Abstract
In order to analyse the specificity of human anti-ribosomal P protein antibodies, anti-ribosomal P protein antibodies were affinity-purified from the sera of lupus patients. Their binding capacity towards recombinant SmD protein and recombinant SmB/B′ protein was evaluated by immunoblot and ELISA. Epitope mapping of SmD was performed by means of synthetic peptides. Anti-ribosomal P protein antibodies bound recombinant SmD (5/10) and SmB/B′ (4/10) on immunoblot; 6/10 showed binding capacity to SmD on ELISA. Inhibition experiments using ELISA confirmed the specificity of this binding. Our data indicate the cross-reactivity of spontaneously developed anti-ribosomal P protein antibodies with the B/B′ and D constituents of the Sm complex. The coexistence of anti-Sm and anti-ribosomal antibodies in lupus sera may therefore be due, at least in part, to the reactivity of a single autoantibody population.
Keywords: anti-ribosomal antibodies, systemic lupus erythematosus, autoantibodies, anti-Sm antibodies, anti-P antibodies
INTRODUCTION
Systemic lupus erythematosus (SLE) is characterized by the presence of various autoantibodies directed against a large number of intracellular antigens [1]. Among the antinuclear antibodies, anti-dsDNA (anti-double-stranded DNA) and anti-Sm antibodies are highly specific for the disease. Some autoantibodies are directed against cytoplasmic antigens, among which those antibodies directed against ribosomal P proteins show a high degree of specificity for lupus [2]. The main targets of anti-ribosomal antibodies are the three P proteins, P0, P1 and P2, with molecular weights of 38, 19 and 17 kD, respectively. They are located in the large ribosomal subunit and share the same C-terminal amino acid sequence. This sequence represents the dominant epitope in the anti-P protein response [3]. Thus, the reactivity of sera with the three P proteins is explained by the sharing of a sequence which forms the target of the autoantibodies.
Both in humans and in murine models of SLE, the anti-P protein antibodies appear more frequently in sera containing anti-Sm antibodies; conversely, anti-Sm antibodies are more often detected in sera containing anti-ribosomal antibodies [4]. However, the reasons for this association are unknown. We addressed this issue by analysing the specificity of affinity-purified anti-P antibodies and found that some of them cross-react with proteins forming the Sm complex.
MATERIALS AND METHODS
Sera from SLE patients
Ten sera containing anti-P antibodies from 10 different SLE patients were considered for analysis. SLE diagnosis was made according to ARA criteria [5]. Out of 10, seven reacted with Sm antigens either in counterimmunoelectrophoresis or in immunoblot on rabbit thymus. Three SLE sera containing anti-Sm antibodies but negative on P peptide were also tested.
ELISA for anti-P protein antibodies
Anti-P antibodies were detected by a previously described ELISA assay [6]. Briefly, we used a multiple antigen peptide (MAP) carrying four copies of the antigenic sequence shared by the three ribosomal P proteins (Glu-Glu-Glu-Asp-Glu-Asp-Met-Gly-Phe-Gly Leu-Phe-Asp) as antigen for the coating of plates (Nunc Maxisorp F96; Roskilde, Denmark) at 1 μg/ml in PBS. Saturation was carried out with gelatine 1% in PBS for 1 h at room temperature. The serum samples were diluted 1:300 in PBS–gelatine 0.5%–Tween 20 0.05%. Purified antibodies were diluted in the same medium and tested at different concentrations (20–0.3 μg/ml). The samples were incubated for 3 h at room temperature, then washed and anti-human IgG (Fab2 fragment) labelled with alkaline phosphatase was used as the second step antibody for 3 h at room temperature or overnight at 4°C. After a new washing step, plates were developed with freshly prepared p-nitrophenyl-phosphate substrate.
Purification of anti-P protein antibodies
Cyanogen bromide (CnBr)-activated Sepharose (Sigma Chemical Co., St Louis, MO) was conjugated with the ribosomal MAP (16 mg/g resin), and the residual active groups of the resin were blocked by glycine 0.2 m. Immunoglobulins from 10 sera containing anti-P antibodies and from three sera negative for anti-P antibodies were precipitated with ammonium sulphate.
The precipitates were dissolved in PBS buffer, dialysed, and applied to the column. For each serum the flow-through was collected, and the antibodies binding to the column were eluted by 0.1 m glycine buffer pH 2.8, neutralized and dialysed in PBS. The eluates containing anti-P antibodies were tested on ELISA and on immunoblot (IB) using ribosomal proteins purified as described elsewhere [6]. After each elution the column was washed with high salt acid and alkaline buffers, as recommended by the manufacturers.
ELISA for anti-SmD antibodies
A recombinant SmD protein (rSmD) [7] (a kind gift from Prof. van Venrooij) was used to coat polystyrene plates (Nunc Maxisorp F96) at 1 μg/ml in carbonate buffer. Plates were saturated with PBS–bovine serum albumin (BSA) 3% for 1 h at room temperature, and the samples were diluted in PBS–BSA 1%–Tween 0.05%. The samples were incubated for 3 h at room temperature and after washing, incubated for 3 h either at room temperature or overnight at 4°C with the detection antibody anti-human IgG (Fab2 fragment) labelled with alkaline phosphatase. After a new washing step, plates were developed with freshly prepared p-nitrophenyl-phosphate substrate.
Anti-SmD activity was also measured by an ELISA assay employing seven synthetic peptides of 20 amino acids each, overlapping by four amino acids, and corresponding to the whole length of SmD [8]. The peptides were used at 10 μg/ml in PBS for the coating, and the ELISA assay was carried out as described above for the recombinant SmD protein. When the C-terminal 95–117 peptide was used, 5% dry non-fat milk was substituted for BSA in the saturation and sera dilutions in order to reduce the background caused by the strong positive charge of this sequence.
All samples were tested in duplicate. One positive and one negative control were included in each plate. All samples were also tested for non-specific binding to saturated, non-antigen-coated plates. The absorbance obtained on non-coated plates, which was always < 5%, was subtracted from the results obtained for the coated plates.
Inhibition experiments
For the inhibition experiments, the amount of anti-P antibodies that gave 50% of the maximum binding on the solid phase was determined. This amount was then preincubated with different amounts of synthetic peptides or recombinant protein for 1 h at room temperature, before being transferred to antigen-coated plates. The assay was carried out as for the direct binding assay.
Immunoblot
Anti-P antibodies were tested on rSmD protein. The lysates of untransfected and transfected bacteria were dissolved in sample buffer containing SDS, 1 μg/mm2 was loaded, run on a 15% polyacrylamide gel [9] and then electrically transferred to a nitrocellulose sheet in Tris–glycine–methanol buffer [10].
Recombinant SmB/B′ fusion protein and its control [11] (a kind gift from Dr S. Hoch, Agouron Institute, La Jolla, CA), dissolved in sample buffer containing SDS, were run on a 10% polyacrylamide gel and then electrically blotted on a nitrocellulose sheet.
Anti-P antibodies were tested on rabbit thymus extract. Briefly, 100 mg of rabbit thymus (rabbit thymus acetone powder; Pel-Freeze) were dissolved in 2 ml of PBS with NaCl raised to 0.35 m and PMSF 2 mm and sonicated; it was centrifuged for 15 min at 7000 g in Microfuge, the supernatant was run on a 15% polyacrylamide gel and then electrically transferred to a nitrocellulose sheet in Tris–glycine–methanol buffer.
The same immunoblotting procedure was applied for all the antigens: nitrocellulose strips were cut, quenched by 5% non-fat milk in Tris-buffered saline (TBS) for 1 h at room temperature, then incubated with anti-P antibodies diluted in casein 2% in TBS at 25 μg/ml for 3 h at room temperature on a gentle rotating shaker. After extensive washing, the strips were incubated with anti-human IgG antibodies conjugated with alkaline phosphatase, and bound antibodies detected using 5-bromo-4-chloro-indoxyl- phosphate and nitroblue tetrazolium as substrate [12].
Immunofluorescence
Human mesangial cells grown on multiwell slides were fixed in 4% paraformaldehyde for 30 min and permeabilized with saponin 0.1%. After blocking with normal goat serum 1%, BSA 4%, saponin 0.1% in PBS, the cells were incubated with anti-P antibodies or control antibodies (50 μg/ml) for 3 h at room temperature. After three washings with PBS–saponin, the cells were stained with FITC-conjugated goat anti-human κ- and λ-chain antiserum for 90 min at room temperature. After three washings with PBS–saponin followed by three further washings with PBS, the cells were mounted in glycerol.
RESULTS
The polyclonal anti-P antibodies, affinity-purified from anti-P-positive lupus sera, were able to recognize the P protein C-terminal peptide on ELISA, and the P0, P1 and P2 proteins on immunoblot. In contrast, no binding to a Gly-Ala peptide corresponding to the repeats of the EBNA 1 molecule [13] was observed, thereby excluding the copurification of antibodies reactive with glycine-rich sequences. The flow-through of the ribosomal MAP column showed no anti-P activity (data not shown). No antibodies were eluted when immunoglobulins purified from Sm-positive, anti-P-negative sera were absorbed on the ribosomal MAP column.
The reactivity of anti-P antibodies with Sm antigens is presented in Table 1. Five out of 10 anti-P antibodies bound the SmD recombinant protein on immunoblot (Fig. 1). Among the five positive antibodies, three also bound rSmD on ELISA. Moreover, two antibodies that did not bind rSmD on immunoblot were positive on ELISA assay with the same protein.
Table 1.
Reactivity of purified anti-P antibodies with Sm antigens
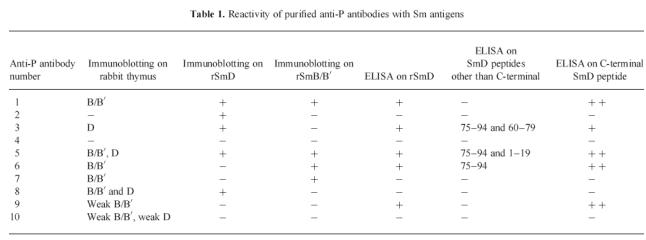
Fig. 1.

Binding of affinity-purified anti-P antibodies to rSmD protein. The lysates from bacteria expressing SmD protein (a) and from untransfected bacteria (b) were run on 15% SDS polyacrylamide gel and transferred to nitrocellulose. The filters were probed with purified anti-P antibodies. Anti-P antibodies in lanes 1–3 detected a band of 18 kD (arrow) corresponding to rSmD. Lane 4 shows anti-P antibodies that did not bind rSmD. No reactivity with the control lysate was observed.
When the antibodies were tested on the seven SmD peptides, five out of 10 showed a strong binding capacity for the SmD C-terminal peptide. Three of them also bound the 75–94 peptide. One of these latter bound also the 1–19 peptide, while another one bound the 60–79 peptide. However, binding to the N-terminal and internal sequences was always weaker than the binding capacity to the C-terminal peptide. The five antibodies that bound the SmD C-terminal peptide also bound the recombinant SmD on ELISA.
In order to evaluate the specificity of this binding, inhibition experiments were performed using two polyclonal anti-P antibodies that demonstrated binding capacity to SmD both on ELISA and on immunoblot. The results obtained with one of the two polyclonal anti-P antibodies are shown in Fig. 2. When the antibodies were preincubated with rSmD, binding to the solid-phase rSmD was inhibited (Fig. 2a). In contrast, preincubation with the ribosomal peptide did not result in more than 50% inhibition of this binding (Fig. 2b). Moreover, preincubation with the control multiple antigen peptide liver-kidney-microsome antigen (LKM) did not exert any inhibition, thereby excluding any reactivity of anti-P antibodies with MAP backbone. A similar inhibition experiment was performed using the C-terminal SmD peptide coated on the solid phase, and it was found that the binding of the anti-P antibodies was effectively inhibited by the same peptide and less effectively inhibited by the ribosomal MAP (Fig. 3a). Conversely, binding to solid-phase ribosomal MAP was fully inhibited by fluid-phase ribosomal MAP and less effectively by the SmD C-terminal peptide (Fig. 3b).
Fig. 2.
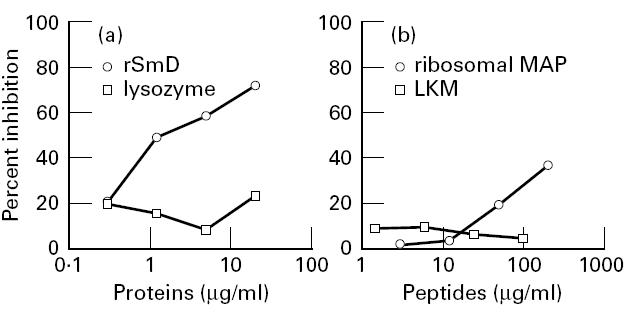
Inhibition of binding to recombinant SmD protein (rSmD). Anti-P antibodies were preincubated with rSmD (a) or ribosomal multiple antigen peptide (MAP) (b) or appropriate controls and were then transferred to rSmD-coated plates. Results are expressed as percentage inhibition: 100 − (100 × absorbance (antibody + peptide)/absorbance (antibody + buffer)).
Fig. 3.
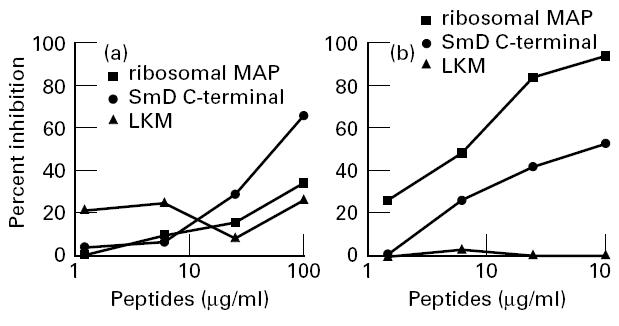
Inhibition of binding to the SmD C-terminal peptide (a) or to the ribosomal multiple antigen peptide (MAP) (b). Anti-P antibodies were preincubated with either ribosomal MAP, or the SmD C-terminal peptide, or a control peptide, and were then transferred to plates coated with SmD C-terminal peptide (a) or ribosomal MAP (b). Results are expressed as percentage inhibition: 100 − (100 × absorbance (antibody + peptide)/absorbance (antibody + buffer)).
The anti-P antibodies were tested by immunoblot on the recombinant SmB/B′ protein. Four out of 10 anti-P antibodies showed binding capacity toward the recombinant protein (Fig. 4). Two of these positive antibodies also bound the rSmD protein.
Fig. 4.
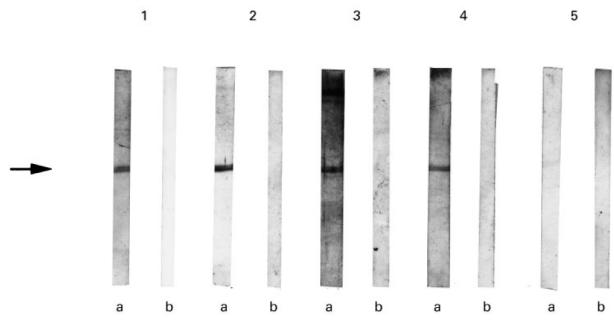
Binding of affinity-purified anti-P antibodies to the recombinant SmB/B′ protein. The lysates from bacteria expressing recombinant SmB/B′ protein (a) and from control bacteria (b) were run on 15% SDS polyacrylamide gel and were transferred to nitrocellulose. The filters were probed with purified anti-P antibodies. Anti-P antibodies in lanes 1–4 detected a band of 62 kD (arrow) corresponding to the recombinant SmB/B′. No reactivity with the control lysate was observed.
The anti-P antibodies were also tested by immunoblot on rabbit thymus. The B/B′ doublet was detected on thymus extract by all the antibodies reactive with the recombinant B/B′ and by three antibodies not reactive with the recombinant protein. Four anti-P antibodies detected the D protein; three of them were also reactive with blotted recombinant SmD (Table 1).
The reactivity with nuclear proteins was further evaluated by immunofluorescence. The two polyclonal anti-P antibodies that demonstrated binding capacity to SmD both on ELISA and on IB were tested on cultured human mesangial cells. The two antibodies stained both the cytoplasm (mainly the perinuclear area) of permeabilized cells and, although more weakly, the nucleus (data not shown).
DISCUSSION
Our results show that a certain proportion of anti-ribosomal antibodies can also bind the D and/or B/B′ proteins of the Sm complex. These conclusions are based on an analysis of the binding ability of affinity-purified anti-ribosomal antibodies to recombinant proteins of the Sm complex, to Sm proteins present in thymus extract and to synthetic peptides of SmD.
Four polyclonal anti-ribosomal antibodies bound recombinant SmB/B′ protein on immunoblot. Five out of 10 anti-P antibodies bound the rSmD protein on immunoblot and five on ELISA. The lack of full concordance between the results of the IB and the results of ELISA on rSmD was to be expected. IB is less sensitive than ELISA, so it is reasonable that some antibodies would be detected on ELISA but not by IB. On the other hand, the type and degree of unfolding of the protein may differ in the two procedures. Although in the transfer to the nitrocellulose sheet some proteins may be partially renatured, their conformation is less affected by coating to polystyrene plates than by gel separation and transfer. Thus, it is conceivable that the antibodies to linear epitopes may be better detected by IB.
The reactivity of anti-P antibodies with Sm proteins was confirmed by IB on rabbit thymus and by immunofluorescence on human mesangial cells. The anti-P antibodies that reacted with recombinant Sm proteins detected the homologous protein also in rabbit thymus, with one exception (eluate 2). In three cases (eluates 8, 9, 10), the anti-P antibodies reacted with B/B′ or D from rabbit thymus, but not with the recombinant proteins, suggesting that epitopes of the ‘natural’ antigen may be modified in the recombinant one.
The specificity of the SmD binding was verified by inhibition experiments on ELISA. These experiments showed that anti-ribosomal antibodies can bind non-denatured rSmD and SmD peptides. In cross-inhibition experiments, the ribosomal MAP only partially inhibited the binding of anti-P antibodies to rSmD, and only at high concentrations. This suggests that the binding sites for the SmD and ribosomal P proteins are only partially overlapped on the anti-P protein antibodies.
The existence of cross-reactions among the various components of the Sm/RNP complex is well known [14–17]. The B/B′ and D proteins do not share any sequence homology, but there are examples of monoclonal and human antibodies that bind both structures. The anti-Sm MoAb Y12 is able to recognize non-homologous epitopes on the B/B′ and D proteins [16]. Affinity-purified antibodies from human lupus sera have also been observed to bind both the B/B′ and D proteins [15].
Since cross-inhibition experiments were not performed, however, we could not determine whether the anti-ribosomal antibodies that bound both the SmD protein and the SmB/B′ protein contained an antibody with triple specificity or two populations of antibodies, one reacting with the SmD protein and the other reacting with the SmB/B′ protein.
The hypothesis of a cross-reactivity between ribosomal P proteins and the Sm antigen was first proposed in 1989, independently by two groups of researchers. Elkon et al. [4] analysed lupus sera and reported a higher frequency of anti-P protein antibodies in the anti-Sm-positive than in the anti-Sm-negative group. This same association was found in MRL/lpr mice, a murine model of lupus. Anti-Sm and anti-P antibodies had the same subclass distribution in lupus and in murine lupus. The existence of a cross-reactive epitope was analysed by ELISA in two murine sera containing both autoantibody specificities. The authors used an affinity-purified Sm antigen and a synthetic peptide corresponding to the ribosomal P protein C-terminal amino acid sequence. Pre-incubation of the sera with purified Sm antigen inhibited anti-Sm binding activity, but did not inhibit anti-P activity, while preincubation with the P peptide inhibited anti-P activity but did not inhibit anti-Sm activity. Based on these findings the authors excluded the presence of a cross-reactive epitope.
Instead of using purified anti-ribosomal antibodies, however, Elkon et al. used unfractionated serum and an affinity-purified Sm antigen, which represents a complex of Sm proteins. The possible presence in the serum of monospecific (e.g. not cross-reactive with ribosomal proteins) anti-Sm antibodies may have hampered the detection of the cross-reactive antibody population.
In the other study, Nojima et al. [18] affinity-purified autoantibodies directed against another acidic ribosomal protein, RP21, that apparently has no structural relationship with the P proteins. They observed that the anti-RP21 antibodies reacted on a blotted nuclear extract with proteins whose molecular weight was identical to that of the B/B′ and D of the Sm antigen. Moreover, two MoAbs, one directed against the SmD antigen and the other able to bind both the SmD and the SmB/B′ antigens, recognized RP21 on IB. On the basis of inhibition experiments, the authors concluded that there was a cross-reaction between protein antigens of the Sm complex and RP21.
In conclusion, our work demonstrates for the first time that purified anti-P antibodies can cross-react with either the SmD protein or with the SmB/B′ protein. Our results agree with previous observations showing that the autoantibodies developing in spontaneous autoimmune diseases often react with various autoantigens located in the cell or on the cellular surface. Since the C-terminal peptide of the ribosomal P proteins does not show any sequence homology with the SmD and SmB/B′ proteins, a conformational rather than a sequential determinant is probably involved in this cross-reactivity. Our results suggest that, at least in some cases, the presence of multiple specificities in the sera of patients with autoimmune diseases may be due to a limited number of cross-reactive antibody populations.
Acknowledgments
We are grateful to Dr S. Hoch (Agouron Institute, La Jolla, CA) for the SmB/B′ fusion protein, to Dr W. Van Venrooij (Department of Biochemistry, University of Nijmegen, The Netherlands) for the recombinant SmD protein, to Dr P. Rovero (CNR Institute of Mutagenesis, Pisa, Italy) for the synthesis of ribosomal MAP, and to Dr G. Carbone (Istituto di Istologia, Università di Genova, Italy) for performing immunofluorescence labelling.
References
- 1.Steinberg AD, Klinman DM. Pathogenesis of systemic lupus erythematosus. Rheum Dis North Am. 1988;14:25–41. [PubMed] [Google Scholar]
- 2.Elkon KB, Parnassa AP, Foster CL. Lupus autoantibodies target ribosomal P proteins. J Exp Med. 1985;162:459–71. doi: 10.1084/jem.162.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rich BE, Steitz JA. Human acidic ribosomal phosphoproteins P0, P1 and P2: analysis of cDNA clones, in vitro synthesis, and assembly. Mol Cell Biol. 1987;7:4065–74. doi: 10.1128/mcb.7.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elkon KB, Bonfa E, Lovet R, Eisenberg RA. Association between anti-Sm and anti-ribosomal P protein autoantibodies in human systemic lupus erythematosus and MRL/lpr mice. J Immunol. 1989;143:1549–54. [PubMed] [Google Scholar]
- 5.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 6.Caponi L, Pegoraro S, Di Bartolo V, Rovero P, Revoltella R, Bombardieri S. Autoantibodies directed against ribosomal P proteins: use of a multiple antigen peptide as the coating agent in ELISA. J Immunol Methods. 1995;179:193–202. doi: 10.1016/0022-1759(94)00285-5. [DOI] [PubMed] [Google Scholar]
- 7.Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 8.Sabbatini A, Dolcher MP, Marchini B, Bombardieri S, Migliorini P. Mapping of epitopes on the SmD molecule. The use of multiple antigen peptides to measure autoantibodies in systemic lupus erythematosus. J Rheumatol. 1993;20:1679–83. [PubMed] [Google Scholar]
- 9.Laemmli EK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 10.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrilamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rokeach LA, Jannatipour M, Hoch SO. Heterologous expression and epitope mapping of a human small nuclear ribonuclein-associated SmB′/B autoantigen. J Immunol. 1990;144:1015–22. [PubMed] [Google Scholar]
- 12.Blake MS, Johnston KH, Russell-Jones GJ, Gotschlich EC. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on western blots. Anal Biochem. 1984;136:175–91. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- 13.Baboonian C, Venables PJW, Williams DG, Williams RO, Maini RN. Cross reaction of antibodies to a glycine/alanine repeat sequence of Epstein–Barr virus nuclear antigen-1 with collagen, cytokeratine, and actine. Ann Rheum Dis. 1991;50:772–5. doi: 10.1136/ard.50.11.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habets WJ, Hoet MH, de Jong BAW, van der Kemp A, van Venrooij WJ. Mapping of B cell epitopes on small nuclear ribonuclear ribonucleoproteins that react with human autoantibodies as well as with experimentally induced mouse monoclonal antibodies. J Immunol. 1989;143:2560–6. [PubMed] [Google Scholar]
- 15.Habets W, Berden JHM, Hoch SO, Van Venrooij W. Further characterization and subcellular localization of Sm and U1 ribonucleoprotein antigens. Eur J Immunol. 1985;15:992–7. doi: 10.1002/eji.1830151006. [DOI] [PubMed] [Google Scholar]
- 16.Hirakata M, Craft J, Hardin JA. Autoantigenic epitopes of the B and D polypeptides of the U1 snRNP. J Immunol. 1993;150:3592–601. [PubMed] [Google Scholar]
- 17.Bloom DD, Davignon J-L, Retter MW, Schlomchik MJ, Pisetsky DS, Cohen PL, Eisenberg RA, Clarke SH. V region gene analysis of anti-Sm hybridomas from MRL/Mp-lpr/lpr mice. J Immunol. 1993;150:1591–610. [PubMed] [Google Scholar]
- 18.Nojima Y, Minota S, Yamada A, Takaku F. Identification of an acidic ribosomal protein reactive with anti-Sm autoantibody. J Immunol. 1989;143:1915–20. [PubMed] [Google Scholar]


