Abstract
An age-dependent decrease in T cell responsiveness to CD28 costimulation has been described. In order to test the hypothesis that an age-related decrease in CD28 expression by CD8+ T lymphocytes might be involved, we analysed 67 healthy donors ranging in age from 15 to 69 years for their CD8+ T cell expression of CD28 and CD57. We found a statistically significant decrease of CD28 expression through ageing and a significant increase of CD57 expression, both markers being mutually exclusive. Given that cytomegalovirus (CMV) is reported to induce CD57 expression, and since the carrier status for this ubiquitous virus increases with age in the general population, it seemed essential to evaluate whether the phenotypic age-related changes described in CD8high+ cells were not influenced by the CMV carrier status of the individuals. Accordingly, we performed a multivariate analysis to assess the independent association of age and CMV carrier status with CD28 and CD57 expression in CD8high+ cells. Results showed that the progressive decrease in CD8high+ CD28+ CD57− cells was associated only with age, while the expansion of the CD8high+ CD28− CD57+ subset depended both on age and CMV, although mainly on age. We conclude that ageing is accompanied by a progressive loss of CD28 expression in CD8+ T cells and a reciprocal enhancement of CD57 expression, both facts being probably related to the repeated antigenic stimulation occurring throughout life.
Keywords: CD28, CD57, CD8, age, cytomegalovirus
INTRODUCTION
CD28 is a disulphide-linked homodimer expressed by peripheral T lymphocytes, which specifically binds B7-1 (CD80) and B7-2 (CD86), molecules that are expressed on ‘professional’ antigen-presenting cells (APC). The ligation of CD28 by these molecules provides a costimulatory signal which is critical for T cell activation [1]. An age-dependent decrease in T cell responsiveness to CD28 costimulation has been described [2,3]. However, the mechanism remains to be elucidated. It has been speculated that an age-related defect in signal transduction pathways might be involved, but evidence is still lacking [3]. Surprisingly, the hypothesis that CD28 expression in T cells could decrease with age has not been tested yet. There is not a single study dealing with the analysis of CD28 expression through ageing.
It has been reported that CD28 expression excludes the presence of the glycoprotein CD57, and vice versa in CD8+ T cells from HIV, cancer and autoimmune patients [4,5]. Azuma and coworkers have also noted this reciprocal expression in healthy controls, although it has not been extensively tested in a large panel of donors [6]. Given the mutually exclusive expression of CD57 and CD28 in CD8+ T cells, and since it is well documented that CD57 expression increases with age [7–10], an age-dependent decrease of CD28 expression would not be surprising.
Therefore, the current study was undertaken to evaluate the influence of age on the expression of CD28 in CD8+ T cells from healthy donors, excluding CD4+ T cells from the analysis, since virtually all of them express CD28 [6]. As we thought that the relationship between CD28 and CD57 needed a closer study in healthy controls, we performed a simultaneous analysis for both molecules.
Finally, we also tested the influence of cytomegalovirus (CMV) on CD28 expression, given that this ubiquitous virus has been extensively reported to induce CD57 expression in CD8+ T cells [11–13]. If CMV had any effect on CD28 expression, it would influence the age effect, since its carrier status increases with age in the general population.
SUBJECTS AND METHODS
Subjects
EDTA-anticoagulated venous blood was obtained from 67 donors ranging in age from 15 to 69 years. All were healthy at the time of the study and were not taking any drug. All had no history of autoimmune or allergic diseases, or recent infections.
In contrast to many studies dealing with age-dependent variations of immune parameters, we did not compare a group of young subjects with a group of aged subjects. As human ageing is a slow process, we considered that this two-group design was an artificial approach, as it does not allow full evaluation of the changes occurring over time. Accordingly, our donors were all aged between 15 and 69 years, and were not divided into groups for the analysis.
Three-colour flow cytometry
Whole blood was stained with fluorochrome-conjugated MoAbs (anti-CD57–FITC (Leu-7), anti-CD28–PE (Leu-28) and anti-CD8–PerCP (Leu-2a), all purchased from Becton Dickinson (San Jose, CA)). Thereafter blood was submitted to Q-Prep lysing and fixing (Coulter Corp., Hialeah, FL).
We stained whole blood instead of mononuclear cells isolated with density gradients because it has been reported that CD28− T cells show different density properties from CD28+ T cells [14].
Samples were analysed on a Epics XL flow cytometer (Coulter), using the XL2 software. Ten thousand lymphocytes were gated on forward and side scatter (SS) parameters. This gating region was referred to a FL3/SS histogram, where a FL3+ (CD8high+) region was defined. This CD8high+ region was further analysed for the expression of FL1 and FL2.
The fact that CD8high+ subsets were referred to total CD8high+ cells instead of to total lymphocytes excluded the possibility that the results could have been affected by the age-dependent variation of the percentage of CD8+ T cells.
CD8low+ cells were excluded from the analysis since they correspond mainly to natural killer (NK) cells [15].
EIA for CMV-specific IgG antibodies
Screening for IgG antibodies to CMV was performed using a commercially available EIA (IMX System; Abbot Labs, IL). The cutoff established by the manufacturer was at 15 AU/ml.
Statistical analysis
To assess the bivariate associations between age and CD28/CD57 expression in CD8high+ cells or between age and the titre of IgG anti-CMV, Spearman's non-parametric correlation coefficients were computed.
In order to assess the association between age and CD28/CD57 expression in CD8high+ cells, controlling for CMV carrier status, a multiple linear regression model was fitted. CD8 markers were used as the dependent variables and age and the titre of IgG anti-CMV as the independent variables. The validity of the regression model assumptions was assessed by the analysis of residuals. Logarithmic transformations of the variables were checked, but they did not improve the goodness of fit of the model.
P < 0.05 was considered to be statistically significant.
RESULTS
Age-related changes in the phenotype of CD8high+ T cells
As explained in Subjects and Methods, we performed a simultaneous analysis for CD28 and CD57 expression in CD8high+ cells, excluding CD8low+ cells from the study since they correspond mainly to NK cells [15]. Results are shown in Fig. 1.
Fig. 1.
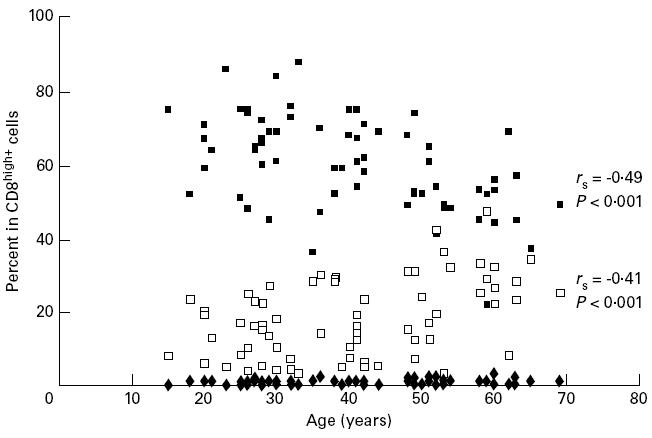
Association between CD28 and CD57 expression in CD8high+ cells and the age of the donors (n = 67). ▪, CD28+ CD57−; □, CD28− CD57+; ♦, CD28+ CD57+.
On one hand, the reciprocal expression of CD28 and CD57 must be noted: only 0.79% (range 0–3%) of CD8high+ cells were CD28+ CD57+, while 18% (range 3–47%) were CD28− CD57+ and 60% (range 22–88%) were CD28+ CD57−.
On the other hand, CD8high+ CD28+ CD57− cells correlated negatively with age (rSpearman= −0.49, P < 0.001), while CD8high+ CD28− CD57+ cells showed a significant positive correlation with age (rSpearman= 0.41, P < 0.001).
Detection of CMV-specific IgG antibodies
Given that CMV induces CD57 expression in CD8+ cells [11–13] and since the presence of CD57 is related to the absence of CD28, it can be deduced that CMV might have some effect on CD28 expression. For this reason, we tested all the donors for the presence of IgG anti-CMV. Results are depicted in Fig. 2.
Fig. 2.
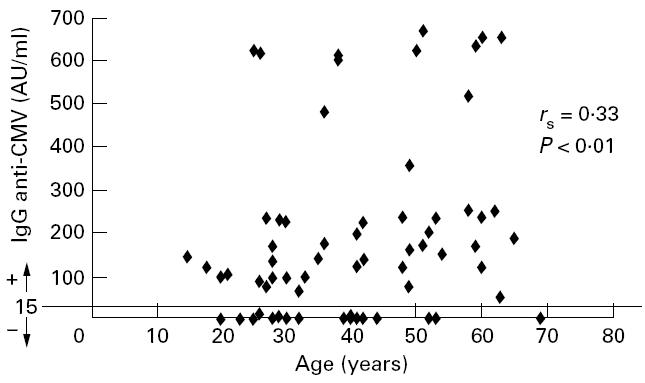
Association between the titre of IgG anti-cytomegalovirus (CMV) and the age of the donors (n = 67).
IgG antibodies to CMV were detectable by EIA in 49 out of 67 individuals (73%; range 48–665 AU/ml). The remaining 18 donors (27%) were negative (< 15 AU/ml).
It is interesting to note that we found a statistically significant correlation between the titre of IgG anti-CMV and the age of the donors (rSpearman= 0.33, P < 0.01).
Independent effects of CMV and age on CD28 and CD57 expression in CD8high+ cells
As we explained above, CMV induces CD57 expression in CD8+ cells, which theoretically means that CMV would down-regulate CD28 expression in these cells. Given that we had found an age-dependent increase in CMV carrier status, we could not be sure whether the previously described age-related increase in CD8high+ CD28− CD57+ cells was an effect of age or CMV infection, or both. For this reason, we tried to assess the independent association of these two factors (age and titre of IgG anti-CMV) with CD8high+ CD28− CD57+ as well as CD8high+ CD28+ CD57− subpopulations, by means of a multivariate analysis.
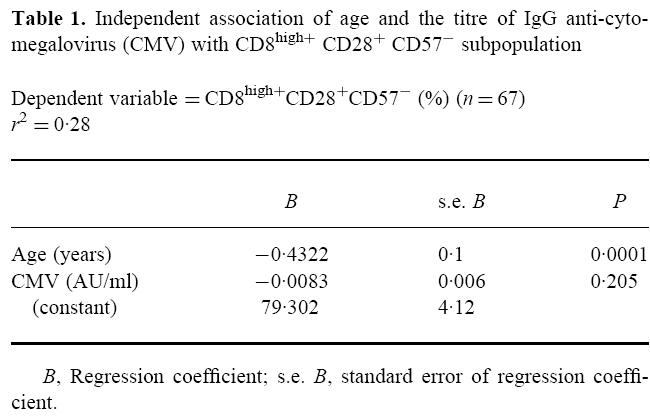
As shown in Table 1, the observed decrease in CD8high+ CD28+ CD57− cells was influenced only by age (B = –0.43% per year, P < 0.001) and not significantly by CMV (B = –0.008% per AU/ml, P = 0.2). The age of the donors explained 26.5% of the variability of this subpopulation and both variables explained 28.2%.
Table 1.
Independent association of age and the titre of IgG anti-cytomegalovirus (CMV) with CD8high+ CD28+ CD57− subpopulation
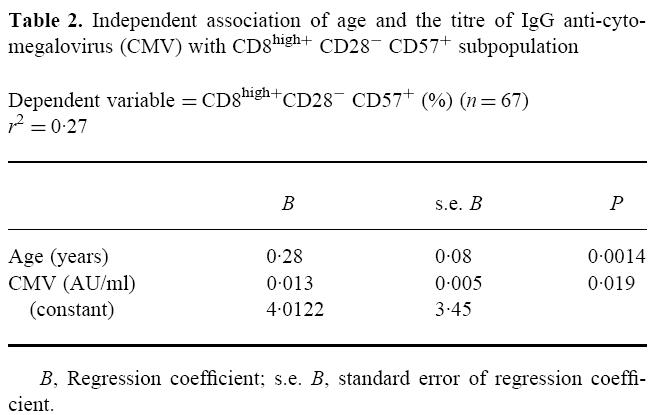
However, the increase in CD8high+ CD28− CD57+ cells depended both on age (B = 0.28% per year, P < 0.01) and CMV carrier status (B = 0.013% per AU/ml, P < 0.05) (Table 2). Both factors altogether explained 26.9% of the variability of this subpopulation (r2= 0.27). When studied separately, we found that age accounted for 20.3% of the variability and CMV added the remaining 6.6%.
Table 2.
Independent association of age and the titre of IgG anti-cytomegalovirus (CMV) with CD8high+ CD28− CD57+ subpopulation
DISCUSSION
The first major conclusion that can be drawn from this study is that CD8high+ cells show an age- and CMV-dependent increase of CD57 expression and an age-dependent decrease of CD28 expression. We did not find any significant association between CD28 expression and CMV carrier status. Although it can not be excluded that we might have lacked the statistical power to detect this association, we assume it to be very weak if it exists.
With regard to CD57, it is well documented that CMV carrier status is associated with significant increases in the number of T cells expressing this molecule [11–13]. On the other hand, several authors had reported that age increases CD57 expression in CD8+ T cells [7–10]. However, none of these studies analysed simultaneously the influence on CD57 expression of both age and CMV. This simultaneous analysis seemed essential to us, since, as described in this study and extensively reported by others [16,17], CMV carrier status increases with age. Taking it into account, it could have been possible that the CD57 induction attributed to age was in fact an indirect effect due to CMV carrier status. To evaluate this possibility, we performed a multivariate analysis of the independent relationship of age and CMV on the expression of CD57 by CD8high+ cells, concluding that both factors are associated with its increase, although to a different extent. The association of age with CD57 expression was stronger than the association of this marker with CMV carrier status.
Concerning the observed decrease in CD28 expression by CD8high+ cells, we found an association only with age, and not with CMV. To our knowledge, this is the first study that evaluates the effect of age on CD28 expression by T lymphocytes. The observed age-dependent decrease in CD28 expression could contribute to explain the diminished T cell responsiveness to CD28 costimulation that Engwerda et al. have reported in relation to age [2,3]. It also could account for the substantial variability described by Sunder-Plassmann and coworkers in the individual response to CD28. These authors tested a large panel of adult healthy controls for their T cell response to CD28 stimulation and reported great variation between donors, attributing it to genetic or other, so far unidentified, factors [18]. As we have demonstrated in this study, age is one of these factors.
The second conclusion that can be drawn is the reciprocal expression of CD28 and CD57 in healthy CD8+ T cells. Only around 1% of them express both markers simultaneously. Azuma et al. also described this phenomenon in healthy controls [6], but an extensive testing of a large panel of donors was lacking. Most studies dealing with the simultaneous analysis of CD28 and CD57 in CD8+ T cells have been performed in HIV patients [4] as well as in cancer or autoimmune patients [5]. In fact, the expansion of the subset CD8high+ CD28− CD57+ has been considered as associated with pathological conditions [5]. From our results it can be deduced that this expansion takes place as part of the normal process of ageing. It does not seem surprising, since human T cells have been shown to down-regulate CD28 expression after stimulation by antigen [19,20]. The repeated antigen stimulation occurring throughout life could lead to a permanent loss of CD28 expression. In fact, CD8+ CD28− cells are considered as terminally differentiated T cells [21], showing shortened telomeric lengths [22] and a diminished proliferative capacity [23]. In a reciprocal manner, repeated antigenic exposures throughout life could lead to an enhancement of CD57 expression, given that the presence of this molecule is also considered a late differentiation event of T cells [23,24].
Further studies will be required in order to elucidate whether CD28 down-regulation and CD57 up-regulation in CD8+ T cells are linked by some regulatory pathway, or are simply coincidental events responding to the same cause, namely a repeated antigenic stimulation.
References
- 1.June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–31. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 2.Engwerda CR, Handwerger BS, Fox BS. Aged T cells are hyporesponsive to costimulation mediated by CD28. J Immunol. 1994;152:3740–7. [PubMed] [Google Scholar]
- 3.Engwerda CR, Handwerger BS, Fox BS. An age-related decrease in rescue from T cell death following costimulation mediated by CD28. Cell Immunol. 1996;170:141–8. doi: 10.1006/cimm.1996.0144. [DOI] [PubMed] [Google Scholar]
- 4.Lewis DE, Ng Tang DS, Adu-Oppong A, Schober W, Rodgers JR. Anergy and apoptosis in CD8+ T cells from HIV-infected persons. J Immunol. 1994;153:412–20. [PubMed] [Google Scholar]
- 5.Kern F, Ode-Hakim S, Vogt K, Höflich C, Reinke P, Volk HD. The enigma of CD57+ CD28− T cell expansion—anergy or activation? Clin Exp Immunol. 1996;104:180–4. doi: 10.1046/j.1365-2249.1996.d01-635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azuma M, Phillips JH, Lanier LL. CD28− T lymphocytes. J Immunol. 1993;150:1147–59. [PubMed] [Google Scholar]
- 7.Malinowski K, Papaport FT. Effects of ageing upon the expression of differentiation and class II MHC antigens on the surface of T lymphocytes from normal human subjects. Cell Immunol. 1995;162:68–73. doi: 10.1006/cimm.1995.1052. [DOI] [PubMed] [Google Scholar]
- 8.Hannet I, Erkeller-Yuksel F, Lydyard P, Deneys V, DeBruyère M. Developmental and maturational changes in human blood lymphocyte subpopulations. Immunol Today. 1992;13:215–8. doi: 10.1016/0167-5699(92)90157-3. [DOI] [PubMed] [Google Scholar]
- 9.Abo T, Cooper MD, Balch CM. Postnatal expansion of the natural killer and killer cell population in humans identified by the monoclonal HNK-1 antibody. J Exp Med. 1982;155:321–6. doi: 10.1084/jem.155.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ligthart GJ, Van Vlokhoven PC, Schuit HR, Hijmans W. The expanded null cell compartment in ageing: increase in the number of natural killer cells and changes in T-cell and NK-cell subsets in human blood. Immunology. 1986;59:353–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Lenkei R, Andersson B. High correlations of anti-CMV titres with lymphocyte activation status and CD57 antibody-binding capacity as estimated with three-colour, quantitative flow cytometry in blood donors. Clin Immunol Immunopathol. 1995;77:131–8. doi: 10.1006/clin.1995.1136. [DOI] [PubMed] [Google Scholar]
- 12.Gratama JW, Naipal AMIH, Oosterveer MAP, et al. Effects of herpes virus carrier status on peripheral T lymphocyte subsets. Blood. 1987;70:516–23. [PubMed] [Google Scholar]
- 13.Gratama JW, Kardol M, Naipal AMIH, et al. The influence of cytomegalovirus carrier status on lymphocyte subsets and natural immunity. Clin Exp Immunol. 1987;68:16–24. [PMC free article] [PubMed] [Google Scholar]
- 14.Nakata M, Kawasaki A, Azuma M, et al. Expression of perforin and cytolytic potential of human peripheral blood lymphocyte subpopulations. Int Immunol. 1992;4:1049–54. doi: 10.1093/intimm/4.9.1049. [DOI] [PubMed] [Google Scholar]
- 15.Norment AM, Littman DR. A second subunit of CD8 is expressed in human T cells. EMBO J. 1988;7:3433–9. doi: 10.1002/j.1460-2075.1988.tb03217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold E, Nankervis GA. Cytomegalovirus: descriptive epidemiology. In: Evans AS, editor. Viral infections of humans. Epidemiology and control. 3. New York: Plenum Medical Book Co.; 1989. pp. 171–5. [Google Scholar]
- 17.Britt WJ, Alford ChA. Cytomegalovirus: epidemiology. In: Fields BN, Knipe DM, Howley PM, editors. Fields virology. 3. Vol. 2. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 2494–6. [Google Scholar]
- 18.Sunder-Plassmann R, Pickl WF, Majdic O, Knapp W, Holter W. Crosslinking of CD27 in the presence of CD28 costimulation results in T cell proliferation and cytokine production. Cell Immunol. 1995;164:20–27. doi: 10.1006/cimm.1995.1138. [DOI] [PubMed] [Google Scholar]
- 19.Linsley PS, Bradshaw J, Urnes M, Grosmaire L, Ledbetter JA. CD28 engagement by B7/BB-1 induces transient down-regulation of CD28 synthesis and prolonged unresponsiveness to CD28 signalling. J Immunol. 1993;150:3161–9. [PubMed] [Google Scholar]
- 20.Lake RA, O'Hehir RE, Verhoef A, Lamb JR. CD28 mRNA rapidly decays when activated T cells are functionally anergized with specific peptide. Int Immunol. 1993;5:461–6. doi: 10.1093/intimm/5.5.461. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd TE, Yang L, Ng Tang D, Bennett T, Schober W, Lewis DE. Regulation of CD28 costimulation in human CD8+ T cells. J Immunol. 1997;158:1551–8. [PubMed] [Google Scholar]
- 22.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28− CD8+ T cells imply a replicative history that is distinct from their CD28+ CD8+ counterparts. J Immunol. 1996;156:3587–90. [PubMed] [Google Scholar]
- 23.Morley JK, Batliwalla FM, Hingorani R, Gregersen PK. Oligoclonal CD8+ T cells are preferentially expanded in the CD57+ subset. J Immunol. 1995;154:6182–90. [PubMed] [Google Scholar]
- 24.Dupuy d'Angeac A, Monier S, Pilling D, Travaglio-Encinoza A, Rème T, Salmon M. CD57+ T lymphocytes are derived from CD57− precursors by differentiation occurring in late immune responses. Eur J Immunol. 1994;24:1503–11. doi: 10.1002/eji.1830240707. [DOI] [PubMed] [Google Scholar]


