Abstract
The present study analyses the ability of HIV-1 to modulate IL-10 production in cells of monocyte-macrophage lineage cultured in the presence of macrophage colony-stimulating factor (M-CSF). Both monocytes and macrophages spontaneously produced low amount of IL-10. Lipopolysaccharide (LPS) induced a strong IL-10 response in fresh monocytes and in M-CSF-treated macrophages. In contrast, macrophages cultured in the absence of M-CSF exhibited a marked decrease in their susceptibility to LPS stimulation. M-CSF increased the IL-10 response of macrophages to LPS by enhancing both the expression of membrane-bound CD14, the protein that serves as LPS receptor, and the sensibility of CD14-expressing cells to LPS stimulation. Neither spontaneous nor LPS-induced expression of IL-10 was modulated in monocytes and macrophages by infection with eight monocytotropic strains, as demonstrated by ELISA and cytofluorimetric analysis. In contrast, all the HIV-1 strains primed macrophages for an increased IL-6 response to LPS stimulation. To determine whether IL-10 production was associated with in vivo infection, monocytes from AIDS individuals were analysed for IL-10 production. We found that neither spontaneous nor LPS-induced IL-10 production were different between healthy controls and HIV-infected patients. Taken together, these data strongly suggest that HIV-1 infection of monocytes-macrophages does not play a significant role in the regulation of IL-10 in infected patients. This study also emphasizes the role of M-CSF activation in the regulation of the cytokine response in macrophages.
Keywords: IL-10, monocytes-macrophages, macrophage colony-stimulating factor, lipopolysaccharide
INTRODUCTION
IL-10 is a recently identified cytokine produced by T and B lymphocytes as well as monocytes-macrophages [1–4]. IL-10 inhibits T cell proliferation and cytokine production [5–8], and it is thought to play an important role in T cell dysfunction and cytokine imbalance observed in HIV-1-infected patients [9–13]. IL-10 levels were found to be significantly elevated in phytohaemagglutinin (PHA)-stimulated peripheral blood lymphocytes and in cell populations isolated from lymph nodes of HIV-infected individuals, compared with healthy controls [13–15]. In addition, serum IL-10 levels were found higher in HIV-infected subjects than in uninfected controls [16].
Although several studies reported that the major source of IL-10 in HIV-infected patients are T lymphocytes [14,15], it has been recently hypothesized that also monocyte-macrophage cells, a major target for HIV-1 in vivo [17–21], could contribute to IL-10 dysregulation. However, while some studies have described an over-production of IL-10 by monocyte-macrophage cells exposed to HIV-1 products (recombinant gp120) or infected by HIV-1 [22–25], others reported contrasting results [26,27]. Resolving which cells are over-producing IL-10 in HIV-1-infected patients may have a potential profound impact on the design of therapeutic strategies.
Following this line of research we have tested, in vitro, several HIV-1 isolates (primary clinical isolates and laboratory-adapted strains) for their ability to induce IL-10 in monocytes and macrophages activated by macrophage colony-stimulating factor (M-CSF) which in vivo regulates monocyte-macrophage differentiation and function [28–31]. Then, to determine whether IL-10 production was associated with in vivo HIV-1 infection, monocytes from AIDS individuals were analysed for IL-10 production.
MATERIALS AND METHODS
Cells
Peripheral blood obtained from HIV− donors was enriched for mononuclear cells (PBMC) by centrifugation over Ficoll–Hypaque. The PBMC were then further enriched for monocytes by elutriation as described by Gerrard et al. [32]. Cells obtained by this method are > 90% monocytes and > 80% CD14+ as determined by FACS analysis.
Compounds
Recombinant M-CSF was kindly provided by Genetic Institute (Cambridge, MA). The product concentration was 0.78 mg/ml, and the specific activity 1.9 × 106 U/mg protein (one unit equals half maximal stimulation in the Murine Bone Marrow Colony Assay). Lipopolysaccharide (LPS) from Escherichia coli 0111/B4 was purchased from Sigma Chimica (Milan, Italy). LPS (100 ng/ml) was used to stimulate the cells.
Viruses
A laboratory strain (Ba-L) and seven clinical isolates of HIV-1 were used to infect macrophages. Supernatants of infected macrophages were used as the source of HIV-1 Ba-L; these were filtered and stored in liquid nitrogen before use. The clinical isolates of HIV-1 were obtained from seven HIV antibody-seropositive individuals. Isolation of these strains from the plasma was performed in PBMC cultures, and the supernatants of these cultures were used as the source of the virus. Titration to determine infectivity of Ba-L and primary isolates was performed, respectively, in a primary macrophage system or in PBMC as previously described [33,34]. The titre of the virus stocks, expressed as 50% tissue culture infectious dose (TCID50), was determined as previously described [35].
Viral detection
HIV-p24 antigen production in supernatants was assessed by a sandwich ELISA (Abbott, Pomezia, Italy).
ELISA immunoassay
Commercially available sandwich ELISA kits (R&D Systems Minneapolis, MN) were used to determine the concentration of IL-10 and IL-6. The detection limits of these ELISAs are 7.8 pg/ml (IL-10) and 3.13 pg/ml (IL-6). According to the manufacturer's specifications, these ELISAs are specific for the relative interleukin. All the samples were determined in duplicate, in a single analytical set. The intra-series variation coefficient was < 15%.
Assessment of interleukin production by monocytes and macrophages infected or not by HIV-1
After purification (day 0), monocytes, stimulated or not with LPS, were cultured in the presence or in the absence of 1000 U/ml of M-CSF in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 20% heat-inactivated fetal calf serum (FCS), 2 mml-glutamine, 50 U/ml penicillin and 50 μg/ml streptomycin (complete medium), at 37°C in a humidified atmosphere of 5% CO2 in air, in 48-well plates (Costar, Cambridge, MA) at a concentration of 2 × 105 cells/well per ml. After 48 h of incubation the supernatants were harvested and stored at −80°C. Alternatively, just after purification monocytes were infected with either 1000 TCID50 of the different HIV-1 clinical isolates or with 300 TCID50 of HIV-1 Ba-L. After 2 h of incubation the cells were extensively washed to remove excess virus and cultured for 48 h as described above. Then the supernatants were collected and stored at −80°C.
For the determination of IL-10 production by macrophages, 5 × 105 monocytes were cultured for 21 days in 48-well plates in 1 ml of complete medium in the presence or absence of M-CSF. The cells were washed and fed every 7 days. At different time points the cultures were washed, refed with fresh medium (containing or not M-CSF as needed) and stimulated or not with LPS. After 48 h of incubation the supernatants were harvested and stored at −80°C. Alternatively, the cells were cultured for 7 days and then infected as described before. At day 21, cultures were washed and refed with fresh medium (containing or not M-CSF as needed) and stimulated or not with LPS. After 48 h of incubation the supernatants were collected and stored at −80°C.
Cytofluorimetric (FACS) analysis
FACS analysis was performed using a previously described method [36]. In short, macrophages were cultured in 25-cm2 flasks (Corning 25102-25) and infected as described above. At day 21, the cultures were washed and refed with complete medium containing or not M-CSF and LPS, in the presence of 2.5 μg/ml of the protein transport inhibitor brefeldin A (Sigma Chimica). After 24 h incubation, the cells were washed twice in PBS, detached by gentle scraping, collected by centrifugation and stained for 15 min with R-PE-cyanine 5 (PE-Cy5) conjugated anti-CD14 MoAb (Immunotech, Marseille, France) for determination of their surface phenotype. The cells were then washed twice in PBS and fixed in ice-cold PBS containing 4% paraformaldehyde. After two further washes in PBS, 2 × 105 cells were resuspended for 30 min at room temperature in 30 μl PBS containing 0.1% saponin (Sigma Chimica), 1% bovine serum albumin (BSA) (Sigma Chimica) and 0.5 μg/1 × 106 cells of the following MoAbs: PE-conjugated mouse anti-HIV-p24 antigen (Immunotech) or PE-conjugated MoAb against IL-10 (Pharmingen, San Diego, CA), or both. Paired isotype-specific control antibodies (Pharmingen) were run with each sample. As a last step, the cells were washed twice in PBS containing 0.01% saponin, resuspended in PBS and analysed by a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). Five thousand to 10 000 cells were computed in list mode and analysed using the FACScan research software (Becton Dickinson).
Assessment of IL-10 production by monocytes from HIV-infected individuals
Twenty HIV-1+ individuals (13 male and seven female, mean age 37 years, range 25–49 years) were enrolled in this study, 10 healthy HIV− donors were included as controls. All the seropositive subjects had had at least one AIDS-defining illness, but had no active opportunistic diseases. All patients were repeatedly positive for HIV-1 antibodies by ELISA, confirmed by Western blot analysis. Patients were classified according to their absolute T CD4 cell counts: < 200/mm3 (n = 10, mean T CD4 cell counts 84 ± 63, range 11–183), > 200/mm3 (n = 10, mean T CD4 cell counts 317 ± 73, range 250–442). All patients were receiving anti-retroviral drug therapy but not cytokines. Informed consent was obtained from all participants.
Peripheral blood was obtained by venipuncture. Monocytes were obtained as described above and then further purified by immunomagnetic depletion of T and B lymphocytes, using Dynabeads M-450 Pan-T (CD2) and Pan-B (CD19) (Dynal AS, Oslo, Norway). The procedure was carried out as indicated by the manufacturer. Cells obtained by this method are > 99% pure as determined by FACS analysis. After purification, 2 × 105 monocytes were cultured for 48 h as described above in the presence or absence of LPS. Then the supernatants were harvested and stored at −80°C.
Statistical analysis
Student's t-test was used to analyse data.
RESULTS
Effect of M-CSF on IL-10 production by monocytes and macrophages
We initially evaluated the effect of M-CSF on IL-10 production by freshly elutriated monocytes (day 0) and macrophages at different stages of maturation, in the absence of LPS stimulation (Fig. 1a). M-CSF treatment did not modify IL-10 production by monocytes, yet it induced some cytokine release in macrophages starting from day 7 of culture. Since M-CSF causes proliferation of macrophages [28], we wondered if the augmented IL-10 levels found in the supernatants were simply due to the M-CSF-induced increase in cell number. Indeed, the ratio between the number of cells and the levels of IL-10 was quite similar in the cultures exposed to M-CSF compared with those unexposed (not shown).
Fig. 1.
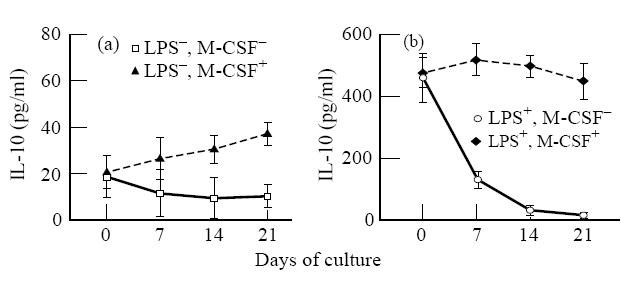
IL-10 production by monocytes and macrophages stimulated or not with lipopolysaccharide (LPS) and macrophage colony-stimulating factor (M-CSF). At given time points, macrophage cultures were washed, refed with fresh medium with or without LPS and M-CSF. After 48 h of incubation, the supernatants were obtained for IL-10 determination. The data represent the mean of three experiments each carried out in duplicate. Each experiment was performed with cells from a single donor. The error bars represent s.e.m.
We then analysed the ability of M-CSF to modulate the response of monocytes and macrophages to LPS. As shown in Fig. 1b, M-CSF significantly primed macrophages, but not monocytes, to an enhanced IL-10 response to LPS. It should be noted that IL-10 levels in M-CSF-treated macrophages at days 7, 14 and 21 were, respectively, 4, 26 and 28 times greater than in untreated cultures, whereas the increase in cell number was 1.5-, 2.4- and 3.4-fold. Thus, it is unlikely that the increase in cell number induced by M-CSF can account for the enhanced IL-10 production.
Effect of M-CSF on surface CD14 and intracellular IL-10 expression
Cytofluorimetric analysis was carried out to determine whether the ability of M-CSF to enhance macrophage IL-10 response to LPS might be due to the up-regulation of CD14, the cellular receptor for LPS [37], to a different ability of macrophages to respond to the LPS signal, or both. Membrane CD14 expression and intracellular IL-10 production were studied at single-cell level by using a recently developed cytofluorimetric technique [36]. As shown in Table 1, M-CSF treatment increased both the percentage of cells expressing CD14 and the percentage of CD14-bearing cells that produced IL-10 in response to LPS compared with untreated cells. Taken together, these data suggest that M-CSF modulates IL-10 production in LPS-stimulated macrophages by increasing both the expression of surface-bound CD14 and the sensitivity of the CD14-expressing cells to LPS stimulation.
Table 1.
Cytofluorimetric evaluation of surface CD14 and intracellular IL-10 expression in macrophages stimulated by lipopolysaccharide (LPS)
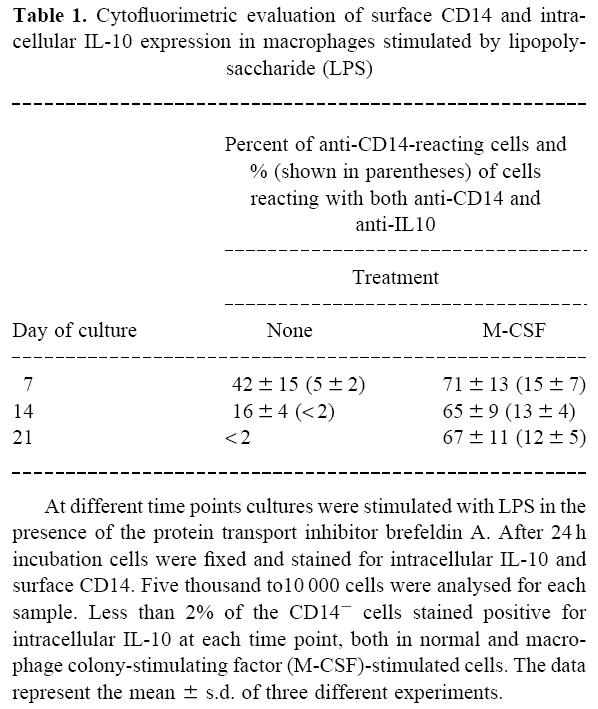
At different time points cultures were stimulated with LPS in the presence of the protein transport inhibitor brefeldin A. After 24 h incubation cells were fixed and stained for intracellular IL-10 and surface CD14. Five thousand to10 000 cells were analysed for each sample. Less than 2% of the CD14− cells stained positive for intracellular IL-10 at each time point, both in normal and macrophage colony-stimulating factor (M-CSF)-stimulated cells. The data represent the mean ± s.d. of three different experiments.
HIV-1 infection of monocytes and macrophages
A productive infection (HIV-p24 antigen production) was consistently obtained with all the HIV-1 isolates in both normal and M-CSF-treated macrophages. However, both the HIV-p24 antigen production in the supernatants and the percentage of HIV-producing cells were higher in M-CSF-treated macrophages than in normal cells (Table 2). These data agree with those from other groups [38] and indicate that M-CSF strongly up-modulates HIV-1 replication in macrophages.
Table 2.
HIV-1 infection of normal and macrophage colony-stimulating factor (M-CSF)-treated macrophages
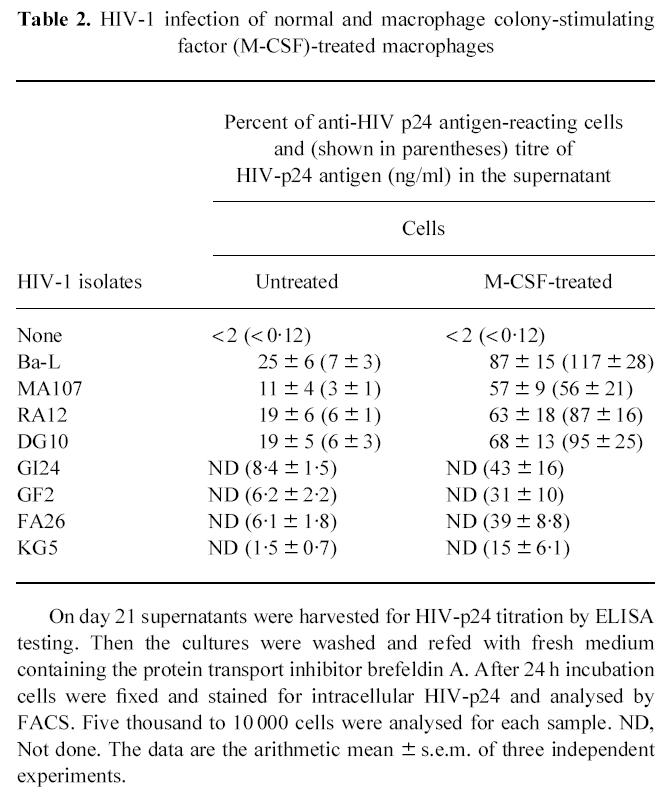
On day 21 supernatants were harvested for HIV-p24 titration by ELISA testing. Then the cultures were washed and refed with fresh medium containing the protein transport inhibitor brefeldin A. After 24 h incubation cells were fixed and stained for intracellular HIV-p24 and analysed by FACS. Five thousand to 10 000 cells were analysed for each sample. ND, Not done. The data are the arithmetic mean ± s.e.m. of three independent experiments.
IL-10 production by HIV-infected monocytes-macrophages
Just purified monocytes (day 0) were infected with the HIV-1 isolates in the presence or absence of LPS. Alternatively, the cells were infected on day 7 and then stimulated by LPS when a productive infection became established (day 14 after infection).
None of the eight HIV-1 isolates significantly modulated the production of IL10 in monocytes or in macrophages, both in the presence or absence of LPS or M-CSF stimulation (Fig. 2a,b).
Fig. 2.
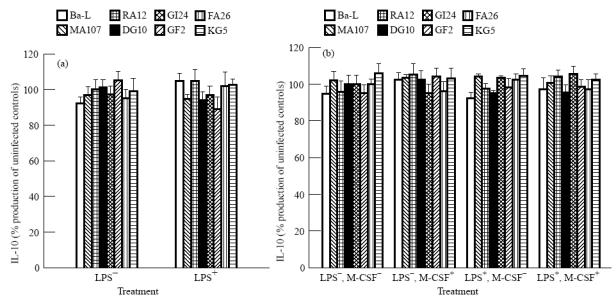
IL-10 production by monocytes and macrophages infected with different HIV-1 isolates. (a) Monocytes. (b) Macrophages. The levels of IL-10 in uninfected controls were the same as in Fig. 1a,b. Cell cultures were considered infected if the levels of HIV-p24 antigen in the supernatants were equal to or more than those presented in Table 2. The data represent the mean of three experiments each carried out in duplicate. The error bars represent s.e.m.
For comparison, we evaluated under our experimental conditions the ability of HIV-1 to induce the production of another LPS-induced cytokine such as IL-6 [39]. IL-6 production was measured in the same macrophage culture supernatants that were used to determine IL-10 levels. We found that all the HIV-1 isolates primed these cells for an augmented IL-6 response to LPS stimulation (Fig. 3).
Fig. 3.
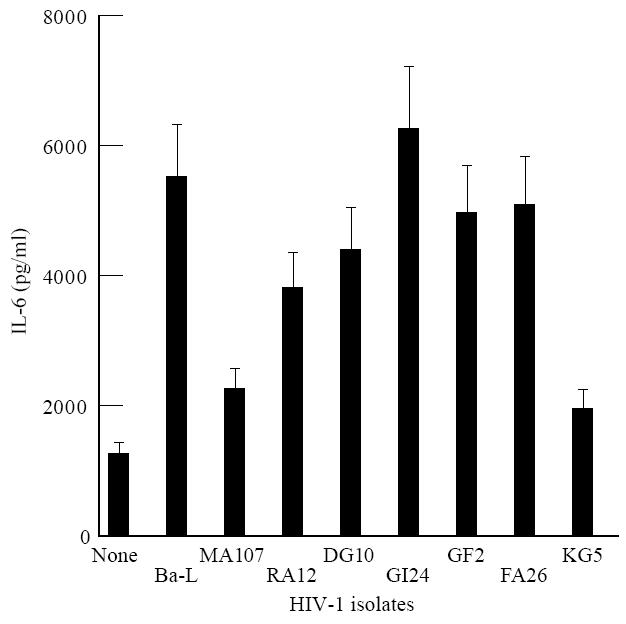
IL-6 production by HIV-infected macrophages. The cells were cultured in the presence of macrophage colony-stimulating factor (M-CSF) and stimulated by lipopolysaccharide (LPS) when HIV-p24 antigen in the supernatants was equal to or more than that presented in Table 2. The supernatants were harvested after 24 h of incubation. The data represent the mean of three experiments each carried out in duplicate. The error bars represent s.e.m.
IL-10 production by HIV-1-infected macrophages was also analysed at single-cell level by FACS analysis (Fig. 4). Both untreated and M-CSF-treated cells were stimulated by LPS and analysed for contemporary HIV-p24 antigen and IL-10 production at day 14 after infection. Intracellular p24 antigen was detected in both untreated and M-CSF-treated cells. The percentage of HIV-p24+ cells was 21% (Fig. 4b) and 93% (Fig. 4d), respectively. HIV-1 infection did not induce IL-10 production in both untreated and M-CSF-treated cells. Indeed, the percentage of IL-10-producing cells in uninfected and infected cultures was < 2% versus < 2% in untreated macrophages (Fig. 4a,b) and 14.3 ± 4% versus 15.8 ± 8% in M-CSF-treated macrophages (Fig. 4c,d).
Fig. 4.
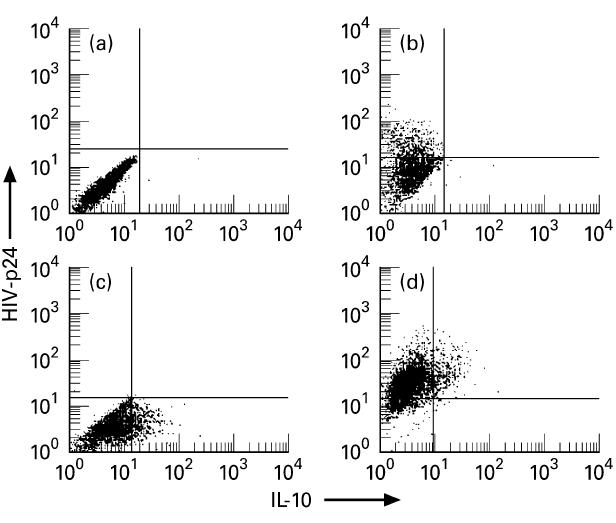
Cytofluorimetric assessment of individual IL-10- and HIV-p24 antigen-producing cells in lipopolysaccharide (LPS)-stimulated macrophages by specific two-colour, intracellular staining. (a,b) Uninfected and HIV-Ba-L-infected macrophages cultured in the absence of macrophage colony-stimulating factor (M-CSF). (c,d) Uninfected and HIV-Bal-infected macrophages cultured in the presence of M-CSF. The data are displayed as bivariate dot plots. The quadrants were set according to the negative controls (< 1% of the isotype control cells appeared positive). Low left quadrants, unstained cells; upper left quadrants, HIV-p24-stained cells; low right quadrants, IL-10-stained cells; upper right quadrants, cells stained for both IL-10 and HIV-p24. Five thousand to 10 000 cells were analysed for each sample. The data refer to a typical experiment of three performed with similar results.
IL-10 production by monocytes from HIV-1-infected patients
The studies described above demonstrate that monocytes-macrophages did not produce IL-10 in response to HIV-1 infection in vitro. To determine whether IL-10 production was associated with in vivo infection, we analysed highly purified monocytes from AIDS patients.
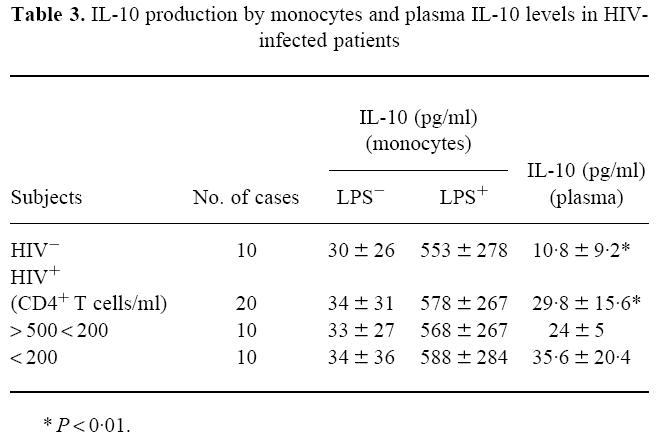
IL-10 production by monocytes, both in the absence and presence of LPS stimulation, did not differ between 10 controls and 20 patients, whether they were stratified according to their CD4+ T cells or not (Table 3). However, as shown in Table 3, IL-10 plasma levels were significantly lower in controls than in HIV-1-infected patients. Moreover, in the HIV-infected group, plasma IL-10 levels were particularly high in patients with low CD4+ T cell counts.
Table 3.
IL-10 production by monocytes and plasma IL-10 levels in HIV-infected patients
DISCUSSION
In the current study we have demonstrated that IL-10 production in monocytes-macrophages is dramatically modulated by LPS and M-CSF stimulation. In the absence of M-CSF, macrophages exhibited a marked decrease of both the expression of membrane CD14 and the sensibility of CD14-expressing cells to LPS stimulation. These results are consistent with those from other authors which reported that monocytes show a time-dependent CD14 loss, and a decreased ability to produce cytokines in response to LPS activation [40–42]. M-CSF treatment significantly enhanced IL-10 production by macrophages by increasing both the expression of membrane-bound CD14 and susceptibility to LPS stimulation. These data confirm and extend the results of a recent report which shows that M-CSF may prime macrophages to an enhanced cytokine release under LPS stimulation, possibly by influencing the expression of CD14 receptor [43].
M-CSF is a haematopoietic growth factor that supports the proliferation and differentiation of bone marrow progenitor cells and enhances the function of mature cells such as macrophages [28–31]. Bioassays performed on blood have shown that endogenous M-CSF levels are very similar to those used here [44,45]. Also, recombinant M-CSF is currently undergoing clinical trials as an anticancer drug [46]. In light of these observations, our data suggest that the effect of M-CSF should be taken into account when cytokine production is studied in monocyte-macrophage cells.
A great number of observations now suggest the critical role of cytokines in the pathogenesis of HIV-1 infection [9–16,39,47,48]. In particular, recent in vitro observations have indicated a potential role for IL-10 in HIV-induced immune dysfunction [13–15], and some studies have described the induction of IL-10 during in vitro infection of monocytes-macrophages [23–25]. We have tested several viral isolates (primary clinical isolates and laboratory-adapted strains) for their ability to induce IL-10 in monocytes and macrophages, under a broad range of experimental conditions. Our results clearly show that HIV-1 infection did not modulate IL-10 production in these cells. Such differences may rest on the mode of isolation and activation of monocytes-macrophages. Indeed, differently from all the other authors who isolated cells by adhesion, a procedure which led to the recovery of mature and activated monocyte subpopulations, we obtained monocytes by elutriation. Since elutriation is a gentle process that uses physiological media, normal cell viability and function are maintained. Consequently, the cells are not activated or artificially stimulated. Thus, cells purified by elutriation are suitable for studies, such as those on cytokine induction, where cell purity and unaltered cell function are critical.
Another possible explanation for the discrepancy between our results and those reported by others might be that the frequency of infected macrophages was below the threshold required for measurable IL-10 release. This is improbable, however, since IL-10 secretion was not increased also in cultures with > 90% of productively HIV-1-infected macrophages.
Additional experiments with cells from HIV-1-infected individuals were carried out to find out if monocytes contribute or not to IL-10 dysregulation in vivo. In accordance with previously reported data [16], we detected significant changes in IL-10 plasma levels in HIV patients. However, monocytes from the same subjects were not found to produce more IL-10 than the uninfected controls. Thus, other cells, such as T lymphocytes, may be responsible for the increased IL-10 levels found in HIV-infected subjects, as previously reported [14,15]. In this regard, in preliminary experiments with multiparametric, three-colour cytofluorimetric analysis, we found that CD8 T cells are the main source of IL-10 in infected patients (A. Bergamini et al., unpublished observations).
Taken together, our data do not support the hypothesis of a direct role of HIV-1 in modulating IL-10 production in monocytes-macrophages both in vitro and in vivo. Moreover, our study emphasizes the role of growth factor activation in the regulation of the cytokine response in monocytes-macrophages.
Acknowledgments
This work was supported by grants of the Italian Istituto Superiore di Sanità, IX Progetto di ricerche sull'AIDS. The authors acknowledge Mss Enrica Piscitelli and Mirella Lupi for excellent technical assistance.
References
- 1.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cells. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–7. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maggi E, Giudizi MG, Biagiotti R, et al. Th2-like CD8+ T cell showing B cell helper function and reduced cytolytic activity in human immunodeficiency virus type 1 infection. J Exp Med. 1994;180:489–95. doi: 10.1084/jem.180.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Garra A, Stapleton G, Dhar V, et al. Production of cytokine by mouse B cells. B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2:821–6. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- 4.De Waal Malefyt R, Abrams J, Bennet B, Frigdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ralph P, Nakoinz I, Sampson-Joannes A, et al. IL-10, T lymphocyte inhibitor of human blood cell production of IL-1 and tumor necrosis factor. J Immunol. 1992;148:808–13. [PubMed] [Google Scholar]
- 6.Fiorentino DF, Zlotnik A, Vieira P, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–50. [PubMed] [Google Scholar]
- 7.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard M, O'Garra A, Ishida H, de-Waal-Malefyt R, de Vries J. Biological properties of interleukin 10. J Clin Immunol. 1992;12:239–47. doi: 10.1007/BF00918147. [DOI] [PubMed] [Google Scholar]
- 9.Shearer GM, Clerici M. Abnormalities of immune regulation in human immunodeficiency virus infection. Pediatr Res. 1993;33:S71–S75. doi: 10.1203/00006450-199305001-00410. [DOI] [PubMed] [Google Scholar]
- 10.Weiss RA. How does HIV cause AIDS? Sci. 1993;260:1273–8. doi: 10.1126/science.8493571. [DOI] [PubMed] [Google Scholar]
- 11.Maggi E, Macchia D, Parronchi P, et al. Reduced production of interleukin 2 and interferon gamma and enhanced helper activity for IgG synthesis by cloned CD4 T cells from patients with AIDS. Eur J Immunol. 1987;17:1685–90. doi: 10.1002/eji.1830171202. [DOI] [PubMed] [Google Scholar]
- 12.Clerici M, Herkin FT, Venzon DJ, Hendrix GW, Wynn TA, Shearer GM. Changes in interleukin 2 and interleukin 4 production in asymptomatic human immunodeficiency virus seropositive individuals. J Clin Invest. 1993;91:789–95. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz-Mitoma F, Kumar A, Karimi S, et al. Expression of IL-10, IL-4 and interferon gamma in unstimulated and mitogen stimulated peripheral blood lymphocytes from HIV seropositive patients. Clin Exp Immunol. 1995;102:31–39. doi: 10.1111/j.1365-2249.1995.tb06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clerici M, Wynn TA, Berzofsky JA, et al. Role of interleukin 10 in T helper dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Invest. 1994;93:768–75. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graziosi C, Pantaleo G, Gantt KR, et al. Lack of evidence for the dichotomy of Th1 and Th2 predominance in HIV-infected individuals. Sci. 1994;265:248–51. doi: 10.1126/science.8023143. [DOI] [PubMed] [Google Scholar]
- 16.Ameglio F, Cordiali Fei P, Solmone M, et al. Serum IL-10 levels in HIV-positive subjects: correlation with CDC stages. J Biol Regul Homeost Agents. 1994;8:48–52. [PubMed] [Google Scholar]
- 17.McErlath MJ, Pruett JE, Chon ZA. Mononuclear phagocytes of blood and bone marrow: comparative roles as viral reservoirs in human immunodeficiency virus type 1 infection. Proc Natl Acad Sci USA. 1989;86:675–9. doi: 10.1073/pnas.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Embretson J, Zupanic M, Ribas JL, et al. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–65. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 19.Salahuddin SZ, Rose RM, Groopman JE, Markham PD, Gallo RC. Human T-lymphotrophic virus type III infection of human alveolar macrophages. Blood. 1986;68:281–95. [PubMed] [Google Scholar]
- 20.Hoda SA, Gerber MA. Immunohistochemical studies of human immunodeficiency virus type I in liver tissues of patients with AIDS. Clin Pathol. 1990;93:452–8. [PubMed] [Google Scholar]
- 21.Koenig S, Gendelman HE, Orenstein JM, et al. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Sci. 1986;233:1089–93. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 22.Hagiwara E, Sacks T, Leitman-Klinman SF, Klinman DM. Effect of HIV infection on the frequency of cytokine-secreting cells in human peripheral blood. AIDS Res Hum Retrovir. 1996;12:127–33. doi: 10.1089/aid.1996.12.127. [DOI] [PubMed] [Google Scholar]
- 23.Borghi P, Fantuzzi L, Varano B, et al. Induction of interleukin 10 by human immunodeficiency virus type 1 and its gp120 protein in human monocytes/macrophages. J Virol. 1995;69:1284–7. doi: 10.1128/jvi.69.2.1284-1287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akridge RE, Oyafuso LKM, Reed SG. IL-10 is induced during HIV-1 infection and is capable of decreasing viral replication in human macrophages. J Immunol. 1994;153:5782–9. [PubMed] [Google Scholar]
- 25.Yoo J, Chen H, Kraus T, et al. Altered cytokine production and accessory cell function after HIV-1 infection. J Immunol. 1996;157:1313–20. [PubMed] [Google Scholar]
- 26.Chehimi J, Ma X, Chouaib S, et al. Differential production of interleukin 10 during human immunodeficiency virus infection. AIDS Res Hum Retrovir. 1996;12:1141–9. doi: 10.1089/aid.1996.12.1141. [DOI] [PubMed] [Google Scholar]
- 27.Dereuddre-Bosquet N, Clayette P, Martin M, et al. Lack of interleukin 10 expression in monocyte-derived macrophages in response to in vitro infection by HIV type 1 isolates. AIDS Res Hum Retrovir. 1997;13:961–6. doi: 10.1089/aid.1997.13.961. [DOI] [PubMed] [Google Scholar]
- 28.Chen BDM, Clark CR, Chou Th. Granulocyte/macrophage colony stimulating factor stimulates monocyte and tissue macrophage proliferation and enhances their responsiveness to macrophage colony-stimulating factor. Blood. 1988;71:997–1002. [PubMed] [Google Scholar]
- 29.Ampel NM, Wing EJ, Waheed A, Shadduck RK. Stimulatory effects of purified macrophage colony stimulating factor on murine resident peritoneal macrophage. Cell Immunol. 1986;97:344–9. doi: 10.1016/0008-8749(86)90405-3. [DOI] [PubMed] [Google Scholar]
- 30.Young DA, Lowe LD, Clark CS. Comparison of the effects of IL-3, granulocyte-macrophage colony stimulating factor and macrophage colony stimulating factor in supporting monocyte differentiation in culture. Analysis of macrophage antibody-dependent cellular cytotoxicity. J Immunol. 1990;145:607–15. [PubMed] [Google Scholar]
- 31.Becker S, Warren MK, Haskill S. Colony stimulating factor-induced monocyte survival and differentiation into macrophages in serum free cultures. J Immunol. 1987;139:3703–9. [PubMed] [Google Scholar]
- 32.Gerrard TL, Jurgensen CH, Fauci AS. Differential effect of monoclonal anti DR antibody on monocytes in antigen- and mitogen-stimulated response: mechanisms of inhibition and relationship to interleukin 1 secretion. Cell Immunol. 1983;82:394–402. doi: 10.1016/0008-8749(83)90172-7. [DOI] [PubMed] [Google Scholar]
- 33.Bergamini A, Capozzi M, Ghibelli L, et al. Cystamine potently suppresses in vitro HIV replication in acutely and chronically infected human cells. J Clin Invest. 1994;93:2251. doi: 10.1172/JCI117223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreoni M, Sarmati L, Parisi SG, Ercoli L, Rocchi G. Efficient and reproducible new semimicromethod for the detection and titration of HIV in human plasma. J Med Virol. 1992;38:207–13. doi: 10.1002/jmv.1890380310. [DOI] [PubMed] [Google Scholar]
- 35.Karber G. Beitrag zur kollektiven Behandlung Pharmakologischer Reihenversuche. Arch Exp Pharmakol. 1931;162:480–3. [Google Scholar]
- 36.Sander B, Andersson J, Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 37.Dentener MA, Bazil V, Von Asmuth EJU, Ceska M, Buurman WA. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-α, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1992;150:2885–91. [PubMed] [Google Scholar]
- 38.Crowe S, Mills J, McGrath MS. Quantitative immunocytofluorographic analysis of CD4 surface antigen expression and HIV infection of human peripheral blood monocyte/macrophage. AIDS Res Hum Retrovir. 1987;3:135–41. doi: 10.1089/aid.1987.3.135. [DOI] [PubMed] [Google Scholar]
- 39.Gan H, Ruef C, Hall BF, Tobin E, Remold HG, Mellors JW. Interleukin-6 expression in primary macrophages infected with human immunodeficiency virus-1 (HIV-1) Aids Res Hum Retrovir. 1991;7:671–9. doi: 10.1089/aid.1991.7.671. [DOI] [PubMed] [Google Scholar]
- 40.Koyanagi Y, O'Brien W, Zhao JQ, Golde DW, Gasson JC, Chen ISY. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Sci. 1988;241:1673–5. doi: 10.1126/science.241.4873.1673. [DOI] [PubMed] [Google Scholar]
- 41.Arenzana-Seisdedos F, Virelizier JF, Fiers W. Interferons as macrophage activating factors. III. Preferential effects of IFN-γ on the interleukin 1 secretory potential of fresh or aged human monocytes. J Immunol. 1985;134:2444–7. [PubMed] [Google Scholar]
- 42.Arend WP, Gordon DF, Wood WM, Janson RW, Joslin FG, Jameel S. IL-1β production in cultured human monocytes is regulated at multiple levels. J Immunol. 1989;143:118–25. [PubMed] [Google Scholar]
- 43.Asakura E, Hanamura T, Umemura A, Yada K, Yamauchi T, Tanabe T. Effects of macrophage colony stimulating factor (M-CSF) on lipopolysaccharide (LPS)-induced mediator production from monocytes in vitro. Immunobiol. 1996;195:300–13. doi: 10.1016/S0171-2985(96)80047-7. [DOI] [PubMed] [Google Scholar]
- 44.Furosawa S, Komatsu H, Saito K, Enokihara H, Hirose K, Shishido H. Effect of normal human serum on granulocyte colony formation by human bone marrow cells. J Clin Med. 1978;91:377–82. [PubMed] [Google Scholar]
- 45.Chan SH, Metcalf D, Stanley ER. Stimulation and inhibition by normal human serum of colony formation in vitro by human bone marrow cells. Br J Haematol. 1971;20:329–34. doi: 10.1111/j.1365-2141.1971.tb07043.x. [DOI] [PubMed] [Google Scholar]
- 46.Weiner LM, Li W, Holmes M, et al. Phase I trial of recombinant macrophage colony-stimulating factor and recombinant gamma interferon: toxicity, monocytosis and clinical effect. Cancer Res. 1994;54:4084–90. [PubMed] [Google Scholar]
- 47.Nakajima K, Martinez-Maza O, Hirano T, et al. Induction of IL-6 (B cell stimulatory factor-2/IFN-β2) production by HIV. J Immunol. 1989;142:531–6. [PubMed] [Google Scholar]
- 48.Merril JE, Koyangi Y, Chen ISY. Interleukin 1 and tumor necrosis factor-alpha can be induced from mononuclear phagocytes by human immunodeficiency virus type 1 binding to the CD4 receptor. J Virol. 1989;63:4404–8. doi: 10.1128/jvi.63.10.4404-4408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


