Abstract
An immunodominant region recognized by serum autoantibodies has been defined on the autoantigen thyroid peroxidase (TPO) using recombinant human TPO-specific Fab or a panel of mouse MoAbs. We have now analysed the epitopic relationships between the four recombinant Fab that identify the A and B domains of the TPO immunodominant region and (i) the mouse TPO MoAb as well as (ii) nine new TPO-specific Fab isolated independently. Competition between mouse MoAbs and recombinant Fab for binding to 125I-TPO revealed three patterns. First, for MoAbs 15, 59, 64 and 18, TPO binding was virtually abolished (≈ 90%) by Fab which define the A domain of TPO, with less inhibition by B domain Fab. Second, for MoAbs 2, 9 and 47, the Fab competed much less for TPO binding, and, when detectable, inhibition was predominantly with B domain Fab (65–20%). Third, for MoAbs 53, 30, 1, 24 and 40, none of the Fab competed effectively for 125I-TPO binding. Thus, the epitopes for MoAbs 18, 59, 64 and 15 correspond to those of the A domain defined by the human Fab, and the epitopes for MoAbs 2, 9 and 47 correspond to those of the B domain. In the second part of the study, competition studies demonstrated that the epitopes of nine new Fab corresponded to those of the four Fab that define the immunodominant region. For four new Fab, TPO binding was inhibited to a greater extent by B- than by A- domain Fab (65–95% versus < 50%). In contrast, for five new Fab the A-domain Fab were more effective inhibitors (≈ 90%) than the B-domain Fab. In addition, consistent with previous observations, all five new Fab with 02/012 κ L chains, but none of the new Fab with non-O2/O12 l chains, interacted with A-domain epitopes. In conclusion, we have established the epitopic relationships between recombinant human Fab and mouse MoAbs that define the TPO immunodominant region on TPO. Further, analysis of recombinant TPO Fab isolated from patients on three continents strengthens the paradigm of a relationship between autoantibody epitopic recognition and immunoglobulin gene usage.
Keywords: immunoglobulin genes, epitopes, recombinant Fab, thyroid peroxidase autoantibodies, combinatorial immunoglobulin gene libraries, human monoclonal antibodies
INTRODUCTION
Serum autoantibodies to thyroid peroxidase (TPO) are polyclonal and interact predominantly with conformational epitopes on the TPO molecule (reviewed in [1]). Mouse MoAbs to TPO have provided important information on TPO recognition by patients' autoantibodies [2]. In particular, the epitopes of most serum TPO autoantibodies correspond to those of two mouse MoAbs [3]. However, human monoclonal TPO autoantibodies, derived from patients' B cells, are the ultimate tools for defining the B cell epitopes on TPO in thyroid autoimmunity and are indispensable for determining the immunoglobulin genes that encode TPO autoantibodies.
From a large repertoire of human TPO monoclonal autoantibodies isolated using the combinatorial immunoglobulin gene library approach [4–8], four representative autoantibodies (expressed as Fab) have been used to define two overlapping domains (A and B) on the surface of native TPO [6] (Fig. 1). These domains are recognized by TPO autoantibodies in all 195 patients' sera so far analysed and by ≈ 80% of TPO autoantibodies within each individual serum. Consequently, the A + B domains represent the immunodominant region on TPO [6]. When used separately, the four TPO-specific human autoantibodies to the immunodominant region can be used to determine a quantitative ‘epitopic fingerprint' for TPO autoantibodies in the sera of individual patients [9–12].
Fig. 1.
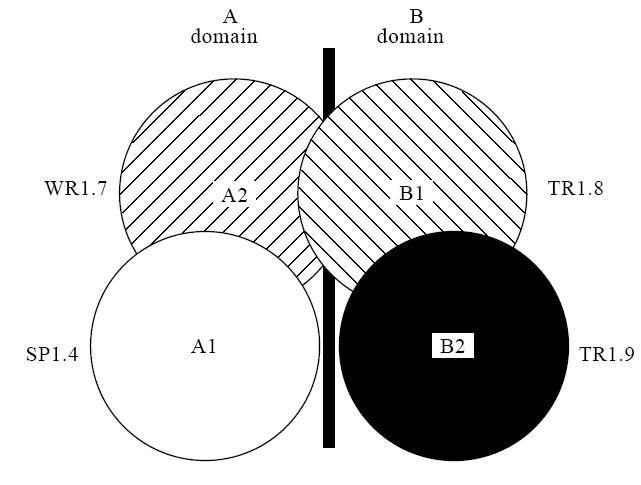
The thyroid peroxidase (TPO) immunodominant region defined by TPO-specific recombinant human Fab [6]. Fab SP1.4 and WR1.7 define subdomains A1 and A2 and Fab TR1.8 and TR1.9 define subdomains B1 and B2, respectively.
An important question that arises is whether or not the immunodominant region defined by recombinant TPO Fab [6] corresponds to the single, dominant TPO domain defined by two mouse MoAbs [3]. Moreover, the immunoglobulin gene combinatorial library approach has been used by other laboratories to isolate human monoclonal TPO autoantibody Fab [13, 14]. These Fab also interact with a restricted region on the TPO molecule recognized by serum autoantibodies [13, 14], and the epitopes of many of these Fab [15] have been compared with those of the mouse MoAbs to TPO [2].
The purpose of the present study was to analyse the epitopic relationship between the recombinant human Fab used to identify the TPO immunodominant region (Fig. 1) and (i) a panel of mouse TPO MoAbs [2], as well as (ii) TPO-specific Fab isolated independently in a separate laboratory [14, 15]. Furthermore, information on a large number of TPO autoantibody Fab isolated from different patients on three continents provided an opportunity to test the previous concept [6, 8, 16, 17] of a relationship between autoantibody epitopic recognition and immunoglobulin gene usage.
MATERIALS AND METHODS
Preparation of soluble Fab
TPO-specific Fab SP1.4, WR1.7, TR1.8 and TR1.9 were expressed by plasmid-bearing XL1-blue cells as previously described [9]. In brief, transformed cells were grown in a shaker (225 rev/min, 37°C) in SuperBroth medium with 100 μg/ml ampicillin, 20 mm MgCl2, 1% glucose until an optical density (OD) of 0.2 was attained. Protein synthesis was induced with 1 mm isopropyl-thio-galacto-pyranoside (Sigma Chemical Co., St Louis, MO) overnight at 27°C. The cells were pelleted, resuspended in PBS pH 7.5 containing 2 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 0.1 mm phenylmethylsulphonyl fluoride (all from Sigma) and frozen (−80°C). After thawing, the suspension was sonicated, the membranes pelleted by centrifugation at 30 000 g and the Fab were affinity-purified using Protein G Sepharose (Pharmacia, Piscataway, NJ) or an anti-H + L column (Zymed, South San Francisco, CA). Purified Fab were analysed by SDS–PAGE under reducing conditions. A similar approach was used to express the panel of new Fab. However, in this case, supernatant obtained after freezing and thawing from the pelleted cells was used as a source of Fab without purification [14].
Relationship between epitopes recognized by mouse MoAb and recombinant human Fab to the immunodominant region
Mouse MoAb binding of 125I-TPO was examined in the absence and presence of recombinant TPO Fab that define the immunodominant region (Fig. 1) as follows. Duplicate aliquots of MoAbs were incubated with 125I-TPO (20 000 ct/min; 1 h, room temperature; labelled using iodogen [5] to a specific activity of ≈ 50 μCi/μg) alone or with Fab SP1.4, WR1.7, TR1.8 or TR1.9 (4 × 10−8 m) in a total volume of 200 μl. To precipitate the antigen–antibody complex, anti-mouse IgG coupled to a solid phase (Sac Cel; 100 μl; IDS, Bolden, Tyne & Wear, UK) was added and the incubation continued for 30 min. After addition of 1 ml assay buffer (0.15 m NaCl, 10 mm Tris–HCl pH 7.5, 0.1% Tween 20 and 0.5% bovine serum albumin (BSA)), the mixture was vortexed, centrifuged for 30 min at 1000 g (4°C), supernatants removed by aspiration and radiolabelled TPO remaining in the pellets counted. Preliminary experiments were performed to determine the MoAb dilutions required to provide binding values of ≈ 15% in the absence of TPO Fab. Such dilution was necessary to attain maximal inhibition of TPO binding by the addition of an excess concentration of Fab. Non-specific 125I-TPO binding by Fab in the absence of MoAb (≈ 3% of total ct/min) was subtracted from the values obtained with autoantibody in calculating the percentage inhibition by the TPO-specific Fab.
Relationships between epitopes recognized by recombinant human TPO-specific Fab from different laboratories
Fab binding to 125I-TPO was measured as described [5] by incubating the Fab (1 h, ≈ 20°C) with 125I-TPO (≈ 20 000 ct/min) and murine monoclonal anti-human κ-chains (QE11; Recognition Sciences, Birmingham, UK) in a total volume of 200 μl. Subsequently, 100 μl donkey anti-mouse immunoglobulin Sac-Cel (IDS) were added, and the incubation was continued for 30 min. After addition of 1 ml assay buffer (see above) and vortexing, the mixture was centrifuged (25 min, 1000 g) to sediment the immune complexes, which were then counted to determine the percentage 125I-TPO bound.
A modification of this assay [6] was used to determine test Fab interaction with epitopes recognized by Fab that define the immunodominant SP1.4 and WR1.7 (TPO A domain) and Fab TR1.8 and TR1.9 (TPO domain B). Briefly, the test Fab was first immobilized by incubation with murine monoclonal anti-human κ-chains (QE11) (1 h, ≈ 20°C). After addition of Sac-Cel (100 μl, 30 min, room temperature), the immobilized Fab complex was diluted in assay buffer (see above) and centrifuged at 1000 g (25 min, 4°C). The pellets were resuspended in normal human serum diluted 1:30 in assay buffer to saturate remaining anti-κ binding sites. In a separate set of tubes, 125I-TPO (≈ 20 000 ct/min) was preincubated with or without ‘free’ Fab (4 × 10−8 m) for 1 h at room temperature. Duplicate aliquots (100 μl) were then incubated for 30 min with the immobilized test Fab, washed with assay buffer and radioactivity bound to the Sac-Cel was counted. Non-specific binding (≈ 3% of total counts added) was subtracted to provide values for specific binding to TPO.
RESULTS
Epitopic relationship between mouse MoAbs and recombinant human Fab to the immunodominant region
The epitopes of mouse MoAbs to TPO and the human Fab to the TPO immunodominant region (Fig. 1) were compared by competition for binding to 125I-TPO. Mouse MoAb–TPO complexes were precipitated using an Fc-specific anti-mouse IgG that does not precipitate the human Fab (present at high concentration, 4 × 10−8 m). The results defined three categories of mouse MoAb. In the first group (MoAbs 15, 59, 64, 18), TPO binding was virtually completely inhibited (≈ 90%) by Fab SP1.4 and WR1.7 to the A domain of the immunodominant region, with little, if any, inhibition by B domain Fab (Fig. 2, top panel).
Fig. 2.
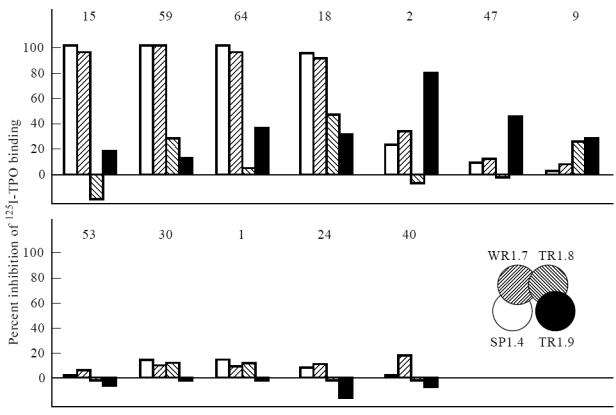
Epitopic relationship between mouse MoAbs [2] and recombinant human Fab [6] to the immunodominant region. Mouse MoAb binding to 125I-thyroid peroxidase (TPO) was measured in the absence and presence of Fab SP1.4, WR1.7, TR1.8 or TR1.9 (each at 4 × 10−8 m). The percentage inhibition of radiolabelled TPO binding is represented by a bar with shading corresponding to that in the inset. Binding for the 12 MoAbs without Fab averaged 10.4 ± 4.4% (mean ± s.d.).
With the second group of MoAbs (2, 47, 9), the human Fab competed much less for TPO binding. When detectable, inhibition was predominantly with B-domain Fab. Thus, Fab TR1.9 inhibited binding of these three MoAbs by 65%, 38% and 20%, respectively (Fig. 2, top panel). Fab TR1.8 also inhibited MoAb 9 binding to TPO by about ≈ 20%. Poor inhibition by Fab of MoAb 9 binding to TPO may be related to poor recognition by this MoAb of 125I-TPO [2]. In contrast, the recombinant Fab interacted very well with iodinated TPO [6]. In the third group of mouse MoAbs (53, 30, 1, 24, 40), none of the Fab competed effectively for 125I-TPO binding (Fig. 2, lower panel).
Modest inhibition by human Fab TR1.9 (B2 domain; Fig. 1) of MoAb 47 binding to TPO was surprising in view of the previous observation that the MoAb 47 epitope did not lie within the TPO immunodominant region [18, 19]. Because inhibition in the present study was observed at a higher Fab concentration (4 × 10−8 m) than used previously (up to 10−9 m), we repeated the analysis using two lower concentrations of recombinant Fab (Fig. 3). Indeed, consistent with the previous study, lower concentrations of Fab were unable to compete for MoAb 47 binding to TPO. Poor competition by the human Fab for MoAb 47 binding to TPO could be explained if the affinity of MoAb 47 was even higher than that of the TPO Fab TR1.9 (Kd of ≈ 10−10 m). However, this was not the case; the affinity of MoAb 47 (and MoAb 15) was too low to measure with the amount of TPO available (Fig. 4).
Fig. 3.
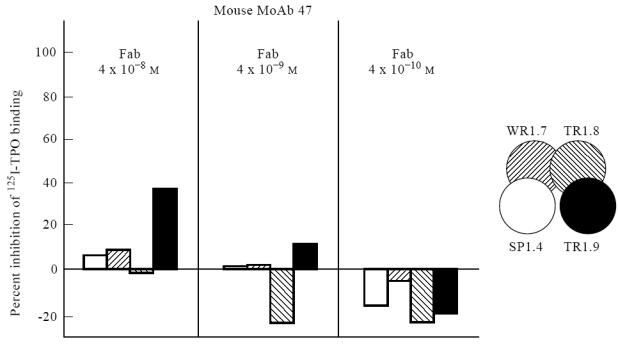
High concentrations of recombinant Fab are required to compete for MoAb 47 [2] binding to thyroid peroxidase (TPO). 125I-TPO binding by MoAb 47 was measured without or with decreasing concentrations (4 × 10−8–4 × 10−10 m) of Fab SP1.4, WR1.7, TR1.8 or TR1.9. The percentage inhibition is represented by a bar with shading corresponding to that in the inset. In the absence of competing Fab, binding by MoAb 47 was 14.3%.
Fig. 4.
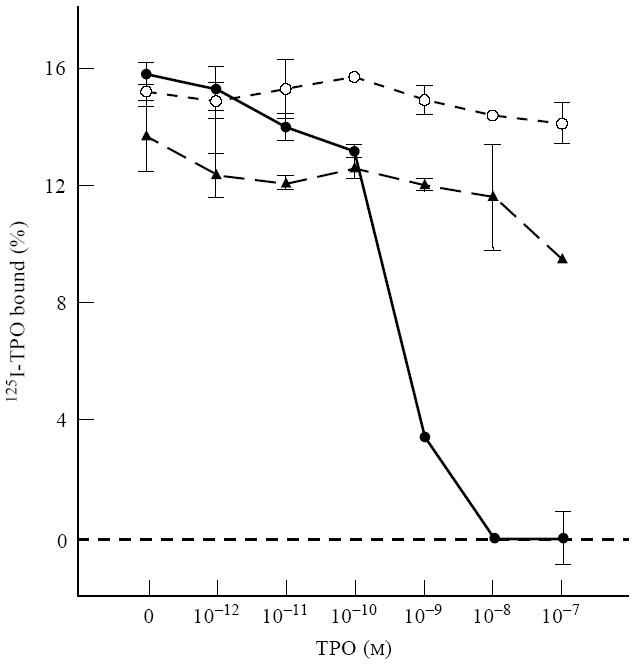
The affinity of MoAb 47 for thyroid peroxidase (TPO) is lower than that of recombinant Fab. Unlike Fab TR1.9, 125I-TPO binding by MoAb 47 (and also by MoAb 15) is not inhibited by unlabelled TPO up to concentrations of 10−7 m. Data are shown as the mean ± range of duplicate determinations. Binding in the absence of unlabelled TPO was 15.9%, 15.2% and 13.6% for Fab TR1.9 (•), MoAb 47 (○) and MoAb 15 (▴), respectively.
Epitopic relationship between recombinant human TPO-specific Fab from different laboratories
The four TPO-specific Fab to the immunodominant region (Fig. 1) were tested for their ability to compete for 125I-TPO binding by nine representative recombinant Fab recently cloned in the UK [14]. Two patterns of inhibition were observed. In one group (four test Fab) (Fig. 5, right panel), binding was markedly inhibited (65–95%) by Fab TR1.9 and TR1.8, the two Fab that define epitopes in the TPO B domain. Much less inhibition (20–50%) was induced by Fab WR1.7 and SP1.4 with epitopes in the A domain. In contrast, for the second group (five Fab) (Fig. 5, left panel), the B-domain Fab, in particular TR1.8 (B1 subdomain), were less effective inhibitors than the A-domain Fab. Indeed, Fab SP1.4 and WR1.7 almost totally inhibited TPO binding by the test Fab.
Fig. 5.
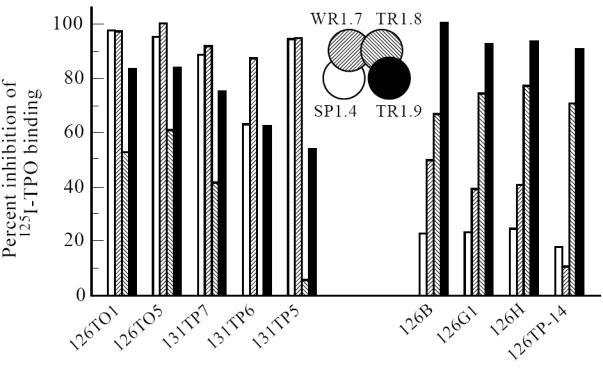
Epitopic relationship between recombinant human thyroid peroxidase (TPO)-specific Fab from different laboratories. 125I-TPO binding by nine recombinant Fab recently cloned in the UK [14] was measured in the absence and presence of Fab SP1.4, WR1.7, TR1.8 or TR1.9 (each at 4 × 10−8 m). The percentage inhibition of radiolabelled TPO binding is represented by a bar with shading corresponding to that in the inset. In the absence of free competing Fab, average binding of the nine test Fab was 4.9 ± 1.5% (mean ± s.d.).
DISCUSSION
Four recombinant human Fab (SP1.4, WR1.7, TR1.8, TR1.9) define overlapping epitopes in an immunodominant region recognized by TPO autoantibodies in patients' sera ([6] and reviewed in [20]) (Fig. 1). Previously, TPO binding by several mouse MoAbs was inhibited by a pool of Hashimoto or Graves' sera [2] (Fig. 6a). More recently, when tested with serum from individual patients, most autoantibodies were found to recognize a region on TPO close to, or identical with, the epitope for mouse MoAb 9 and, to a lesser extent, for MoAb 2 [3]. In the present study, we have been able to relate the autoantibody immunodominant regions characterized by these two sets of MoAbs (human and mouse).
Fig. 6.
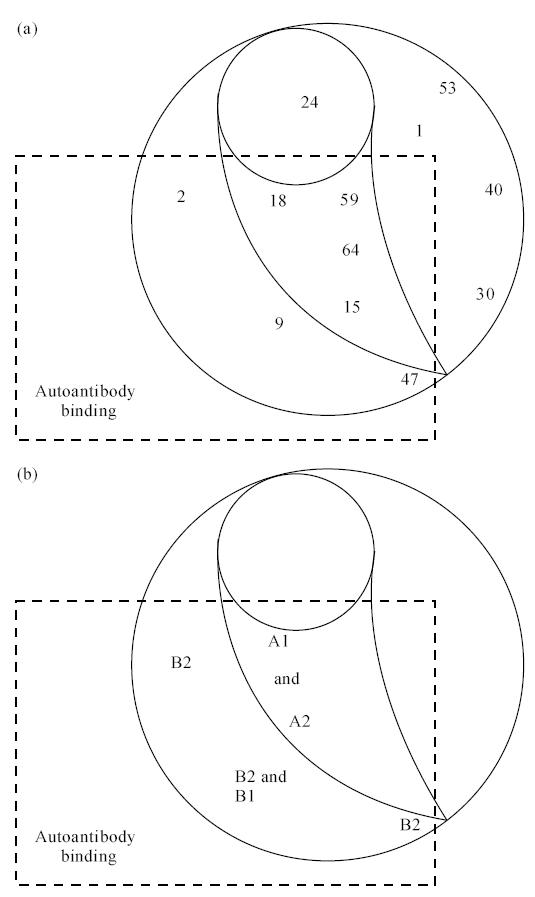
(a) Map of epitopes on thyroid peroxidase (TPO) recognized by mouse MoAb (adapted from [2] with permission). (b) Relationship between mouse MoAb epitopes and A1, A2, B1 and B2 subdomains on the immunodominant region defined by recombinant human TPO-specific Fab.
The epitopes for mouse MoAbs 9 and 2 correspond to those of the human Fab that define the B1 and B2 subdomains of the TPO immunodominant region (Fig. 6b). In addition, the epitopes for mouse MoAbs 18, 59, 64 and 15 correspond to those of the human Fab to the A1 and A2 subdomains on TPO. Of the four Fab to the immunodominant region, the epitope for TR1.8 (B1 subdomain) is recognized by human autoantibodies to the greatest extent [9]. Remarkably, this epitope corresponds to that of mouse MoAb 9, which also defines the primary epitope recognized by patients' autoantibodies [3]. Thus, mouse MoAb A domain is the human recombinant Fab B domain, and vice versa.
It should be emphasized that there is no overlap between the epitopes recognized by these four TPO-specific Fab and the epitopes of five other mouse MoAbs (53, 30, 1, 24, 40) (Fig. 6). Our findings mirror the observations from previous studies comparing recognition of thyroglobulin [21] and TPO [2, 3] by serum autoantibodies versus mouse MoAbs. Thus, antibodies artificially generated by immunizing mice interact with a wide range of epitopes, while spontaneously arising IgG class autoantibodies recognize restricted, overlapping epitopes on the same antigen.
In the second part of our study, we evaluated the epitopes of a new set of human TPO-specific Fab [14] with Fab that define the TPO immunodominant region of human autoantibodies [6]. All nine new Fab analysed had epitopes corresponding to the four original Fab, further validating the latter as defining the immunodominant region on TPO. Of the newly isolated Fab, four recognized epitopes predominantly in the B domain, and five in the A domain, as defined by the human MoAb Fab. These findings correlate closely with the comparison made between the new Fab and the mouse MoAb [15].
A major advantage of using monoclonal human TPO autoantibodies (in contrast to mouse MoAb) is that it permits analysis of the relationship between epitopes and the immunoglobulin genes encoding the patients' autoantibodies [22]. Previous evidence suggested a relationship between κ light (L) chain genes and recognition of the A domain in the immunodominant regions defined by the recombinant Fab. Thus, all TPO-specific Fab with O2/12 l chains studied previously interacted with the TPO A domain [6, 16]. Conversely, TPO-specific Fab with κ L chain genes other than 02/012 [6, 16, 17], as well as λ L chain genes [8], recognized epitopes in the B domain. The present study extends the number of human TPO Fab whose genes have been characterized and whose epitopes have been related to the same set of standards. Remarkably, all nine newly isolated Fab fell into the same paradigm (Table 1). Thus, the five new Fab with 02/012 κ L chains interacted with A-domain epitopes, and the four new Fab with non-02/012 κ L chains recognized epitopes predominantly in the B domain. It is also noteworthy that this finding reflects genes cloned from thyroids or lymph nodes of patients with either Graves' disease or Hashimoto's thyroiditis from Italy [4], USA [6], Japan [17] and the UK [14]. These data provide strong support for the relevance of TPO autoantibody Fab obtained by the combinatorial immunoglobulin gene library approach, although the validity of this methodology has been questioned [23].
Table 1.
Summary of the relationship between the light chain genes encoding recombinant thyroid peroxidase (TPO)-specific Fab and recognition of the A or B domains in the immunodominant region
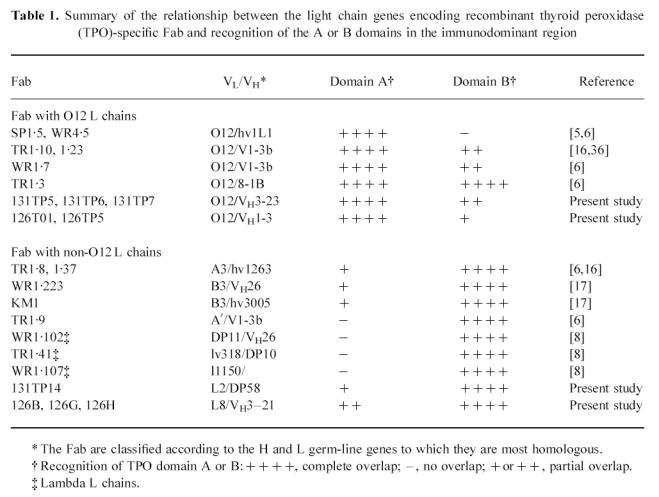
Like most serum TPO autoantibodies [24–26], most recombinant TPO Fab isolated to date have κ L chain genes (reviewed in [20]) [14]. It is interesting that TPO-specific Fab with κ L chains derived from the 02/O12 gene been isolated in three laboratories [6, 13, 14]. This finding may reflect over-usage of the 012 gene in the expressed κ chain repertoire generally [27] (reviewed in [28]).
Among the murine MoAbs considered in the present study, MoAb 47 is of particular interest for two main reasons. First, MoAb 47 is the only antibody whose epitope could be determined (at least in part) by screening a TPO random fragment cDNA library [29]. Identification of this linear amino acid sequence was possible because MoAb 47 is the only member of this panel whose binding to TPO could not be abolished by dithiothreitol treatment to denature the protein [30]. Importantly, most serum autoantibodies [31–33], as well as recombinant TPO-specific Fab [5, 6, 8, 13, 15], interact predominantly with conformationally intact TPO. Second, despite its ability to recognize non-conformational epitopes, there is overlap between the MoAb 47 epitope and those of some autoantibodies in patients' sera [2, 34].
Previous studies indicated that the MoAb 47 epitope lay outside the immunodominant region. Thus, targeted mutations of TPO in the region of its epitope (residues 713–721) retained recognition by TPO-specific Fab that define the immunodominant region [19]. Furthermore, these TPO-specific Fab did not compete for MoAb 47 binding to TPO [18]. In agreement with these earlier studies, the new panel of TPO-specific Fab was also unable to inhibit MoAb 47 binding to TPO [15]. However, in competition studies performed in the reverse manner, MoAb 47 did inhibit Fab binding to TPO, leading to the suggestion that the MoAb 47 epitope might, indeed, be involved in the immunodominant region [15].
The present studies re-examine this confusing issue and demonstrate that: (i) the suggestion [15] that the inability of immunodominant region Fab to compete for MoAb 47 binding was due to the latter's very high affinity for TPO can be excluded; and (ii) only very high concentrations of one Fab (TR1.9) produce partial inhibition of low-affinity MoAb 47 binding to native TPO. A possible explanation for MoAb 47 inhibiting Fab binding, and not vice versa, is steric hindrance by the much larger immunoglobulin molecule. Further, MoAb 47 may interact with TPO differently when the antigen is in solution ([18]; present study) and when it is adherent to an ELISA plate [3].
Taken together, the data past and present suggest that the MoAb 47 epitope (amino acids 713–721) is not part of the immunodominant region. However, B cell epitopes generally comprise more than nine amino acids [35] and, as originally suggested [29], other parts of the folded protein may contribute to the MoAb 47 epitope. The MoAb 47 epitope may therefore lie on the fringe of the immunodominant region, or form part of a larger conformational epitope. Resolution of this issue awaits determination of the three-dimensional structures of TPO complexed to Fab that define the immunodominant region.
In conclusion, we have established the epitopic relationship between recombinant human Fab and mouse TPO MoAb that define an immunodominant region on TPO recognized by serum autoantibodies. Further, analysis of recombinant TPO Fab isolated from patients on three continents strengthens the paradigm of a relationship between autoantibody epitopic recognition and immunoglobulin gene usage.
Acknowledgments
This research was supported by National Institutes of Health Grant DK36182.
References
- 1.McLachlan SM, Rapoport B. The molecular biology of thyroid peroxidase: cloning, expression and role as autoantigen in autoimmune thyroid disease. Endocr Rev. 1992;13:192–206. doi: 10.1210/edrv-13-2-192. [DOI] [PubMed] [Google Scholar]
- 2.Ruf J, Toubert M, Czarnocka B, Durand-Gorde J, Ferrand M, Carayon P. Relationship between immunological structure and biochemical properties of human thyroid peroxidase. Endocrinol. 1989;125:1211–8. doi: 10.1210/endo-125-3-1211. [DOI] [PubMed] [Google Scholar]
- 3.Czarnocka B, Pastuszko D, Carayon P, Ruf J, Gardas A. Majority of thyroid peroxidase in patients with autoimmune thyroid disease are directed to a single TPO domain. Autoimmunity. 1996;23:145–54. doi: 10.3109/08916939608995338. [DOI] [PubMed] [Google Scholar]
- 4.Portolano S, Seto P, Chazenbalk GD, Nagayama Y, McLachlan SM, Rapoport B. A human Fab fragment specific for thyroid peroxidase generated by cloning thyroid lymphocyte-derived immunoglobulin genes in a bacteriophage lambda library. Biochem Biophys Res Commun. 1991;179:372–9. doi: 10.1016/0006-291x(91)91380-u. [DOI] [PubMed] [Google Scholar]
- 5.Portolano S, Chazenbalk GD, Seto P, Hutchison JS, Rapoport B, McLachlan SM. Recognition by recombinant autoimmune thyroid disease-derived Fab fragments of a dominant conformational epitope on human thyroid peroxidase. J Clin Invest. 1992;90:720–6. doi: 10.1172/JCI115943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chazenbalk GD, Portolano S, Russo D, Hutchison JS, Rapoport B, McLachlan SM. Human organ-specific autoimmune disease: molecular cloning and expression of an autoantibody gene repertoire for a major autoantigen reveals an antigenic dominant region and restricted immunoglobulin gene usage in the target organ. J Clin Invest. 1993;92:62–74. doi: 10.1172/JCI116600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prummel MF, Portolano S, Costante G, Rapoport B, McLachlan SM. Isolation and characterization of a monoclonal human thyroid peroxidase autoantibody of lambda light chain type. Molec Cell Endocrinol. 1994;102:161–6. doi: 10.1016/0303-7207(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 8.Portolano S, Prummel MF, Rapoport B, McLachlan SM. Molecular cloning and characterization of human thyroid peroxidase autoantibodies of lambda light chain type. Molec Immunol. 1995;32:1157–69. doi: 10.1016/0161-5890(95)00060-7. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa T, Costante G, Prummel MF, McLachlan SM, Rapoport B. Recombinant thyroid peroxidase autoantibodies can be used for epitopic ‘fingerprinting' of thyroid peroxidase autoantibodies in the sera of individual patients. J Clin Endocrinol Metab. 1994;78:944–9. doi: 10.1210/jcem.78.4.7512572. [DOI] [PubMed] [Google Scholar]
- 10.Jaume JC, Costante G, Nishikawa T, Phillips DIW, Rapoport B, McLachlan SM. Thyroid peroxidase autoantibody fingerprints in hypothyroid and euthyroid individuals. I. Cross-sectional study in elderly women. J Clin Endocrinol Metab. 1995;80:994–9. doi: 10.1210/jcem.80.3.7533777. [DOI] [PubMed] [Google Scholar]
- 11.Jaume JC, Parkes AB, Lazarus JH, et al. Thyroid peroxidase autoantibody fingerprints. II. A longitudinal study in postpartum thyroiditis. J Clin Endocrinol Metab. 1995;80:1000–5. doi: 10.1210/jcem.80.3.7533767. [DOI] [PubMed] [Google Scholar]
- 12.Jaume JC, Burek CL, Hoffman WH, Rose N, McLachlan SM, Rapoport B. Thyroid peroxidase autoantibody epitopic ‘fingerprints’in juvenile Hashimoto's thyroiditis: evidence for conservation over time and in families. Clin Exp Immunol. 1996;104:115–23. doi: 10.1046/j.1365-2249.1996.d01-659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hexham JM, Partridge LJ, Furmaniak J, et al. Cloning and characterisation of TPO autoantibodies using combinatorial phage display libraries. Autoimmunity. 1994;17:167–79. doi: 10.3109/08916939409010651. [DOI] [PubMed] [Google Scholar]
- 14.McIntosh RS, Asghar MS, Kemp EH, et al. Analysis of IgG kappa anti-thyroid peroxidase antibodies from different tissues in Hashimoto's thyroiditis. J Clin Endocrinol Metab. 1997;82:3818–25. doi: 10.1210/jcem.82.11.4348. [DOI] [PubMed] [Google Scholar]
- 15.Czarnocka B, Janota-Bzowski M, McIntosh RS, et al. Immunoglobulin Gκ anti-thyroid peroxidase antibodies in Hashimoto's thyroiditis: epitope mapping analysis. J Clin Endocrinol Metab. 1997;82:2639–44. doi: 10.1210/jcem.82.8.4124. [DOI] [PubMed] [Google Scholar]
- 16.Costante G, Portolano S, Nishikawa T, et al. Recombinant thyroid peroxidase-specific autoantibodies. II. Role of individual heavy and light chains in determining epitope recognition. Endocrinol. 1994;134:25–30. doi: 10.1210/endo.135.1.7516865. [DOI] [PubMed] [Google Scholar]
- 17.Jaume JC, Guo J, Rapoport B, McLachlan SM. The epitopic ‘fingerprint’ of thyroid peroxidase-specific Fab isolated from a patient's thyroid gland by the combinatorial library approach resembles that of autoantibodies in the donor's serum. Clin Immunol Immunopathol. 1997;84:150–7. doi: 10.1006/clin.1997.4383. [DOI] [PubMed] [Google Scholar]
- 18.Chazenbalk GD, Costante G, Portolano S, McLachlan SM, Rapoport B. The immunodominant region on human thyroid peroxidase recognized by autoantibodies does not contain the monoclonal antibody 47/c2l linear epitope. J Clin Endocrinol Metab. 1993;77:1715–8. doi: 10.1210/jcem.77.6.7505290. [DOI] [PubMed] [Google Scholar]
- 19.Nishikawa T, Rapoport B, McLachlan SM. The quest for the autoantibody immunodominant region on thyroid peroxidase: guided mutagenesis based on a hypothetical 3-dimensional model. Endocrinol. 1996;137:1000–6. doi: 10.1210/endo.137.3.8603566. [DOI] [PubMed] [Google Scholar]
- 20.McLachlan SM, Rapoport B. Genetic and epitopic analysis of thyroid peroxidase (TPO) autoantibodies: markers of the human thyroid autoimmune response. Clin Exp Immunol. 1995;101:200–6. doi: 10.1111/j.1365-2249.1995.tb08339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nye L, Pontes de Carvalho LC, Roitt IM. Restrictions in the response to autologous thyroglobulin in the human. Clin Exp Immunol. 1980;41:252–63. [PMC free article] [PubMed] [Google Scholar]
- 22.Rapoport B, Portolano S, McLachlan SM. Combinatorial immunoglobulin gene libraries: new insights into human organ-specific autoantibodies. Immunol Today. 1995;16:43–49. doi: 10.1016/0167-5699(95)80070-0. [DOI] [PubMed] [Google Scholar]
- 23.Tonacchera M, Cetani F, Costagliola S, et al. Mapping thyroid peroxidase epitopes using recombinant protein fragments. Eur J Endocrinol. 1995;132:53–61. doi: 10.1530/eje.0.1320053. [DOI] [PubMed] [Google Scholar]
- 24.Parkes AB, McLachlan SM, Bird P, Rees Smith B. The distribution of microsomal and thyroglobulin antibody activity among the IgG subclasses. Clin Exp Immunol. 1984;57:239–43. [PMC free article] [PubMed] [Google Scholar]
- 25.Weetman AP, Black CM, Cohen SB, Tomlinson R, Banga JP, Reimer CB. Affinity purification of IgG subclasses and the distribution of thyroid autoantibody reactivity in Hashimoto's thyroiditis. Scand J Immunol. 1989;30:73–82. doi: 10.1111/j.1365-3083.1989.tb01190.x. [DOI] [PubMed] [Google Scholar]
- 26.Kotani T, Kato E, Hirai K, Kuma K, Ohtaki S. Immunoglobulin G subclasses of anti-thyroid peroxidase autoantibodies in human autoimmune thyroid diseases. Endocrinol Japon. 1986;33:505–10. doi: 10.1507/endocrj1954.33.505. [DOI] [PubMed] [Google Scholar]
- 27.Cox JPL, Tomlinson IM, Winter G. A directory of human germ-line Vκ segments reveals a strong bias in their usage. Eur J Immunol. 1994;24:827–36. doi: 10.1002/eji.1830240409. [DOI] [PubMed] [Google Scholar]
- 28.Burton DR, Barbas Iiicf. Human antibodies from combinatorial libraries. Adv Immunol. 1994;57:191–281. doi: 10.1016/s0065-2776(08)60674-4. [DOI] [PubMed] [Google Scholar]
- 29.Finke R, Seto P, Ruf J, Carayon P, Rapoport B. Determination at the molecular level of a B-cell epitope on thyroid peroxidase likely to be associated with autoimmune thyroid disease. J Clin Endocrinol Metab. 1991;73:919–21. doi: 10.1210/jcem-73-4-919. [DOI] [PubMed] [Google Scholar]
- 30.Czarnocka B, Ruf J, Ferrand M, Carayon P. Immunochemical properties of hTPO. In: Carayon P, Ruf J, editors. Thyroperoxidase and thyroid autoimmunity. London: John Libbey & Co. Ltd; 1990. pp. 59–67. [Google Scholar]
- 31.Kajita Y, Morgan D, Parces AB, Rees Smith B. Labelling and immunoprecipitation of thyroid microsomal antigen. FEBS Letters. 1985;187:334–8. doi: 10.1016/0014-5793(85)81271-0. [DOI] [PubMed] [Google Scholar]
- 32.Gardas A, Domek H. The effect of sulphydryl reagents on the human thyroid microsomal antigen. J Endocrinol Invest. 1988;11:385–8. doi: 10.1007/BF03349061. [DOI] [PubMed] [Google Scholar]
- 33.Finke R, Seto P, Rapoport B. Evidence for the highly conformational nature of the epitope(s) on human thyroid peroxidase that are recognized by sera from patients with Hashimoto's thyroiditis. J Clin Endocrinol Metab. 1990;71:53–59. doi: 10.1210/jcem-71-1-53. [DOI] [PubMed] [Google Scholar]
- 34.Libert F, Ludgate M, Dinsart C, Vassart G. Thyroperoxidase, but not the thyrotropin receptor, contains sequential epitopes recognized by autoantibodies in recombinant peptides expressed in the pUEX vector. J Clin Endocrinol Metab. 1991;73:857–60. doi: 10.1210/jcem-73-4-857. [DOI] [PubMed] [Google Scholar]
- 35.Davies DR, Padlan E. Antibody–antigen complexes. Annu Rev Biochem. 1990;59:439–73. doi: 10.1146/annurev.bi.59.070190.002255. [DOI] [PubMed] [Google Scholar]
- 36.Portolano S, McLachlan SM, Rapoport B. High affinity, thyroid-specific human autoantibodies displayed on the surface of filamentous phage use V genes similar to other autoantibodies. J Immunol. 1993;151:2839–51. [PubMed] [Google Scholar]


