Abstract
Clinical improvement has been described in AIDS patients submitted to zinc therapy, but the mechanisms involved are not well understood. In order to evaluate the effect of the zinc ions in the enhancement of the immune response, we tested its role in the lymphoproliferative response to a mitogen, as well as in the prevention of apoptosis. The mitogenic effect of zinc (10−4 m ZnCl2) on the lymphocyte proliferative response was observed in healthy controls as well as in HIV-1+ asymptomatic individuals. Very low stimulation index could be observed in AIDS patients (CD4+ < 200/mm3). However, zinc treatment of phytohaemagglutinin (PHA; 5 μg/ml)-stimulated PBMC cultures significantly enhanced 3H-thymidine incorporation in both asymptomatic and symptomatic groups. A decreased percentage of apoptotic cells could be identified in cell cultures from HIV-1+ individuals submitted to zinc treatment compared with cells treated only with PHA, as detected by both flow cytometry and agarose gel electrophoresis. Further studies with zinc supplementation associated to anti-retroviral therapy would be of great interest to evaluate the in vivo role of this oligoelement in the improvement of the immunological functions of HIV-1-infected individuals and AIDS patients.
Keywords: AIDS, zinc, mitogen, lymphoproliferative response, immunomodulation and apoptosis
INTRODUCTION
Cell-mediated immunity is usually impaired in zinc-efficient patients, as shown by a decreased response to phytohaemagglutinin (PHA) stimulation, thymic atrophy and decreased natural killer (NK) activity [1, 2]. The same features have also been described by experimental studies of zinc depletion in mice [3]. It is interesting to note that the above dysregulations, occurring both in humans and animals, could be reversed by zinc supplementation [4–6]. Reduction in nucleoside phosphorylase (NPase) activity, a zinc-dependent enzyme, adversely affect cell-mediated immunity through the modulation of intralymphocytic nucleotide levels, as has been reported in zinc-deficient individuals. Indeed, the guanosine triphosphate (GTP) accumulation verified among those individuals could also be related to the reduction of the lymphocyte proliferative response [7]. Therefore, zinc is a very important trace element in normal cellular function, with an extraordinary impact upon the immune system.
Several studies have evaluated the use of zinc as a therapeutic agent for a wide variety of human diseases. Its potential antiviral capacity was also demonstrated against HIV [8]. Indeed, zinc can act as an inhibitor of the HIV protease, an enzyme responsible for processing the p17/p24 gag precursor protein, which has been suggested to be associated with the clinical improvement observed in some HIV-infected individuals during zinc therapy [9].
One important fact to be considered is that HIV-1 shows significant zinc concentration in gag proteins [10–12], suggesting an intracellular consumption of this oligoelement during virus replication. Decreased serum zinc levels have been reported in AIDS patients [13–15], which could also contribute to the impairment of cell-mediated immunity to specific antigens and mitogens. Nevertheless, zinc can also prevent DNA fragmentation by inhibition of endonuclease activity [16, 17], potentially controlling the apoptosis of T lymphocytes, associated to cell depletion in AIDS patients [18–20].
In order to assess the potential role of zinc in the improvement of immunological functions in HIV-1 infection, we investigated the in vitro effect of zinc as a mitogen, its synergistic effect on PHA lymphoproliferative response as well as its role in the inhibition of cell apoptosis.
PATIENTS AND METHODS
Study group
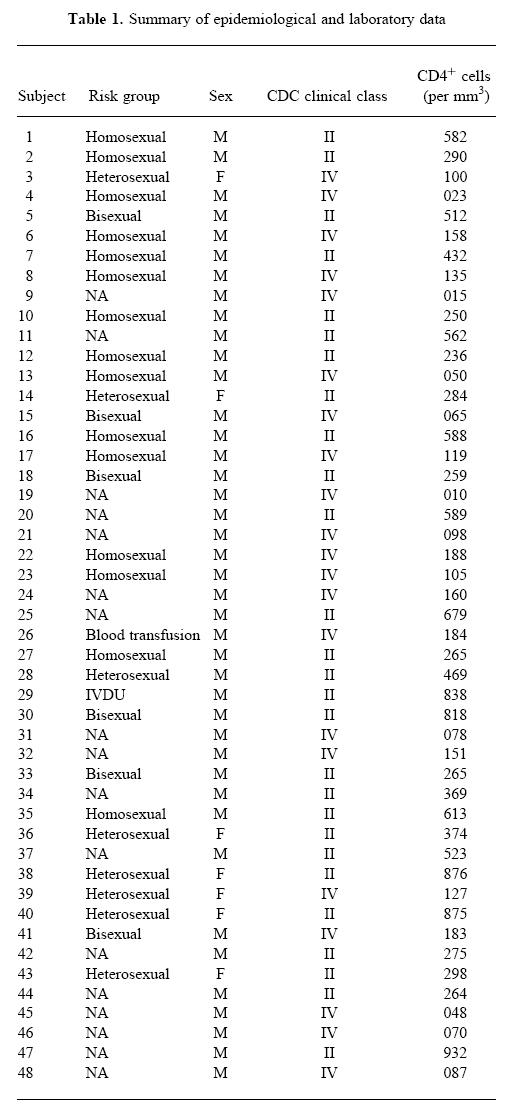
The HIV-1+ patients involved in this study were recruited from the Evandro Chagas Hospital cohort (Institute Oswaldo Cruz, Fiocruz, Rio de Janeiro, Brazil) after written consent. The 48 individuals included in this study were evaluated by clinical and laboratory parameters, as shown in Table 1. Twenty-one were clinically symptomatic AIDS patients, scored as group IV of the Centers for Disease Control (CDC) clinical classification, whereas 27 were asymptomatic HIV-1+ individuals (CDC group II). Moreover, they were also classified in three immunological groups, based on CD4+ T cell counts, as < 200 cells/mm3 (n = 21), 200–500 cells/mm3 (n = 14) and > 500 cells/mm3 (n = 13). The mean age of these groups was 37 years. Ten HIV-1− volunteers, without any risk behaviour, matched for sex and age, were also evaluated as healthy controls. All HIV-1+ individuals were submitted to clinical evaluation and serological tests. Peripheral blood lymphocyte (PBL) subsets were determined by flow cytometry using anti-CD4+/CD3+, anti-CD8+/CD3+, anti-CD14+/CD45+ and negative control double-labelled (RD1 and FITC) MoAbs (Coulter, Hialeah, FL).
Table 1.
Summary of epidemiological and laboratory data
Lymphoproliferative response assay
PBMC from healthy controls and patients were isolated from heparinized venous blood. Following density gradient centrifugation on Ficoll–Hypaque (Sigma Chemical Co., St Louis, MO) for 30 min at 400 g, cells were washed twice and resuspended in RPMI 1640 (Sigma), supplemented with 10% fetal bovine serum (FBS; Sigma), 2 mml-glutamine (Sigma) and 2 mm 2-mercaptoethanol (2-ME; Merck Chemical Co., Rio de Janeiro, Brazil), hereafter called complete medium. For the lymphoproliferative response (LPR) assay, 2 × 105 PBMC/well were stimulated for 72 h at 37°C in a humid atmosphere of 5% CO2, respectively, with 1 × 10−4 m ZnCl2, 5 μg/ml PHA and using both reagents together at the same concentration.
DNA labelling technique and flow cytometry analysis
The evaluation of the percentage of apoptotic cells was done as described elsewhere [17]. Briefly, after cell culture the cells were centrifuged at 200 g, resuspended in cold 80% ethanol with vigorous mixing to a final density of 1 × 106/ml. The cells were incubated at 4°C for a minimum of 30 min. The ethanol-fixed cells were then centrifuged and resuspended in 1 ml of the propidium iodide (PI; Sigma) staining reagent (PBS 0.15 m pH 7.4, 0.1% Triton X-100 (Merck, Darmstadt, Germany), 0.1 mm EDTA disodium salt (Sigma), 50 μg/ml RNase A (Sigma) and 50 μg/ml PI). Samples were stored in the dark at room temperature until analysis, carried out within 24 h. Determinations of cell cycle distribution were carried out using an Epics 751 Flow Cytometer (Coulter). PI was excited using the 488 nm line of an argon ion laser and emission was detected at 620–700 nm. Apoptotic cells were determined as the sub-G0/G1 peak.
DNA electrophoresis
After cell culture, the DNA samples were prepared from cells incubated with culture medium, as well as from those stimulated with PHA and PHA + zinc. Cells (4 × 105) were washed with PBS 0.15 m pH 7.4. The pellets were resuspended with 20 μl of DNA buffer containing 500 μg proteinase K (Sigma), 10 mm EDTA, 50 mm Tris–HCl pH 8.0, 0.5% SDS, and incubated at 50°C for 30 min. After incubation, 10 μl of 500 μg/ml RNase A (Sigma) in DNA buffer were added to each sample and incubated for a further 30 min, as described [17]. After mixing with 5 μl of gel loading buffer (10 mm EDTA pH 8.0, 0.25% bromophenol blue and 40% sucrose), the samples were heated at 65°C for 10 min and electrophoresis was performed in 1.8% agarose gel at 30 V for 5 h. DNA was visualized by staining with ethidium bromide under ultraviolet light and the DNA fragmentation was characterized by the formation of a DNA ‘ladder’ with fragments in multiples of 200 bp.
Statistical analysis
Results were analysed by a computer program (GraphPad Instat, GraphPad Software V2.05a, 1994), using non-parametric tests (Wilcoxon's signed rank test and Mann–Whitney's test). A two-tailed P value < 0.05 was considered significant.
RESULTS
Zinc as a mitogen
Preliminary experiments were performed to discover whether the addition of ZnCl2 was able to induce in vitro a lymphoproliferative response of PBMC taken from HIV-1-infected individuals and AIDS patients. A dose–response curve was initially established with PBMC from healthy individuals in order to determine the zinc mitogenic activity concentration (Fig. 1a). Indeed, mitogenic activity as measured by thymidine incorporation was verified using only 1.25–1.0 × 10−4 m, for 7 days. Based on the cell stimulation dose of 10−4 m we verified a strong mitogenic capacity in the induction of lymphoproliferative responses in healthy controls (P < 0.01) as well as in asymptomatic HIV-1+ individuals (P < 0.005), but not in symptomatic AIDS patients (Fig. 1b). Similar results were also observed when HIV-1+ individuals were grouped based on CD4+ T cell counts. No mitogenic stimulation was observed in patients with CD4+ T cell counts < 200/mm3 (P = 0.06), whereas statistically significant stimulation indexes (SI) were verified with patients with higher T cell counts at > 200 < 500/mm3 (P < 0.001) and at > 500/mm3 (P < 0.005).
Fig. 1.
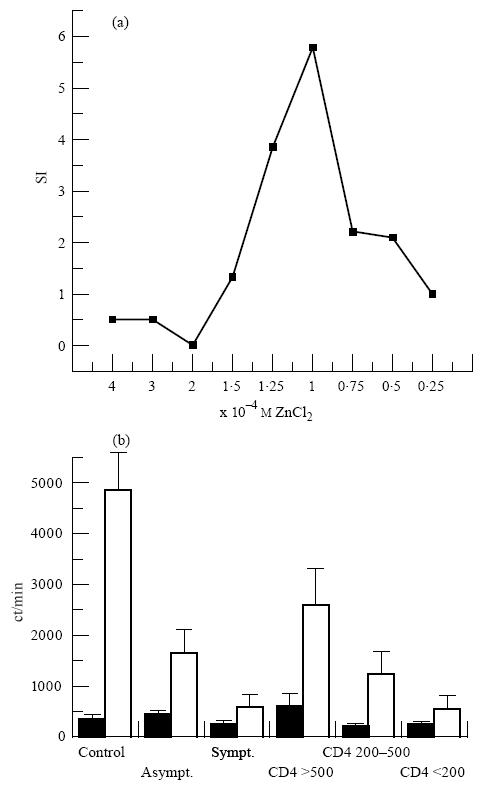
Mitogenic capacity of zinc in the induction of the lymphoproliferative response. (a) Stimulation index (SI) to define the optimal mitogenic concentration in healthy controls. (b) 3H-thymidine incorporation (ct/min) evaluating mitogenic stimulation of PBMC from HIV-1+ individuals as well as from healthy controls. ▪, Mean ± s.e.m. of non-stimulated cells; □, mean ± s.e.m. of zinc (10−4 m)-stimulated PBMC after 7 days of cell cultures. Statistical analysis of the results was performed using paired non-parametric Wilcoxon's signed rank test.
The role of zinc ions on the LPR
The improvement of the LPR to PHA, which acts as a mitogen, preferentially on T lymphocytes, was also verified upon zinc addition to PHA-stimulated cell cultures. The addition of 10−4 m ZnCl2 to PHA-stimulated cell cultures significantly enhanced the uptake of 3H-thymidine of PBMC from healthy controls (P = 0.001), as well as from asymptomatic (P < 0.001) and symptomatic HIV-1+ patients (P = 0.001). As presented in Fig. 2, similar results were obtained when HIV-1+ patients were grouped based on CD4+ T cell counts in < 200/mm3 (P < 0.0001), > 200 < 500/mm3 (P < 0.001) and > 500/mm3 (P < 0.001), confirming the role of zinc as an amplifier of the LPR in the course of HIV-1 infection.
Fig. 2.
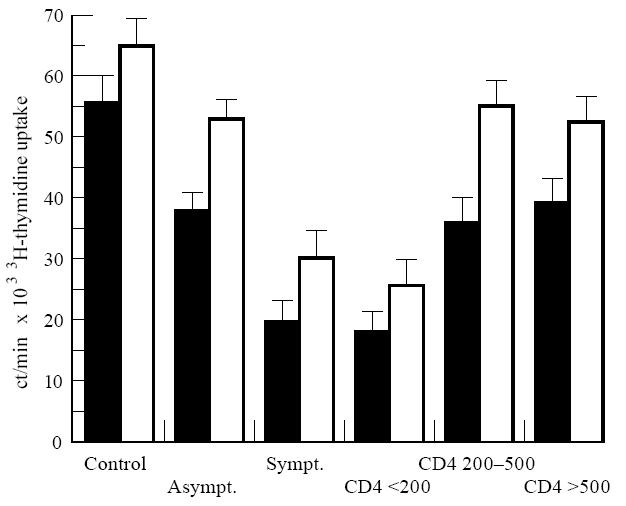
Enhanced uptake of 3H-thymidine upon addition of ZnCl2 to phytohaemagglutinin (PHA)-stimulated PBMC. Results are expressed as ct/min. ▪, Mean ± s.e.m. of the PHA-stimulated PBMC; □, mean ± s.e.m. of PHA plus zinc (10−4 m)-stimulated PBMC. Statistical analysis as in Fig. 1.
Apoptosis evaluation in PBMC cultures from HIV-infected patients
PBMC from HIV-1+ patients and healthy controls were cultivated in vitro in the presence or not of PHA and in its association with 10−4 m ZnCl2 in order to evaluate apoptosis induction after T cell stimulation. Figure 3 shows an example of a flow cytometric analysis of DNA fragmentation based on the appearance of a peak in the sub-G0/G1 region corresponding to the apoptotic cells. An increase in the sub-G0/G1 peak was observed when PBMC were cultivated in the presence of PHA (Fig. 3b), which was reduced after PHA stimulation in the presence of zinc ions (Fig. 3c). Spontaneous apoptosis after 72 h of cell culture without any stimulation was detected by flow cytometric analysis of PBMC from all HIV-1+ groups compared with healthy controls (P < 0.001). Apoptosis was enhanced upon PHA stimulation in the HIV-1+ group with CD4+ counts > 200 < 500/mm3 (P < 0.05), although such an enhancement could not be verified in patients with CD4+ cell counts < 200/mm3 (Fig. 4). Moreover, the inhibitory role of the zinc on apoptosis of PHA-stimulated lymphocytes was also observed in the former group (P < 0.05), but not in the latter one, possibly due to the high percentage of apoptotic cells already determined without mitogenic stimulation of PBMC obtained from patients in more advanced stages of the disease. Indeed, the inhibitory effect of zinc could be verified in 66% of patients included in this group (Fig. 4), although, possibly due to the high dispersion of the results, no statistical significance was verified when compared with cells stimulated with PHA or without stimulation.
Fig. 3.
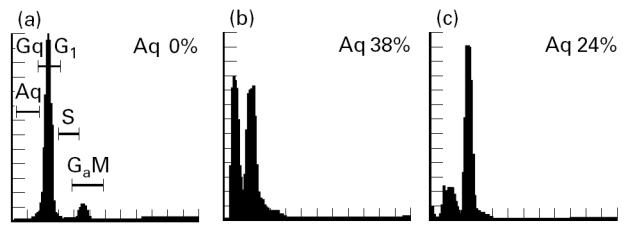
Flow cytometric cell cycle histograms of propidium iodide (PI)-stained PBMC incubated with medium (a), phytohaemagglutinin (PHA) (b), or PHA plus ZnCl2 (c). Determination of cell cycle distribution was carried out using an EPICS 751 Flow Cytometer (Coulter Corp., Hialeah, FL). Apoptotic cells were determined at the sub-G0/G1 peak after PI labelling.
Fig. 4.
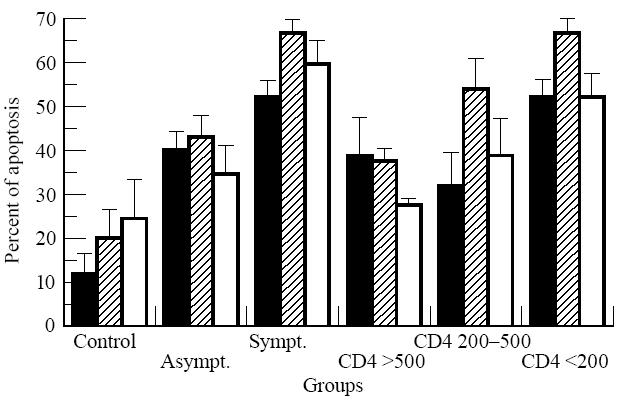
Zinc prevention of apoptosis of phytohaemagglutinin (PHA)-stimulated cells of HIV-1+ individuals but not of AIDS patients. Results are expressed as percentage of apoptotic cells detected in the sub-G0/G1 peak by flow cytometry. HIV-1+ patients were classified based on the CD4+ counts/mm3. Bars are representative of the different cell stimulations as: ▪, non-stimulated cells;  , 5 μg/ml PHA; □, PHA plus 10−4 m ZnCl2, after 3 days culture. Statistical analysis as in Fig. 1.
, 5 μg/ml PHA; □, PHA plus 10−4 m ZnCl2, after 3 days culture. Statistical analysis as in Fig. 1.
Accumulation of apoptotic cells in the sub-G0/G1 peak was consistent with the presence of DNA fragmentation in whole cell lysates as evaluated on agarose gel electrophoresis, as shown in Fig. 5. Increase of DNA degradation could be detected after cell stimulation with PHA in sets 2, 4 and 5 (lanes b). Moreover, apoptosis inhibition upon zinc treatment could be observed in the DNA samples applied in lanes c from those sets, but not in sets 1, 3, and 6 obtained, respectively, from asymptomatic and symptomatic HIV-1+ patients (sets 1 and 3) and from a healthy control (set 6), for which apoptosis was not increased after PHA mitogenic stimulation.
Fig. 5.
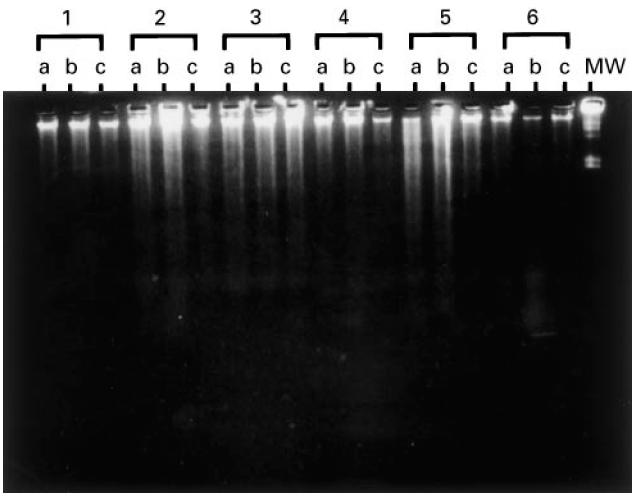
Demonstration of apoptosis in PBMC by gel electrophoresis. PBMC were cultured in medium alone (lane a), phytohaemagglutinin (PHA) (lane b) and PHA plus ZnCl2 (lane c) for 3 days. Sets 1 and 4 correspond to DNA from asymptomatic patients, whereas sets 2, 3 and 5 correspond to DNA from symptomatic patients and set 6 to a healthy control. The λ Hind III digest was used as molecular marker (MW).
DISCUSSION
Impairment of the lymphoproliferative response to mitogens and recall antigens is a well known phenomenon during HIV-1 infection, even before the onset of AIDS. In order to improve the immune response in HIV-infected individuals, oral zinc supplementation has been considered as a potential therapeutical approach [21, 22]. However, very few cases have been reported so far, and the mechanisms involved in the restoration of the immune system are not well understood.
In order to evaluate the effect of zinc in the improvement of the immune response in HIV-1+ individuals and AIDS patients, we tested its role as a mitogen in the proliferative response to PHA, as well as in the prevention of apoptosis. Considering the heterogeneity of the patient population in terms of zinc status, its would be difficult for comparative analysis to employ different zinc doses administered to each patient included in this study. In order to define the optimal dose to be used for lymphocyte stimulation we previously established a dose–response curve using lymphocytes from HIV− individuals, and we found a narrow range of zinc reactivity from 1.25 to 1.0 × 10−4 m, which agrees with previously published data [23, 24]. Under these conditions, zinc was able to induce proliferation in lymphocytes from asymptomatic patients without immunological impairment, but not in AIDS patients. Despite this fact, this dose was able to enhance the lymphoproliferative response to PHA in all patients, independent of their clinical or immunological status, possibly by a synergistic effect. Indeed, the mitogenic effect of zinc occurs within 6 days [23], whereas in our experiments the enhancement of the proliferative response was verified in only 3 days, which is a current time for lymphocyte stimulation with PHA, showing that the observed effect was synergistic and not merely accumulative. The same effect was observed even using a 10-fold lower concentration (10−5 m) of zinc ions (data not shown).
Previous studies have demonstrated that zinc treatment enhanced the in vitro proliferation of T cells, but not of B cells. Both CD4+ and CD8+ T cells are stimulated, though the proliferative response is mainly due to CD4+ cells [23]. Zinc has a great capacity to amplify concanavalin A (Con A)-stimulated PBMC from healthy donors, and this enhancement could be related to the capacity of this oligoelement in increasing the synthesis of some cytokines, such as IL-1, IL-2, IL-4, and the expression of the high-affinity receptor for IL-2 [24]. In addition, zinc is a specific growth factor for the T helper cell line HUT-78, which affects deoxithymidine kinase transcription [25]. Therefore, the immune effects of zinc are very interesting in HIV-infected individuals.
The mechanism of CD4+ cell loss, which is the main immunological feature of HIV-1-infected patients, is not completely understood in AIDS pathogenesis. Viral infection, as well as several mechanisms such as autoreactivity, cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC), seem to be implicated in the cell death of HIV-1+ patients [26]. Recently, the HIV-1 transactivator Tat protein was also shown to induce apoptosis in both PBMC from seronegative individuals and T cell lines. The Tat-induced apoptosis was verified after cell activation, suggesting an alteration of the cell cycle [27]. The induction of apoptosis as a mechanism of cell death upon T cell stimulation was also verified in our experiments. However, this apoptosis could be prevented when cells were stimulated in the presence of zinc. This phenomenon was associated with the enhancement of the proliferative response, suggesting a synergistic effect of zinc on the PHA stimulation of PBMC or a direct action of zinc on the inhibition of viral proteins. Indeed, in vitro studies showed the potential role of zinc as an inhibitor of the HIV-1 protease, which could explain some of the beneficial effects seen in AIDS patients submitted to zinc treatment [8].
The results of the present study have shown in vitro improvement of the LPR and reduction of the percentage of apoptotic cells, suggesting that zinc not only can prevent DNA fragmentation by inhibition of endonuclease activity [16, 17], but has also a role in immunomodulation, possibly acting in conjunction with the cytokine network. Based on these data, zinc therapy could be very helpful for HIV-infected individuals with impaired immune functions. Further studies with oral and i.v. zinc supplementation, associated with anti-retroviral therapy, will be of great interest to evaluate the in vivo role of this oligoelement in the improvement of the immunological functions of HIV-infected individuals and AIDS patients.
Acknowledgments
We are indebted to Drs Vera Bongertz and Luiz Roberto Castello-Branco for the review of this manuscript. I.N. is a Fellow of the FAPERJ.
References
- 1.Willians RD, Loeb LA. Zinc requirement for DNA replication in stimulated human lymphocytes. Cell Biol. 1992;58:594–601. doi: 10.1083/jcb.58.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tapazoglou E, Prasad AS, Hill G, Brewer GJ, Kaplan J. Decreased natural killer cell activity in patients with zinc deficiency with sickle cell disease. J Lab Clin Med. 1985;105:19–22. [PubMed] [Google Scholar]
- 3.Dardenne M, Pléau JM, Savino W, Prasad AS, Bach JF. Biochemical and biological aspects of the interaction between thymulin and zinc. Prog Clin Biol Res. 1993;380:23–32. [PubMed] [Google Scholar]
- 4.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:70–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 5.Dardenne M, Boukaiba N, Gagnerault MC, Homo-Delarche F, Chappuis P, Lemonneir D, Savino W. Restoration of thymus in aging mice by in vivo zinc supplementation. Clin Immunol Immunopathol. 1993;66:1–8. doi: 10.1006/clin.1993.1016. [DOI] [PubMed] [Google Scholar]
- 6.Allen JI, Kay N, McClain C. Severe zinc deficiency in humans: association with a reversible T-lymphocyte dysfunction. Ann Intern Med. 1981;95:154–7. doi: 10.7326/0003-4819-95-2-154. [DOI] [PubMed] [Google Scholar]
- 7.Prasad M, Prasad AS. Nucleotides in lymphocytes of human subsets with zinc deficiency. J Lab Clin Med. 1989;114:114–9. [PubMed] [Google Scholar]
- 8.Sergio W. Zinc salts that may be effective against the AIDS virus HIV. Med Hypotheses. 1988;26:251–3. doi: 10.1016/0306-9877(88)90128-4. [DOI] [PubMed] [Google Scholar]
- 9.Zhang ZY, Reardon IM, Hui JO, et al. Zinc inhibition of renin and protease from human immunodeficiency virus type 1. Bioch. 1991;36:8717–21. doi: 10.1021/bi00100a001. [DOI] [PubMed] [Google Scholar]
- 10.South TL, Blake PR, Sowder RC, III, Arthur LO, Henderson LE, Summer MF. The nucleocapsid protein isolated from HIV-1 particles binds zinc and forms retroviral-type zinc finger. Bioch. 1990;29:7786–9. doi: 10.1021/bi00486a002. [DOI] [PubMed] [Google Scholar]
- 11.Burke CJ, Sanyal G, Bruner MW, et al. Structural implication of spectroscopic characterization of a putative zinc finger peptide from HIV-1 integrase. J Biol Chem. 1992;267:9639–44. [PubMed] [Google Scholar]
- 12.Terri LS, Blake PR, Sowder RC. The nucleocapsid protein isolated from HIV-1 particles binds zinc and forms retroviral-type zinc fingers. Biochem. 1990;29:7786–9. doi: 10.1021/bi00486a002. [DOI] [PubMed] [Google Scholar]
- 13.Falutz J, Tsoukas C, Gold P. Zinc as a cofactor in human immunodeficiency virus-induced immunosuppression. JAMA. 1988;259:2850–1. doi: 10.1001/jama.259.19.2850. [DOI] [PubMed] [Google Scholar]
- 14.Fabris N, Mocchegiani G, Gall M, Jrato L, Lazzarin A, Moron M. AIDS, zinc deficiency and thymic hormone failure. JAMA. 1988;259:839–40. [PubMed] [Google Scholar]
- 15.Niel MHG, Soresen D, Odaka N, et al. Relationship of serum copper and zinc levels to HIV-1 seropositivity and progressions to AIDS. Acq Immun Defic Syndr. 1991;4:976–80. [PubMed] [Google Scholar]
- 16.Meyaard L, Otto SA, Jonker RR, Minjter MJ, Keet RPM, Miedema F. Programmed death of T cells in HIV-1 infection. Sci. 1992;257:217–9. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 17.Telford WG, King LE, Fraker PJ. Evaluation of glucocorticoid-induced DNA fragmentation in mouse thymocytes by flow cytometry. Cell Prolif. 1991;24:447–59. doi: 10.1111/j.1365-2184.1991.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 18.Gougeon ML, Oliver R, Garcia S, Guetard D, Dragtic T, Dauguet C, Montaigner L. Evidence for an engagement process towards apoptosis in lymphocytes of HIV-1 infected patients. C R Acad Sci Paris. 1991;T.312(série III):529–37. [PubMed] [Google Scholar]
- 19.Groux H, Topier G, Monté D, Mouton Y, Capron A, Ameisen JC. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992;175:331–40. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ameisen JC, Capron A. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol Today. 1991;12:102–5. doi: 10.1016/0167-5699(91)90092-8. [DOI] [PubMed] [Google Scholar]
- 21.Ancarani F, Veccia S, Giacometti A, Mocchegiani G, Marcellini M, Scalise G. Zinc therapy in HIV-infected subjects. 1993. p. 493. Ixth Int Conf on AIDS Berlin.
- 22.Caselli M, Bicocchi R. Taux sérique du zinc chez les malades atteints du syndrome d'immunodéfficit acquis. La Presse Médicale. 1993;37:1877. [PubMed] [Google Scholar]
- 23.Tanaka Y, Shiozawa S, Morimoto I, Fujita I. Role of zinc in interleukin-2 (IL-2) mediated T cell activation. Scan J Immunol. 1992;31:547–52. doi: 10.1111/j.1365-3083.1990.tb02805.x. [DOI] [PubMed] [Google Scholar]
- 24.Malavé I, Rodriguez J, Araujo Z, Rojas I. Effect of zinc on the proliferative response of human lymphocytes: mechanism of its mitogenic action. Immunopharmacol. 1990;20:1–10. doi: 10.1016/0162-3109(90)90002-v. [DOI] [PubMed] [Google Scholar]
- 25.Prasad AS, Beck FWJ, Endre L, Handschu W, Kukuruga M, Kumar G. Zinc deficiency affects cells cycle and deoxithymidine kinase gene expression in HUT-78 cells. J Lab Clin Med. 1996;128:51–60. doi: 10.1016/s0022-2143(96)90113-4. [DOI] [PubMed] [Google Scholar]
- 26.Gougeon ML, Montagnier L. Apoptosis in AIDS. Sci. 1993;260:1269–70. doi: 10.1126/science.8098552. [DOI] [PubMed] [Google Scholar]
- 27.Li CJ, Friedman DJ, Wang C, Metelev V, Pardee AB. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Sci. 1995;268:429–31. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]


