Abstract
Pancreatic cancer is characterized by an increasing incidence and an extremely poor prognosis. It is resistant to most of the conventional treatment modalities. Histomorphologically, it presents with a strong desmoplastic reaction around cancer cells, and lymphocytes are typically localized as aggregates in the fibrotic interstitial tissue. Using the method of multi-epitope imaging with fluorochrome-tagged specific MoAbs which allows the simultaneous localization and characterization of T cells in tissues, we studied phenotypes and distribution of tumour-infiltrating lymphocytes (TIL) in pancreatic cancer. CD3+ T cells comprised up to 90% of the tumour-infiltrating cells which were either CD4+ or CD8+, most of them being memory cells (CD45RO+). In decreasing order of frequency, T lymphocytes carried the markers for CD45RO, CD18, CD103 and TCR γδ. Very few natural killer cells (CD56+) were observed. Twenty percent of CD8+ were labelled with CD103. These CD8+ CD103+ T cells, analogous to the gut intraepithelial lymphocytes (IEL), were found in the fibrous interstitial tissue. Furthermore, an inverse correlation was found between the expression of CD18, the β2-integrin, which mediates adhesion of activated lymphocytes, and CD45RO in the CD8+ subset of TIL (P = 0.046). In conclusion, phenotyping of T lymphocytes in pancreatic cancer raises the possibility that pancreatic cancer cells develop several strategies to escape the T cell-induced cytolysis by (i) the aggregation of cytotoxic CD8+ CD103+ T cells in the fibrous tissue distant from the tumour cells, and (ii) the presence of CD18-bearing cells which lack the expression of the activation marker CD45RO.
Keywords: CD4, CD8, CD103, pancreatic cancer, multi-epitope imaging
INTRODUCTION
Pancreatic cancer is the fifth leading cause of cancer death in the Western world, and it is associated with a poor prognosis and a 5-year survival rate of 3% [1]. Clinically, pancreatic cancer is characterized by rapid tumour progression and unresponsiveness to most conventional treatment modalities [1]. The biology of pancreatic cancer and the reasons for its aggressiveness are poorly understood [2]. Pancreatic cancer cells express a variety of growth factors, especially of the epidermal growth factor (EGF), platelet-derived growth factor (PDGF) and transforming growth factor-beta (TGF-β) family [3–6]. On the molecular genetic level, the most common genetic abnormalities found are K-ras gene mutations [7], mutations of the p53 tumour suppressor gene [8] and deletions of the DPC-4 tumour suppressor gene [9].
Failure of the body's immune system to detect or destroy tumour cells may be important in understanding the pathogenesis of cancer. Immunophenotypic studies of various carcinomas have demonstrated that CD8+ (cytotoxic/suppressor) T cells are the predominant T cell subset of tumour-infiltrating lymphocytes (TIL) [10,11]. The presence of other markers, such as CD45RO, indicates that these cells are memory cells and are thus tissue-specific [10,11]. Though studies of the function of TIL indicate that these cells, including CD8+ cells, TCR γδ+ and CD56+ cells, can be isolated from human pancreatic cancer, further phenotyping of TIL has to date not been undertaken [12–14].
Using the method of multi-epitope imaging with fluorochrome-tagged specific MoAbs, we characterized and analysed the differential distribution of lymphocytes in pancreatic cancer [15,16]. We now report that CD8+ cells form the predominant T cell subset in pancreatic cancer. However, the aggregation of the T cell subset CD8+ CD103+ in the fibrous tissue distant from the cancer cells and the presence of CD18+ cells lacking the activation marker CD45RO point to cancer cell-dependent strategies which may allow the latter to escape the cytotoxic effects of these TIL.
MATERIALS AND METHODS
Fluorochrome-tagged (FITC or PE) MoAbs against CD3, CD4, CD8, CD18, CD45RO, CD56, CD64, CD103 and TCR γδ were purchased from Coulter Immunotech (Hamburg, Germany). Details of usage and specificities of the employed antibodies are listed in Table 1 and have been previously reported [15,17]. Normal goat serum was obtained from Sigma Pharmaceuticals (Deisenhofen, Germany). All imaging procedures were performed on a Zeiss microscope (Axiophot, Jena, Germany) with a high resolution camera (Photometrics, Munich, Germany) and software from PMIS and IDL (Munich, Germany).
Table 1.
Tissue samples
Pancreatic cancer (n = 8; one female, seven male) tissues were obtained from patients undergoing pancreatic surgery. Normal pancreatic tissues were obtained from eight individuals (four male, four female) through an organ donor programme. To assure sampling uniformity, tissue samples from organ donors and pancreatic cancer patients were always obtained from the head of the pancreas. The median ages of the cancer patients and organ donors were 66 years (range 37–74 years) and 38 years (range 14–53 years), respectively.
Immediately following surgical removal, tissue samples were snap-frozen in liquid nitrogen. The cancer samples were classified as pancreatic ductal adenocarcinomas according to the TNM classification for pancreatic tumours [1]. All studies were approved by the Human Subjects Committee of the University of Berne (Berne, Switzerland).
Fluorescent immunohistochemistry
Cryostat sections (5–7 μm) from pancreatic tissue were incubated with fluorochrome-labelled primary antibodies and evaluated by fluorescent microscopy [18]. Non-specific binding was blocked by incubating sections with 10% normal goat serum for 30 min, followed by incubation with the pair of primary antibodies (CD4-PE and CD8-FITC). Details of the specificities and dilutions used are shown in Table 1. CD4 and CD8 T cells observed in 30 high power fields (HPF) were enumerated.
Multi-epitope imaging
Sections were treated as mentioned above. Serial incubation with the following primary conjugated antibodies (CD4-PE and CD8-FITC, CD3-PE and TCR γδ-FITC, CD56-PE and CD45RO-FITC, CD64-PE and CD103-FITC, and CD18-PE) was performed as described [15,19]. In short, immunofluorescent sections were examined with a Zeiss microscope equipped with a 50-W mercury lamp and a 40 × water immersion objective (Zeiss, Jena, Germany). A region of interest (ROI) of the section with ample T cells was then selected for further analysis. Computerized image acquisition was performed with the PMIS package. All images acquired showed the same cells as seen labelled with different antibodies. Each incubation step was followed by complete bleaching of the fluorochrome conjugated to the primary antibody. Thus, the ROI was exposed to light of the wavelength required for the excitation of the respective fluorochrome until no more fluorescence was observed, allowing the application of a new set of fluorochrome-tagged primary antibodies without loss in sensitivity or specificity of the fluorescence seen on binding by the next pair of MoAbs to the same cells. Each set of images was digitized and corrected for pixel shift before analysis [16]. As positive controls, we used MoAbs conjugated with fluorochromes different from those used in the original experiment, e.g. we used a PE-labelled CD4 MoAb and as a control CD4-FITC was employed, which gave the same binding pattern. Further, we repeated incubations with sections from the same specimen after interchanging the pairs of primary antibodies. As negative controls, fluorochrome-tagged IgG mouse MoAbs were employed which did not show any specific binding (data not shown). The specificity of the antibodies was tested by using peripheral blood smears from healthy donors subjected to the same experimental conditions as the pancreatic tissue (data not shown).
Statistical analysis
Statistical analysis was performed using the SPSS package (Version 6.0.1; SPSS Inc., Chicago, IL). All results are expressed as mean with s.e.m. Student's t-test was used for statistical analysis, with P < 0.05 taken as the level of significance. Pearson's test for examining correlations was employed [20].
RESULTS
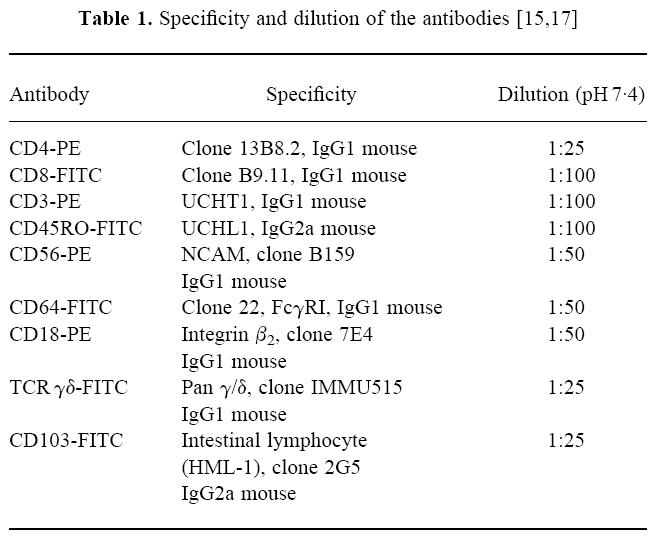
Few CD4+or CD8+ T lymphocytes were found in sections of normal pancreatic tissue. Due to the small numbers of T cells found in normal pancreatic tissue, no further characterization of these cells was performed. In sections of pancreatic cancer, aggregates of T cells were observed in the fibrotic interstitial tissue (Fig. 1A), few lymphocytes were seen in the periductal regions and intraepithelial lymphocytes (IEL) intercalating between the epithelial cells were rarely observed. There were wide variations in the numbers of CD4+ and CD8+ cells in sections of pancreatic cancer, ranging from 24 to 141 CD4+ cells (77.5 ± 16 (mean ± s.e.m.)) and 22 to 151 CD8+ cells (79.9 ± 17) observed in a total of 30 HPF. In five of eight cases examined, CD8+ formed the predominant T cell subset (Fig. 1B).
Fig. 1.
Fluorescent immunohistochemistry. (A) Localization of CD4+ and CD8+ T cells in the fibrous interstitial tissue in pancreatic cancer by immunofluorescence. CD4+ and CD8+ cells are seen in clusters; there is no distinct pattern noticeable in the distribution of the two T cell subsets. This image was produced by the superimposition of the grey scale fluorescent images obtained for binding with the antibodies against CD4 and CD8, respectively, on the phase contrast image. Each image was assigned a separate colour channel (red, CD4; green, CD8) and the phase contrast image was ascribed the colour blue. (B) Image acquired by superimposition of the fluorescent images obtained on binding with the CD8 (red) and TCR γδ+ (green) antibodies. White arrows indicate CD8+ TCR γδ+ cells. Due to the exact superimposition of the two images, the areas of overlap appear white.
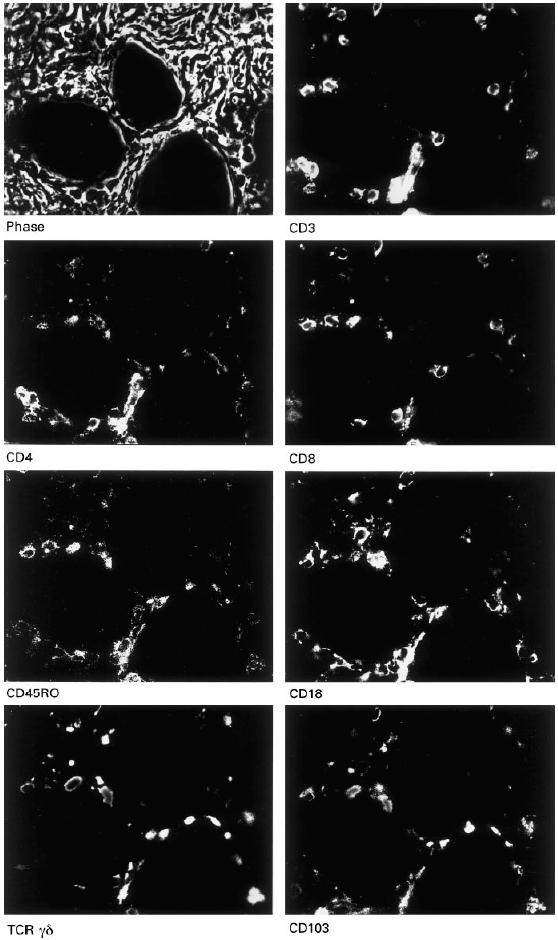
In pancreatic cancer, the CD3+ marker was found on 73% of CD4+ T cells and 82% of CD8+ cells, respectively. Eighty-four percent of the CD4+ and 74% of the CD8+ subset of T lymphocytes expressed CD45RO (memory/activated marker) (Fig. 2). The human mucosal lymphocyte marker, CD103+, also known as HML-1, αEβ7integrin and E-cadherin ligand, was present on 8% of CD4+ and 20% of CD8+ cells. Wide variations were observed in the distribution of CD103 among the CD8+ lymphocytes, varying from 9% to 32% (20.1 ± 7.2%). This T cell subset was localized only in the fibrous interstitial tissue and was not found periductally or intraepithelially (Fig. 3A). Overall, 24.5% of the T cells were CD18+. This subset of CD8+ CD18+ T cells was also located in aggregates of the TIL in the fibrous stroma (Fig. 3B). Furthermore, CD18 was expressed on cells that did not express markers typical of T cells. Statistical analysis using Pearson's coefficient of correlation showed an inverse correlation between the expression of the memory/activation marker (CD45RO) and β2-integrin CD18 in the CD8 subset (P < 0.046).
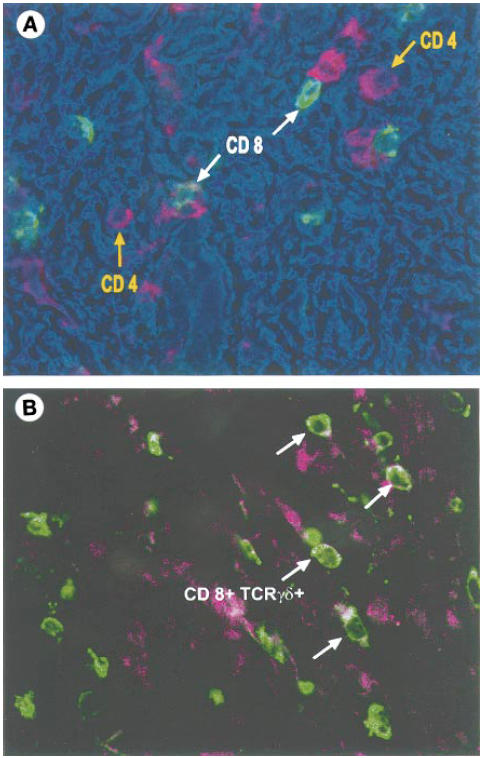
Fig. 2.
Multi-epitope-imaging. The phase contrast image gives the orientation of the lymphocytes. All images depict the same high-power field, i.e. the same lymphocytes as seen labelled with different T cell markers. The panels show the grey scale fluorescent images of a section of pancreatic cancer.
Fig. 3.
Multi-epitope-imaging. (A) Superimposition of the fluorescent images obtained on binding with the CD8 (red) and CD103 (green) antibodies. White arrows indicate CD8+ CD103+ cells. (B) Superimposition of the fluorescent images obtained on binding with the CD8 (red) and CD18 (green) antibodies. The white arrows indicate CD8+ CD18+ cells.
All TCR γδ+ cells were CD8+ (Fig. 1y). TCR γδ+ cells formed only a small proportion of the CD8+ T lymphocytes (mean 14.4%).
Other T cell markers such as CD103 and CD18 were also expressed by CD8+ TCR γδ+ lymphocytes, but no statistical correlation was found between the expression of this T cell receptor and other T lymphocyte markers used in this study.
Only one or two natural killer (NK) cells (CD56+) were observed per section in pancreatic cancer. While most of the macrophages (CD64+) did not carry T cell markers, a few macrophages were also CD4+; these cells were uniformly CD3−.
DISCUSSION
Predominance of the CD8+ T cell subset in TIL is a common feature of many human cancers, including those of the gastrointestinal tract, liver and breast [10,11,21]. A preponderance of CD8 cells was observed in gastric cancer [10] as well as in primary liver carcinoma [11]. Our observation that CD8+ T cells, which are cytotoxic or suppressor cells, are the predominant T lymphocyte subset in pancreatic cancer is in agreement with prior functional studies of TIL in pancreatic cancer [13,14]. Despite the in vitro findings of cytotoxicity of T cells towards pancreatic cell lines [13], the nature of the interaction of CD8+ cells with cancer cells in vivo is unclear.
We have examined, among other T cell markers, the expression of the integrin HML-1 (αEβ7) in this study, to elucidate the nature of the cross-talk between tumour cells and CD8+ cells. HML-1, also known as αEβ7 integrin, was found to be expressed on at least 85% of intestinal IEL (iIEL) and 40% of lamina propria lymphocytes, and only on 2% of peripheral blood lymphocytes (PBL) in healthy individuals [22]. As this integrin is mainly associated with CD8+ cells [23], the CD8+ CD103+ cells observed in our study are analogous to iIEL. HML-1 mediates the interaction between epithelial cells and the IEL [17]. The functions of these cells in the gastrointestinal tract appear to be manifold and are distinct from those of PBL. IEL have been demonstrated to possess spontaneous cytotoxicity against epithelial tumour cell lines, which is distinct from that of NK cells [24]. This finding that IEL-induced cytotoxicity is target cell-restricted would suggest a role in recognizing and destroying transformed epithelial cells [24]. Furthermore, the integrin HML-1 mediates the adhesion of iIEL to epithelial cells [25] and colon carcinoma cells [17]. The ligand for αEβ7 is E-cadherin, which is expressed by pancreatic epithelial cells [26,27]. However, E-cadherin expression is decreased in pancreatic tumours compared with normal pancreas. Furthermore, aberrant expression of E-cadherin in the cytoplasm rather than at intercellular junctions in pancreatic carcinoma has been reported [28]. These observations, taken together with our finding that CD8+ cells are mostly located within the interstitial tissue, imply that tumour cells may avoid cell-to-cell contact with cytolytic CD8+ CD103+ cells via down-regulation of E-cadherin, the ligand for CD103.
The β2-integrin, CD18, comprises one subunit of the leucocyte function-associated antigen 1 (LFA-1), which is expressed by monocytes, T cells and polymorphonuclear leucocytes. This integrin mediates adhesion required for T lymphocyte target cell lysis, T cell proliferation and natural killing [29,30]. The interaction via LFA-1 is mediated by the direct interaction of LFA-1 with intercellular adhesion molecule-1 (ICAM-1), which is over-expressed in pancreatic cancer [31]. However, although ICAM-1 is over-expressed in pancreatic cancer, adhesion as mediated by CD18 is regulated by the state of activation of the T cell expressing this integrin as well [32]. Thus, the inverse correlation between the activation/memory molecule, CD45RO, and CD18 in the CD8+ subset, which we found in this study, could imply a defect in the cytotoxic activity of this CD8+ T cell subset in pancreatic cancer.
TGF-β plays a central role in the mediation of the interaction between pancreatic tumour cells and T lymphocytes. Pancreatic cancer cells have been demonstrated to over-express TGF-β1, TGF-β2 and TGF-β3 [6]. In vitro studies have shown that TGF-β1 can induce the expression of αEβ7 on PBL [33] and can up-regulate this integrin in lymphocytes destined to become IEL [34,35]. However, it also mediates the down-regulation of E-cadherin expression on epithelial cells, as well as suppresses the outgrowth of CD4+ CD45RO+ T cells in vitro [36]. Moreover its antagonistic effect is reflected by the up-regulation of HML-1 and down-regulation of LFA-1 in the IEL microenvironment [35]. TGF-β1 is also instrumental in the stimulation of fibroblast proliferation and formation of the extracellular matrix [37]. Taken together, these findings raise the possibility that TGF-β1 may prevent TIL from coming into direct contact with tumour cells and hence avoid the cytolysis of these cells.
Inasmuch as therapeutical options are limited in pancreatic cancer, new approaches are crucial for the future management of patients suffering from advanced pancreatic cancer [1]. Enhancing the activity of cytotoxic effector cells by induction with IL-2 has been reported recently [38]. Following activation with IL-2 in vitro and in vivo enhanced lymphokine-activated killer (LAK) cell activity is mediated in part by CD3− CD56+ NK cells and CD3+ CD56+ cytotoxic T lymphocytes. Our analysis revealed the presence of only few CD56+ cells in pancreatic carcinoma. Moreover, IL-2 is also instrumental in the induction of HML-1 on PBL destined to become IEL [23]. Thus, in summary, in order to enhance the infiltration of pancreatic cancers by cytotoxic effector cells, the administration of IL-2 in clinical settings may seem beneficial in these patients. In addition, the regulation of the expression of E-cadherin and ICAM-1 on pancreatic cancer cells may present an alternative approach aimed at enhancing the interaction of epithelial cells and lymphocytes in pancreatic cancers in vivo. Thus, several factors, including interferon-gamma (IFN-γ) and IL-2, have been reported to enhance the direct interaction of ICAM-1 and LFA-1 [17]. Furthermore, E-cadherin expression can be induced by IFN-γ, tamoxifen and retinoic acids [39].
In conclusion, our study demonstrates that mainly CD45RO+ T cells of the CD4+ and CD8+ lymphocyte subsets infiltrate the pancreas in human pancreatic cancer, with the CD8+ phenotype representing the predominant T lymphocyte subset. However, a T cell phenotype, analogous to gut intraepithelial lymphocytes bearing markers typical for IEL (CD8+ αEβ7+) is found mainly in the fibrous stroma distant from the cancer cells. These findings, taken together with reports of down-regulation of the adhesion molecule ligand, E-cadherin, on intercellular junctions as well as the over-expression of TGF-β by pancreatic cancer cells, raise the hypothesis that pancreatic cancer cells may mediate the aggregation of cytotoxic T cells in the fibrous tissue and may thus escape their cytolytic effects. Furthermore, the down-regulation of activation markers on cytotoxic CD8+ CD18+ T lymphocytes could result in decreased cytotoxic activity towards pancreatic tumour cells.
Acknowledgments
This work was supported by a grant awarded by the Deutsche Forschungsgemeinschaft (DFG) to W.S. and P.M. (SFB 387, B-1, B-5), the DFG project Schu 627/2-2, and 8-1, the BMBF project 07NBL04, TPB 10 and the Land Sachsen-Anhalt (FK1837A). In addition, this work was supported in part by grants from the Penguin Stiftung (Düsseldorf, Germany) and the Rudolf-Bartling Stiftung (Hannover, Germany) awarded to M.E.
References
- 1.Warshaw AL, Fernandez-Del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–65. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 2.Oertel JE, Heffess CS, Oertel YC. Pancreas. In: Sternberg SS, editor. Diagnostic surgical pathology. New York: Raven Press; 1989. pp. 1057–93. [Google Scholar]
- 3.Lemoine NR, Hughes CM, Barton CM, et al. The epidermal growth factor receptor in human pancreatic cancer. J Pathol. 1992;166:7–12. doi: 10.1002/path.1711660103. [DOI] [PubMed] [Google Scholar]
- 4.Ebert M, Yokoyama M, Kobrin MS, et al. Induction and expression of amphiregulin in human pancreatic cancer. Cancer Res. 1994;54:3959–62. [PubMed] [Google Scholar]
- 5.Ebert M, Yokoyama M, Friess H, et al. Induction of platelet-derived growth factor A and B chains and overexpression of their receptors in human pancreatic cancer. Int J Cancer. 1995;62:529–35. doi: 10.1002/ijc.2910620507. [DOI] [PubMed] [Google Scholar]
- 6.Friess H, Yamanaka Y, Büchler MW, et al. Enhanced expression of transforming growth factor β isoforms in pancreatic cancer correlates with decreased survival. Gastroenterol. 1993;105:1846–56. doi: 10.1016/0016-5085(93)91084-u. [DOI] [PubMed] [Google Scholar]
- 7.Grünewald K, Lyons J, Fröhlich A, et al. High frequency of Ki-ras codon 12 mutations in pancreatic adenocarcinomas. Int J Cancer. 1989;43:1037–41. doi: 10.1002/ijc.2910430614. [DOI] [PubMed] [Google Scholar]
- 8.Simon B, Weinel R, Höhne M, et al. Frequent alterations of the tumor suppressor genes p53 and DCC in human pancreatic carcinomas. Gastroenterol. 1994;106:1645–51. doi: 10.1016/0016-5085(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 9.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Sci. 1996;271:350–3. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 10.Lee WJ, Chang KJ, Lee CS, et al. Selective depression of T lymphocyte subsets in gastric cancer subsets in gastric cancer patients: an implication for immunotherapy. J Surg Oncol. 1994;55:165–9. doi: 10.1002/jso.2930550307. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu Y, Watanabe A, Whiteside TL. Memory T-lymphocytes are the main population of tumor-infiltrating lymphocytes obtained from human primary liver tumors. J Hepatol. 1992;16:197–202. doi: 10.1016/s0168-8278(05)80115-1. [DOI] [PubMed] [Google Scholar]
- 12.Bedossa P, Bacci J, Lemaigre G, et al. Lymphocyte subsets and HLA-DR expression in normal pancreas and chronic pancreatitis. Pancreas. 1990;5:415–20. doi: 10.1097/00006676-199007000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Wahab ZA, Metzgar RS. Human cytotoxic lymphocytes reactive with pancreatic adenocarcinoma cells. Pancreas. 1991;6:307–17. doi: 10.1097/00006676-199105000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Kitayama J, Atomi Y, Nagawa H, et al. Functional analysis of TCRγδ+ T cells in tumor-infiltrating lymphocytes (TIL) of human pancreatic cancer. Clin Exp Immunol. 1993;93:442–7. doi: 10.1111/j.1365-2249.1993.tb08198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schubert W. Multiple antigen mapping microscopy of human tissue. In: Burger G, Oberholzer M, Vooijts GP, editors. Advances in analytical cellular pathology. New York: Elsevier-Excerpta Medica ICS; 1990. pp. 97–98. [Google Scholar]
- 16.Schünemann S, Rethfeldt C, Müller F, et al. Analysis of coupled multi-image information in microscopy. In: Höhne KH, Kikinis R, editors. Lecture notes in computer science 1131: visualization in biomedical computing. Berlin: Springer; 1996. pp. 147–51. [Google Scholar]
- 17.Roberts AI, O'Connell SM, Ebert EC. Intestinal intraepithelial lymphocytes bind to colon cancer cells by HML-1 and CD11a. Cancer Res. 1993;53:1608–11. [PubMed] [Google Scholar]
- 18.Massey RF, Albert WH, Staines NA. Methods of immunological analysis, cells and tissues. Vol. 3. New York: VCH; 1993. [Google Scholar]
- 19.Schubert W, Schwan H. Detection by 4-parameter microscopic imaging: an increase of rare mononuclear blood leucocyte type expressing the Fc gamma RIII receptor (CD16) for immunoglobulin G in human sporadic amyolateral sclerosis (ALS) Neurosci Letters. 1995;198:29–32. doi: 10.1016/0304-3940(95)11956-w. [DOI] [PubMed] [Google Scholar]
- 20.Siegel S. Non-parametric statistics for behavioural sciences. New York: McGraw-Hill; 1956. [Google Scholar]
- 21.Walker RA, Dearing SJ, Gallacher B. Relationship of transforming growth factor beta 1 to extracellular matrix and stromal infiltrates in invasive breast carcinoma. Br J Cancer. 1994;69:1160–5. doi: 10.1038/bjc.1994.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerf-Bensussan N, Jarry A, Brousse N, et al. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987;17:1279–85. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- 23.Schieferdecker HL, Ullrich R, Weiss-Breckwoldt AN, et al. The HML-1 antigen of intestinal lymphocytes is an activation antigen. J Immunol. 1990;144:1541–9. [PubMed] [Google Scholar]
- 24.Taunk J, Roberts AI, Ebert EC. Spontaneous cytotoxicity of human intraepithelial lymphocytes against epithelial cell tumors. Gastroenterol. 1992;102:69–75. doi: 10.1016/0016-5085(92)91785-3. [DOI] [PubMed] [Google Scholar]
- 25.Kelleher D, Murphy A, Lynch S, O'Farrelly C. Adhesion molecules utilized in the binding of intraepithelial lymphocytes to human enterocytes. Eur J Immunol. 1994;24:1013–6. doi: 10.1002/eji.1830240437. [DOI] [PubMed] [Google Scholar]
- 26.Cepek KL, Shaw SK, Parker CM, et al. Adhesion between epithelial cells and T lymphocytes is mediated by E-cadherin and αE7 integrin. Nature. 1994;372:190–3. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 27.Weinel RJ, Neumann K, Kisker O, et al. Expression and potential role of E-cadherin in pancreatic carcinoma. Int J Pancreatol. 1996;19:25–30. doi: 10.1007/BF02788372. [DOI] [PubMed] [Google Scholar]
- 28.Pignatelli M, Ansari TW, Gunther P, et al. Loss of membraneous E-cadherin expression in pancreatic cancer: correlation with lymph node metastasis, high grade, and advanced stage. J Pathol. 1994;174:243–8. doi: 10.1002/path.1711740403. [DOI] [PubMed] [Google Scholar]
- 29.Davignon D, Martz E, Reynolds T, et al. Lymphocyte function-associated antigen 1 (LFA-1): a surface antigen distinct from Lyt-2,3 that participates in T lymphocyte-mediated killing. Proc Natl Acad Sci USA. 1981;78:4535–9. doi: 10.1073/pnas.78.7.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krensky AM, Sanchez-Madrid F, Robbins E, et al. The functional significance of LFA-1, LFA-2 and LFA-3: cell surface antigens associated with CTL–target interactions. J Immunol. 1983;131:611–6. [PubMed] [Google Scholar]
- 31.Shimoyama S, Gansauge F, Gansauge S, et al. Overexpression of intercellular adhesion molecule-1 (ICAM-1) in pancreatic adenocarcinoma in comparison with normal pancreas. Pancreas. 1997;14:181–6. doi: 10.1097/00006676-199703000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Diamond MS, Springer TA. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol. 1993;120:545–56. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mowat AM, Viney JL. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156:145–66. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 34.Cepek KL, Parker CM, Madara JL, et al. Integrin αEβ7 mediates adhesion of T lymphocytes to epithelial cells. J Immunol. 1993;150:3459–70. [PubMed] [Google Scholar]
- 35.Parker CM, Cepek KL, Russell GJ, et al. A family of β7 integrins on human mucosal lymphocytes. Proc Natl Acad Sci USA. 1992;89:1924–8. doi: 10.1073/pnas.89.5.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–36. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massague J, Cheifetz S, Laiho M, et al. Transforming growth factor-β. Cancer Surveys. 1992;12:81–103. [PubMed] [Google Scholar]
- 38.Weidmann E, Bergmann L, Hechler P, Mitrou PS. Cytotoxic activity and phenotypic characteristics of lymphocyte subsets after therapy of cancer patients with interleukin-2. Cancer Immunol Immunother. 1991;33:398–402. doi: 10.1007/BF01741601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang WG, Hiscox S, Hallett MB, et al. Regulation of the expression of E-cadherin on human cancer cells by gamma-linoleinc acid. Cancer Res. 1995;55:5043–8. [PubMed] [Google Scholar]


