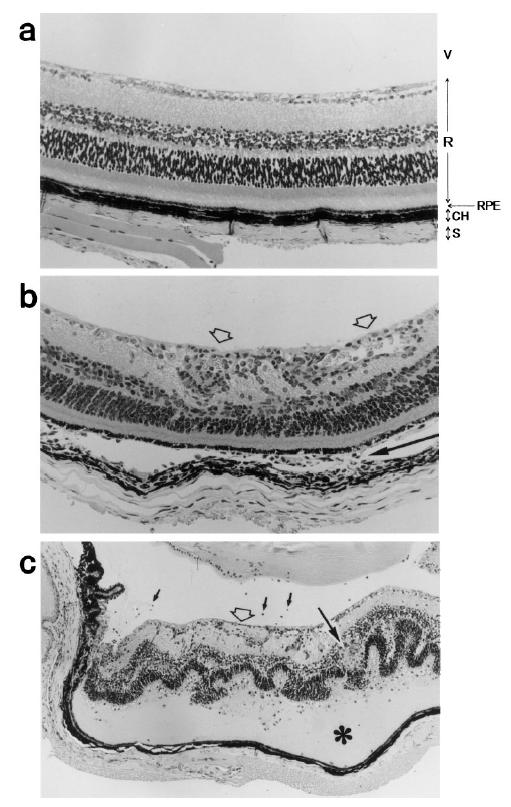
Histopathology of EAU induced by K2 in mice. EAU was not seen in B10.BR mice immunized with K1 peptide (a). V (vitreous), R (retina), RPE (retinal pigment epithelium), CH (choroid) and S (sclera) are indicated (× 50). EAU induced by immunization with K2 peptide is in grade 2 (b). Retinal vasculitis (open arrows) and choroidal inflammation (narrow arrow) which represent grade 4 of EAU are seen (× 50) (c). Vitritis (small arrows), retinal vasculitis (open arrow), retinal folds, retinal granuloma (bold arrow), retinal detachment (*) are indicated (× 25).
Cross-reactivity between K2 and IRBP
In order to determine whether K2 is really a natural epitope of IRBP, B10.BR mice were immunized with bovine IRBP or K2 and the cross-reactivity of the primed T cells was examined. As expected from the observations of EAU induction experiments, K2 stimulated IRBP-primed T cells (Fig. 5a). However, the stimulatory potency of K2 was considerably lower compared with IRBP, and the substantial proliferative responses were detected only at high doses of K2, whereas K2-primed T cells responded to the immunogen K2 at 10-fold higher concentrations than IRBP (Fig. 5b). These findings suggest that K2 peptides were generated from IRBP in vivo by processing in APC and presented on the APC in the context of H-2Ak, even though the K2 might represent a minor epitope. Nevertheless, K2 induced EAU in considerable numbers (8/10) of H-2Ak mice.
Fig. 5.
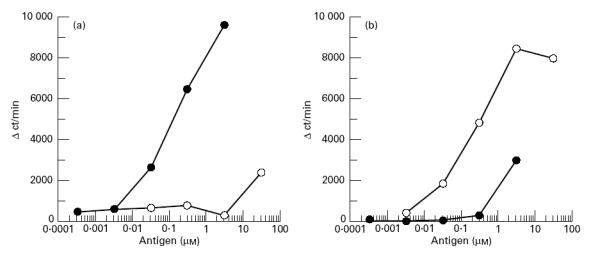
Cross-reactivity of T cells between K2 (○) and interphotoreceptor retinoid-binding protein (IRBP) (•). B10.BR mice were immunized with IRBP (a) or K2 (b). Specificity of T cells from these mice was determined as described in Fig. 2.
Mapping of the core region on K2 that interacts with MHC and T cell receptor
In Fig. 6a, amino acid sequences of 201–216 residues of bovine IRBP and the corresponding site of mouse IRBP are shown. The core region on a peptide that contains both MHC- and T cell receptor (TCR)-binding sites is usually composed of nine amino acids [17–19]. Since in the bovine IRBP 201–216 two H-2Ak binding motifs (residues 202–210 and 204–212) appeared to be present, this peptide might contain two sites that worked as possible core regions. In our previous paper [15] we demonstrated that replacement of negatively charged amino acids at position 1 of a H-2Ak binding peptide by positively charged amino acids significantly reduced the binding capacity of the peptide to H-2Ak and the capacity of eliciting T cell responses. Thus, to determine which site works as the core region, we substituted negatively charged aspartic acid (D) at position 202 or 204 for positively charged lysine (K), 202K or 204K, respectively. When B10.BR mice were immunized with original peptide K2, neither 202K nor 204K elicited significant responses in T cells from these B10.BR mice (Fig. 6b). In addition, 202K showed no significant immunogenicity in B10.BR mice, whereas 204K-primed T cells mounted proliferative responses in an immunogen-specific manner (Fig. 6c, d). When T cells are primed with a peptide antigen or its analogues that are prepared by substitution of amino acid at the TCR-binding site, each peptide antigen stimulates and expands different T cell repertoires which recognize the substituted amino acid [16]. Thus, the responding pattern of T cells shown in Fig. 6b,d suggests that the substituted position 204 is a TCR-binding site. On the other hand, negligible immunogenicity of 202K may be attributed to its low affinity to H-2Ak. It has been reported that the non-responsiveness of T cells is mainly caused by low or no affinity of peptides to MHC molecules [20, 21]. The present results suggest strongly that the residue 202 or 204 works as MHC-binding site or TCR-binding site, respectively. Thus, it seems to us that the core region of K2 peptide is residues 202–210.
Fig. 6.
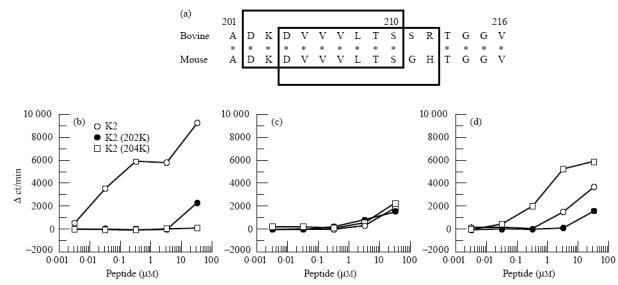
Determination of the core site on K2 peptide. Amino acid sequences of bovine IRBP201–216 and the corresponding site of mouse interphotoreceptor retinoid-binding protein (IRBP) are aligned (a). Two tentative core regions recognized by T cells are present in bovine IRBP201–216. Two squares indicate the tentative core regions. B10.BR mice were immunized with K2 (b), 202K (c) or 204K (d) and specificity of the primed T cells was tested in vitro as described in Fig. 2.
DISCUSSION
In the present study, we attempted to determine uveitogenic peptides deduced from bovine IRBP in mice bearing H-2Ak. Using recombinant proteins of bovine IRBP, Caspi et al. [11] reported that a major pathogenic epitope for mice of the H-2r haplotype resided in the first repeat (domain) of IRBP. However, it was not clear whether the major pathogenic epitope was also located in the first repeat of bovine IRBP in other EAU-susceptible mice. We could identify a uveitogenic peptide 201–216 (K2) derived from IRBP referring to the H-2Ak binding motif (Dxxxxxxxx[A,R,T]) that we had determined previously [15]. Since this peptide sequence was deduced from the first repeat of bovine IRBP, we concluded that at least one of the pathogenic epitopes was present in the first domain of bovine IRBP in H-2Ak mice. However, K2 may not be a major epitope, or other antigenic epitope(s) may be involved in full induction of EAU in H-2k haplotype mice. K2 induced uveoretinitis with a lower frequency (80%) than that (100%) induced by administration of whole IRBP protein [10, 12], although in this study we did not present the experimental results in which uveitogenicity of K2 and that of IRBP was directly compared. Nevertheless, it is anticipated that K2 is useful and advantageous compared with whole IRBP protein for immunological studies of EAU at a molecular level in H-2k haplotype mice.
It has been shown that class II-restricted presentation of uveitogenic antigens must precede subsequent pathogenicity of the antigens in rat and mouse EAU [10]. However, we have shown that non-MHC genes are involved in EAU development in the rat system [22]. Caspi et al. [10] also reported that mice of B10 background were susceptible to EAU. Thus, it appeared that B10 genetic background played certain roles in induction of EAU. Based on these previous studies, we used B10 H-2-congenic mice (B10.A, B10.BR and B10.A(4R)) in the present study. In these mice EAU was successfully induced. Furthermore, generation of EAU in B10.A(4R) mice (Ak, E −) suggested that uveitogenicity of K2 was restricted to H-2Ak, even though involvement of H-2Ek molecules was not formally excluded in the present study. However, successful induction of EAU in B10.A(4R) mice and H-2Ak binding motif on K2 suggest strongly that EAU is restricted to H-2Ak but not H-2Ek, as shown by Caspi et al. [10].
Thus far, only the first domain of mouse IRBP has been sequenced. In Fig. 6a, the amino acid sequence of mouse IRBP is compared with that of bovine IRBP201–216. The bovine IRBP201–216 contains two possible core regions (residues 202–210 and residues 204–212) that may bind to both MHC and TCR. We determined that the former residues 202–210 functioned as the core region. The residues 202–210 possess D at position 202 and serine (S) at position 210. It seems that 202D and 210S work as P1 and P9 anchors for binding to H-2Ak, respectively. Recently, Dadaglio et al. [23] showed that an H-2Ak binding motif contained D at P1 and S at P9. Thus, we concluded that the residues 202–210 of bovine IRBP bound to H-2Ak molecules using P1 and P9 anchors. In addition, we found the same sequence as bovine IRBP202–210 in mouse IRBP. The residues 202–210 of bovine IRBP are also conserved among several other species [24]. Thus, the core region of K2 may be regarded as an autoantigen in H-2Ak mice.
We assume that autoreactive T cells specific for IRBP are circulating in lymphoid tissues. However, the number of IRBP-reactive T cells is not sufficient to circulate through ocular tissue via the blood–retinal barrier. Recently it has been shown that expression of abundant Fas-ligand (FasL) maintains the eye as an immune privileged site [25]. Thus we consider that the blood–retinal barrier consists of both structural and functional barriers. FasL may represent the functional barrier, because the autoreactive T cells entering into the eyes seem to be killed by the Fas–FasL system in normal conditions. However, once mice are immunized with the K2 peptide with potent adjuvants, the increased population of autoreactive T cells may be beyond the capacity of the structural and functional barrier. In any case, the determination of the uveitogenic peptide in the H-2Ak mouse system will expedite the progress of therapeutic strategies for patients with autoimmune uveoretinitis.
Acknowledgments
We thank Dr I. Gery for providing IRBP. This study was supported in part by Grant-in-Aid for Scientific Research by the Ministry of Health and Welfare, and The Ministry of Education, Science, Sports and Culture, Japan, a Grant-in-Aid for Scientific Research (B) and The Special Grant-in-Aid for Promotion of Education and Science in Hokkaido University provided by The Ministry of Education, Science, Sports and Culture, Japan, The East Tomakomai Hospital Foundation, and The Nishimura Aging Fund.
References
- 1.Wacker WB, Donoso LA, Kalsow CM, et al. Experimental allergic uveitis. Isolation, characterization and localization of a soluble uveitopathogenic antigen from bovine retina. J Immunol. 1977;119:1949–58. [PubMed] [Google Scholar]
- 2.Gery I, Mochizuki M, Nussenblatt RB. Retinal specific antigens and immunopathogenic processes they provoke. Prog Retinal Res. 1986;5:75–109. [Google Scholar]
- 3.Caspi RR. Basic mechanisms in immune-mediated uveitic disease. In: Lightman SL, editor. Immunology of eye disease. Lancaster, UK: Kluwer Academic Publishers; 1989. pp. 61–86. [Google Scholar]
- 4.Mochizuki M, Kuwabara T, McAllister C, Nussenblatt RB, Gery I. Adoptive transfer of experimental autoimmune uveoretinitis in rats: immunopathogenic mechanisms and histologic features. Invest Opthalmol Vis Sci. 1985;26:1–9. [PubMed] [Google Scholar]
- 5.Caspi RR, Roberge FG, McAllister CG, et al. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986;136:1928–33. [PubMed] [Google Scholar]
- 6.Gregerson DS, Obritsch WF, Fling SP, Cameron JD. T cell lines recognize peptide fragments of S-antigen and mediate experimental auto-immune uveoretinitis and pinealitis. J Immunol. 1986;136:2875–82. [PubMed] [Google Scholar]
- 7.Caspi RR, Roberge FG, Chan CC, et al. A new model of autoimmune disease: experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol. 1988;140:1490–5. [PubMed] [Google Scholar]
- 8.Pepperberg DR, Okajima TL, Wiggert B, et al. Molecular biology and physiological role in the visual cycle of rhodopsin. Mol Neurobiol. 1993;7:61–85. doi: 10.1007/BF02780609. [DOI] [PubMed] [Google Scholar]
- 9.Borst DE, Redmond TM, Elser JE, et al. Interphotoreceptor retinoid-binding protein: gene characterization, protein repeat structure, and its evolution. J Biol Chem. 1989;264:1115–23. [PubMed] [Google Scholar]
- 10.Caspi RR, Grubbs BG, Chan CC, Chader GJ, Wiggert B. Genetic control of susceptibility to experimental autoimmune uveoretinitis in the mouse model: concomitant regulation by MHC and non-MHC genes. J Immunol. 1992;148:2384–9. [PubMed] [Google Scholar]
- 11.Caspi RR, Silver PB, Chan CC, et al. Immunogenetics of experimental autoimmune uveoretinitis (EAU) Reg Immunol. 1993;6:20–23. [Google Scholar]
- 12.Silver PB, Rizzo LV, Chan C-C, et al. Identification of a major pathogenic epitope in the human IRBP molecule recognized by mice of the H-2r haplotype. Invest Ophthalmol Vis Sci. 1995;36:946–54. [PubMed] [Google Scholar]
- 13.Allen PM, Matsueda DR, Evans RJ, et al. Identification of the T-cell and Ia contact residues of a T-cell antigenic epitope. Nature. 1987;327:713–7. doi: 10.1038/327713a0. [DOI] [PubMed] [Google Scholar]
- 14.Ogasawara K, Wambua PP, Gotohda T, Onoé K. Modification of the T cell responsiveness to synthetic peptides by substituting amino acids on agretopes. Int Immunol. 1989;2:219–24. doi: 10.1093/intimm/2.3.219. [DOI] [PubMed] [Google Scholar]
- 15.Itoh Y, Ogasawara K, Takami K, et al. Determination of amino acids on agretopes of pigeon cytochrome c-related peptides specifically bound to I-A allelic products. Eur J Immunol. 1994;24:76–83. doi: 10.1002/eji.1830240113. [DOI] [PubMed] [Google Scholar]
- 16.Ogasawara K, Maloy WE, Beverly B, Schwartz RH. Functional analysis of the antigenic structure of a minor T cell determinant from pigeon cytochrome c: evidence against an α-helical conformation. J Immunol. 1989;142:1448–56. [PubMed] [Google Scholar]
- 17.Stern LJ, Brown JH, Jardetzky TS, et al. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994;368:215–21. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- 18.Fremont DH, Henderickson WA, Marrack P, Kappler J. Structures of an MHC class II molecule with covalently bound single peptides. Science. 1996;272:1001–4. doi: 10.1126/science.272.5264.1001. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh P, Amaya M, Mellins E, Wiley DC. The structure of an intermediate in class II MHC maturation: CLIP bound to HLA-DR3. Nature. 1995;378:457–62. doi: 10.1038/378457a0. [DOI] [PubMed] [Google Scholar]
- 20.Schaeffer EL, Sette A, Johnson DL, et al. Relative contribution of “determinant selection” and “holes in the T cell repertoire” to T-cell responses. Proc Natl Acad Sci USA. 1989;86:4649–53. doi: 10.1073/pnas.86.12.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogasawara K, Onoé K. MHC-binding motifs and the design of peptide-based vaccines. Trends Microbiol. 1993;1:276–9. doi: 10.1016/0966-842x(93)90051-r. [DOI] [PubMed] [Google Scholar]
- 22.Hirose S, Ogasawara K, Natori T, et al. Regulation of experimental autoimmune uveitis in rats — separation of MHC and non-MHC gene effects. Clin Exp Immunol. 1991;86:419–25. doi: 10.1111/j.1365-2249.1991.tb02947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dadaglio C, Nelson CA, Deck MB, et al. Characterization and quantitation of peptide–MHC complexes produced from hen egg lysozyme using a monoclonal antibody. Immunity. 1997;6:727–38. doi: 10.1016/s1074-7613(00)80448-3. [DOI] [PubMed] [Google Scholar]
- 24.Stanhope MJ, Czelusniak J, Si JS, Nickerson J, Goodman M. A molecular perspective on mammalian evolution from the gene encoding interphotoreceptor retinoid binding protein, with convincing evidence for bat monophyly. Mol Phylogenet Evol. 1992;1:148–60. doi: 10.1016/1055-7903(92)90026-d. [DOI] [PubMed] [Google Scholar]
- 25.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–92. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]


