Abstract
In IBD, the target antigens of anti-neutrophil cytoplasmic autoantibodies (ANCA) have not been fully identified, which limits the analysis of the diagnostic significance as well as of the possible pathophysiological role of these antibodies. In this study, we identify the target antigens of ANCA in large groups of patients with ulcerative colitis (UC) and Crohn's disease (CD). Apart from antibodies against lactoferrin and bactericidal/permeability-increasing protein (BPI), which have been reported before, antibodies against two novel granulocyte antigens were identified: antibodies against a 57/56-kD doublet were found in 38% of samples from UC patients and in 26% of samples from CD patients, whereas antibodies against a 47-kD protein were found in 10% of samples from UC patients and in 18% of samples from CD patients. Partial purification and amino acid sequence analysis identified the 57-kD protein as catalase and the 47-kD protein as α-enolase. This study is the first to report catalase and α-enolase as granulocyte antigens for autoantibodies in IBD.
Keywords: anti-neutrophil cytoplasmic antibodies, inflammatory bowel disease, catalase, α-enolase
INTRODUCTION
Anti-neutrophil cytoplasmic antibodies (ANCA) are autoantibodies directed against constituents of neutrophil granulocytes and monocytes. These antibodies were originally described as markers for systemic vasculitis, in particular Wegener's granulomatosis (WG) [1]. Identification of the target antigens of ANCA, i.e. proteinase 3 (PR3) and myeloperoxidase (MPO), has increased the diagnostic significance of ANCA in these disorders [2]. In addition, several in vitro and in vivo studies have suggested a pathophysiological role for anti-PR3 and anti-MPO antibodies in the vasculitides [3].
ANCA have been shown to occur in many other inflammatory disorders as well, including IBD, autoimmune liver diseases, and rheumatoid arthritis (RA) [4]. In these diseases, the target antigens of ANCA have not been fully identified, which limits the diagnostic significance as well as the analysis of the possible pathophysiological role of these autoantibodies.
In IBD, various antigens are recognized by ANCA+ serum samples, of which lactoferrin [5,6] and bactericidal/permeability-increasing protein (BPI) [7,8] are presently most prominent. Other antigens for ANCA that have been reported in IBD are cathepsin G [9–12], lysozyme [12,13], and β-glucuronidase [14]. Recently, several nuclear non-granulocyte-specific antigens have been found to be recognized by ANCA+ serum samples from patients with ulcerative colitis (UC) [15]. Thus, ANCA in IBD apparently are not directed against one specific antigen.
To identify the target antigens of ANCA in IBD, we tested a large group of patients with UC and Crohn's disease (CD) for antibodies against granulocyte antigens on Western blot, using a crude extract of isolated granulocytes as the source of antigens. Apart from antibodies against lactoferrin and BPI, antibodies against two unidentified polypeptides were detected in considerable numbers of IBD samples. A 57/56-kD polypeptide was identified as catalase, and a 47-kD protein was identified as α-enolase. These antigens have not been reported before as target antigens for ANCA in IBD.
PATIENTS AND METHODS
Patients and controls
We studied plasma samples from 208 consecutive patients with IBD: 96 patients with UC and 112 patients with CD. The diagnosis of UC or CD was based on accepted clinical and endoscopic criteria supported by histopathology [16].
Control plasma samples were obtained from healthy blood bank donors and from patients with ANCA of known specificity as defined by immunoblotting and immunoprecipitation assays (data not shown). Plasma samples were frozen at −20 °C until use.
Indirect immunofluorescence
Detection of ANCA by indirect immunofluorescence (IIF) was performed on ethanol-fixed granulocytes as described before [1,17], with minor modifications [18]. Samples were diluted 1:20 in PBS and tested at two-fold dilutions up to 1:640. A FITC-conjugated rabbit anti-human IgG antibody (F315, dilution 1:100; Dakopatts, Copenhagen, Denmark) was used for detection of bound IgG. Slides for ANCA testing were always read by two independent observers. A titre ≥ 1:40 was considered positive.
Western blotting and immunodetection
Detection of ANCA was also performed by Western blotting followed by immunodetection. A crude extract of isolated granulocytes was used as the source of antigens. To prepare this crude extract, granulocytes were isolated from fresh buffy coats from healthy blood bank donors by density gradient centrifugation, dextran sedimentation, and hypotonic lysis of the remaining erythrocytes. Cells were disrupted by sonication for six periods of 20 s in 1 m NaCl containing 5 mm PMSF (Sigma Chemical Co., St Louis, MO; 2 × 108 cells/ml). Membrane fragments were removed by ultracentrifugation for 2 h at 100 000 g, and an equivalent of 6 × 107 cells was applied on 10% polyacrylamide gels under denaturing but non-reducing conditions. After electrophoresis and transfer to nitrocellulose membranes (Schleicher & Schuell, Keene, NH), the Western blots were incubated with 3% horse serum (CLB, Amsterdam, The Netherlands) to block aspecific reactions. Patient samples were tested at a 1:50 dilution. Immunodetection was performed as described previously [19]. To detect bound IgG, a peroxidase-conjugated rabbit anti-human IgG antibody (P214, dilution 1:250; Dakopatts), a peroxidase-conjugated swine anti-rabbit IgG antibody (P217, dilution 1:250; Dakopatts), or a peroxidase-conjugated rabbit anti-mouse IgG antibody (P260, dilution 1:250; Dakopatts) were used. Molecular weight standards (BioRad Labs, Richmond, CA) were used as references.
To identify the protein bands corresponding to the antigens, lactoferrin, BPI, cathepsin G, PR3, MPO, elastase, catalase, and α-enolase, we used polyclonal and monoclonal antibodies directed against these antigens. Polyclonal rabbit anti-human lactoferrin and rabbit anti-human MPO were purchased from Dakopatts. Polyclonal rabbit anti-human cathepsin G, rabbit anti-human elastase, and rabbit anti-human erythrocyte catalase were purchased from Calbiochem (La Jolla, CA). Monoclonal anti-human PR3 (12.8) was purchased from CLB. MoAb against human BPI 6C2 [20] was a kind gift from Dr J. Meenan (Academic Medical Centre, Amsterdam, The Netherlands). Polyclonal rabbit anti-human BPI [21] was a kind gift from Dr W. A. Buurman and Dr M. A. Dentener (University Hospital Maastricht, The Netherlands). Rabbit anti-human granulocyte catalase [22] was a kind gift from Professor I. Olsson (University Hospital Lund, Sweden). Monoclonal anti-U937 α-enolase [23] was a kind gift from Dr T. Burke (Centre for Thrombosis and Vascular Biology, Cleveland, OH).
ELISAs
ELISAs were performed to confirm the presence of antibodies against lactoferrin and BPI. Lactoferrin (Sigma) was coated at 5 μg/ml in PBS pH 7.4. BPI was isolated from granulocytes accord-ing to Zhao et al. [24] and coated at 1 μg/ml in PBS pH 7.4. Patient samples were applied at a dilution of 1:100 and in two-fold dilutions up to 1:800. Alkaline phosphatase-conjugated goat anti-human IgG (dilution 1:1500; American Qualex, San Clemente, CA) was used for detection of bound IgG in both ELISAs.
A sample was considered positive if the optical density (OD) value obtained exceeded the mean of 25 healthy control samples by > 2 s.d.
Further purification and identification of the 56/57-kD and 47-kD proteins
By immunodetection on Western blot with a crude granulocyte extract as the source of antigens, we demonstrated autoantibodies against two unidentified novel antigens, polypeptides of 57/56 kD and 47 kD. To characterize these polypeptides, granulocytes were isolated from fresh buffy coats and were disrupted by sonication in 1 m NaCl. The disrupted cells were dialysed against 0.01 m phosphate buffer containing 0.075 m NaCl pH 7.4, and were applied to a cation exchange column (Mono-S HR10/10; Pharmacia, Uppsala, Sweden). The unbound fraction contained the 57/56-kD proteins and the 47-kD protein, as confirmed by immunodetection on Western blot with plasma from several patients with IBD. This unbound fraction was applied to an anion exchange column (Mono-Q HR10/10; Pharmacia) in 0.01 m phosphate buffer containing 0.075 m NaCl pH 7.4. The unbound fraction obtained from this second column was strongly enriched for the 57/56-kD and the 47-kD polypeptides, as confirmed by immunodetection on Western blot.
The enriched unbound fraction was subjected to SDS–PAGE on a 12% polyacrylamide gel under reducing conditions. Following electrophoresis the gel was stained with coomassie brilliant blue or was blotted onto PVDF membrane (Immobilon-P; Millipore, Etten-Leur, The Netherlands) as described by Matsudaira [25]. The 57-kD and 47-kD proteins were cut out from the gel or the blot. N-terminal and internal amino acid sequence analyses of the proteins were performed by Eurosequence (Groningen, The Netherlands). N-terminal amino acid sequence analysis was performed with an Applied Biosystems (Warrington, UK) model 477A protein sequencer (pulse-liquid sequenator), on-line connected with a 120 A PTH analyser [26]. Internal amino acid sequence analysis was performed essentially as described by Rosenfeld et al. [27].
RESULTS
Indirect immunofluorescence
ANCA were detected by IIF on ethanol-fixed granulocytes in 56 of 96 UC samples (58%) and in 21 of 112 CD samples (19%). Various staining patterns were observed. Of the 56 UC samples, 52 showed perinuclear staining (P-ANCA), whereas four samples showed an atypical cytoplasmic staining pattern different from the classical C-ANCA pattern specific for anti-PR3 antibodies (C-ANCA). Of the 21 CD samples, 19 showed a P-ANCA pattern, whereas two samples showed atypical cytoplasmic staining.
The median titre for the ANCA+ samples in UC was 1:160, while the median titre for the ANCA+ samples in CD was 1:80. Titres ranged from 1:40 up to 1:640.
Western blotting and immunodetection
By immunodetection on Western blot, we first tested whether the IBD samples contained antibodies to six well defined ANCA antigens. For this test, the protein bands corresponding to PR3, MPO, elastase, cathepsin G, lactoferrin, and BPI were identified by using polyclonal and monoclonal antibodies. In the IBD samples, no antibodies against PR3, MPO, elastase, or cathepsin G were detected. However, 25 (26%) UC samples and 12 (11%) CD samples reacted with the 78–80-kD bands that were identified as lactoferrin, and two (2%) UC samples and six (5%) CD samples reacted with the 55-kD doublet that was identified as BPI (Fig. 1,Table 1).
Fig. 1.
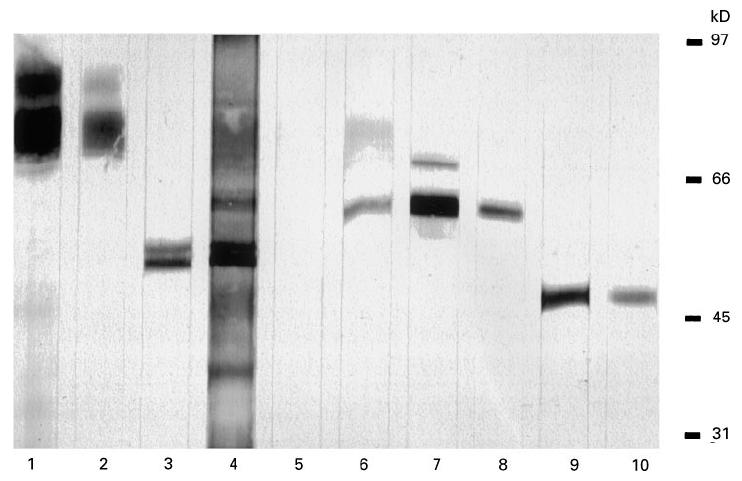
Immunodetection on Western blot using a crude granulocyte extract as antigenic substrate. Lane 1, polyclonal antibody against lactoferrin; lane 2, patient sample reacting with lactoferrin; lane 3, polyclonal antibody against bactericidal/permeability-increasing protein (BPI); lane 4, patient sample reacting with BPI; lane 5, healthy control sample; lane 6, polyclonal antibody against erythrocyte catalase; lane 7, polyclonal antibody against granulocyte catalase; lane 8, patient sample reacting with the 57/56-kD antigen; lane 9, MoAb against U937 enolase; lane 10, patient sample reacting with the 47-kD antigen.
Table 1.
Reactivity of samples from IBD patients and healthy controls with neutrophil proteins as demonstrated by immunodetection on Western blot (some samples were simultaneously positive for more than one antigen)
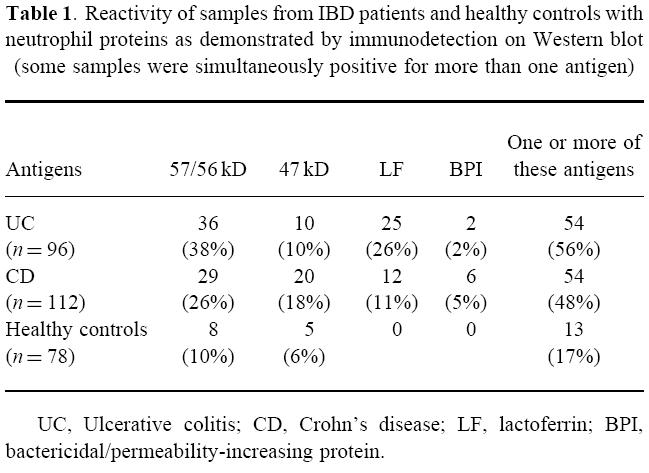
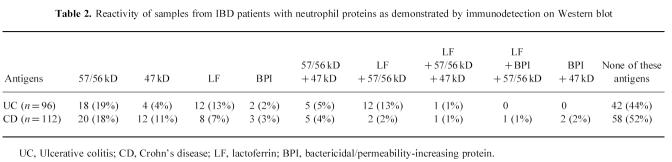
In addition, we tested whether the IBD samples reacted with unidentified protein bands. A considerable number of samples contained antibodies to two novel autoantigens. Thirty-six (38%) UC samples and 29 (26%) CD samples reacted with a polypeptide of 57/56 kD. Ten (10%) UC samples and 20 (18%) CD samples reacted with a polypeptide of 47 kD (Fig. 1,Table 1). Frequently, a single sample contained antibodies to more than one antigen (Table 2).
Table 2.
Reactivity of samples from IBD patients with neutrophil proteins as demonstrated by immunodetection on Western blot
We compared the data obtained by immunodetection with the data obtained by IIF for the two novel antigens. From the samples reacting with the 57/56-kD antigen, 18 of the 36 UC samples and five of the 29 CD samples were positive for ANCA by IIF. As shown in Table 2, 18 UC samples and 20 CD samples reacted only with the 57/56-kD antigen, and not with lactoferrin, BPI, or the 47-kD antigen. Of these 38 monospecific samples, seven samples showed perinuclear staining, one sample showed atypical cytoplasmic staining, and 30 samples were negative by IIF.
From the samples reacting with the 47-kD antigen, five of the 10 UC samples and five of the 20 CD samples were positive for ANCA by IIF. As shown in Table 2, four UC samples and 12 CD samples reacted only with the 47-kD antigen and not with lactoferrin, BPI, or the 57/56-kD antigen. Of these 16 monospecific samples, six samples showed perinuclear staining and 10 samples were negative by IIF.
Healthy donor samples showed reactivity by immunodetection on Western blot in a few cases. Of the 78 control samples tested, eight (10%) reacted with the 57/56-kD doublet, and five (6%) reacted with the 47-kD protein (Table 1). All these samples were negative for ANCA by IIF.
ELISAs
The presence of antibodies against lactoferrin and BPI was confirmed by ELISA. Nineteen of the 25 UC samples and 10 of the 12 CD samples reacting with the 78–80-kD lactoferrin protein bands on Western blot contained antibodies against lactoferrin as measured by ELISA. In addition, both of the UC samples and four of the six CD samples reacting with the 55-kD BPI doublet on Western blot contained antibodies against BPI as measured by ELISA.
Further purification and identification of the 57/56-kD and 47-kD proteins
In an attempt to purify the 57/56-kD and 47-kD polypeptides, we isolated granulocytes from fresh buffy coats and disrupted them by sonication in 1 m NaCl. By successive cationic and anionic exchange chromatography of the crude granulocyte extract, we obtained a fraction that was strongly enriched for the 57/56-kD and 47-kD polypeptides, as confirmed by immunodetection on Western blot with samples from several patients (Fig. 2).
Fig. 2.
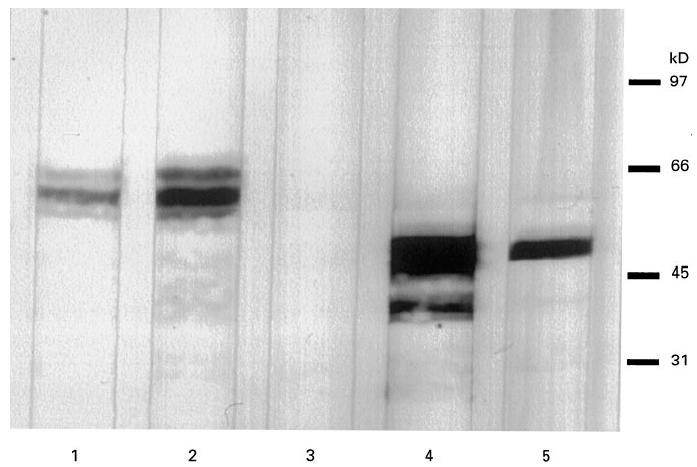
Immunodetection on Western blot using a partially purified extract of granulocytes. Lane 1, polyclonal antibody against granulocyte catalase; lane 2, patient sample reacting with the 57/56-kD antigen; lane 3, healthy control sample; lane 4, MoAb against U937 enolase; lane 5, patient sample reacting with the 47-kD antigen.
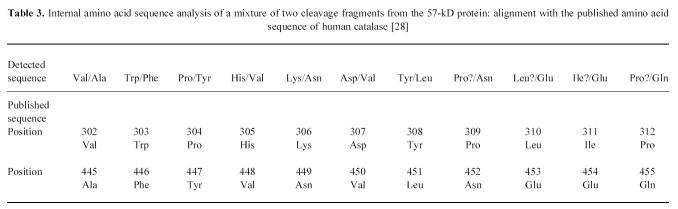
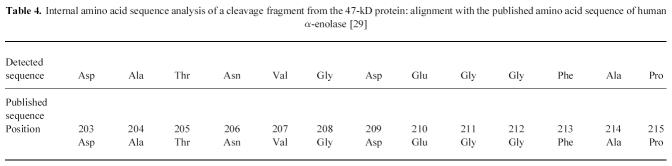
To determine the identity of the novel antigens, amino acid sequence analysis was performed. Direct amino acid sequence analysis of both the 57-kD and the 47-kD polypeptides on PVDF proved that the proteins were blocked at the N-terminus. Therefore, internal amino acid sequence analysis was performed on the protein from the SDS–PAGE gel. For the 57-kD protein, two fragments showed complete homology with peptides from human catalase (Table 3 [28]). For the 47-kD protein, a fragment showed complete homology with a peptide from human α-enolase (Table 4 [29]). The identity of the two novel antigens was confirmed by immunodetection on Western blot. Both a rabbit polyclonal antibody against human erythrocyte catalase and a rabbit polyclonal antibody against human granulocyte catalase reacted with the 57/56-kD doublet recognized by the IBD samples in the crude granulocyte extract (Fig. 1) and in the enriched fraction (Fig. 2). In addition, a MoAb against U937 α-enolase reacted with the 47-kD protein recognized by the IBD samples in the crude extract (Fig. 1) and in the enriched fraction (Fig. 2).
Table 3.
Internal amino acid sequence analysis of a mixture of two cleavage fragments from the 57-kD protein: alignment with the published amino acid sequence of human catalase [28]
Table 4.
Internal amino acid sequence analysis of a cleavage fragment from the 47-kD protein: alignment with the published amino acid sequence of human α-enolase [29]
DISCUSSION
In this study, we describe for the first time the presence of autoantibodies against human granulocyte catalase and α-enolase in plasma samples from patients with IBD.
Autoantibodies to granulocyte antigens (ANCA) have been observed in serum samples from 50–80% of patients with UC and 10–40% of patients with CD [30]. Various target antigens have been described for these ANCA, particularly lactoferrin [5,6] and BPI [7,8]. Cathepsin G [9–12], lysozyme [12,13], and β-glucuronidase [14] have also been reported as target antigens, although these results were not confirmed by others [6,31]. Recent results showed that nuclear antigens are recognized as well by P-ANCA+ serum samples from UC patients [15].
Our data confirm the presence of antibodies against lactoferrin and BPI in plasma samples from patients with IBD. Antibodies against the well defined ANCA antigens PR3, MPO, elastase, and cathepsin G were not detected in IBD samples by immunodetection on Western blot using a crude granulocyte extract as the source of antigens. However, by immunodetection on Western blot we detected antibodies against two novel granulocyte antigens. A 57/56-kD doublet was recognized by 38% of UC samples and 26% of CD samples, whereas a 47-kD protein was recognized by 10% of UC samples and 18% of CD samples. Antibody reactivity to these novel antigens was observed previously by Mulder and coworkers from our group in samples from patients with IBD [6], primary sclerosing cholangitis, autoimmune hepatitis, primary biliary cirrhosis [19], and RA [32]. Erroneously, the 57/56-kD doublet was calculated as 67/66 kD, whereas the 47-kD protein was calculated as 63 kD.
We were able to identify the 57-kD protein as being catalase and the 47-kD protein as being α-enolase. As far as we know, autoantibodies against catalase have not been demonstrated before. Autoantibodies against α-enolase have been demonstrated by Moodie and coworkers [33] in serum samples from patients with vasculitis and systemic lupus erythematosus, but not in IBD.
Catalase [34,35] is a tetrameric molecule of ≈ 240 kD, composed of four identical subunits of ≈ 60 kD. The enzyme catalyses the dissociation of hydrogen peroxide to water and oxygen, thus protecting cytoplasmic structures from the noxious action of hydrogen peroxide. The enzyme is found in virtually all aerobic cells. In mature granulocytes, the enzyme is present in the cytosol [36].
Enolase [37] is a glycolytic enzyme composed of two identical subunits of ≈ 47 kD. It exists as three highly homologous isozymes, α, β and γ; isozyme α is found in most tissues, isozyme β in muscle, and isozyme γ is present mainly in the cytosol of neuronal and neuroendocrine cells.
ANCA with specificity for PR3 and MPO are likely to play a role in the pathophysiology of the disorders associated with these autoantibodies. In vitro, priming of neutrophil granulocytes with tumour necrosis factor-alpha (TNF-α) leads to expression of these antigens on the cellular surface. ANCA are then able to bind their target antigens and thus to stimulate granulocyte degranulation and oxygen radical production [38–40]. In addition, it has been shown that antibodies against PR3 and MPO are able to interfere with physiological functions of their target antigens [41,42]. Finally, animal models have also suggested a role for these antibodies in vivo [43].
Whether these pathophysiological mechanisms are also valid for antibodies against catalase and α-enolase has to be investigated. It is unknown whether cytokine priming leads to expression of the cytosolic proteins catalase and α-enolase on the cellular surface, and whether antibodies to catalase and α-enolase are able to interfere with physiological functions of their target antigens.
Interestingly, when comparing data obtained by IIF with data obtained by immunodetection on Western blot, we found that a considerable number of samples negative by IIF were positive by immunodetection on Western blot. Only 21% of the IBD samples positive for antibodies against catalase and negative for antibodies against lactoferrin, BPI, or α-enolase were positive by IIF. Similarly, only 38% of the IBD samples positive for antibodies against α-enolase and negative for antibodies against lactoferrin, BPI, or catalase were positive by IIF.
This large discrepancy between the two detection methods was not observed for antibodies against other ANCA antigens. This may be due to the difference in cellular localization of the target antigens. The ANCA antigens PR3, MPO, elastase, BPI, cathepsin G and lactoferrin are located in the granules of neutrophils and monocytes. However, both catalase and α-enolase are cytosolic proteins from granulocytes. The fact that antibodies to granule proteins show reactivity by IIF on granulocytes, whereas antibodies to cytosolic proteins, like catalase and α-enolase, show no reactivity, may be due to the relative concentration of the various antigens within granulcoytes. As Moodie and coworkers state in their discussion [33], the ANCA antigens that were originally reported (MPO, PR3, elastase and lactoferrin) are present at high concentration within the total cell volume (1–5% of total cellular protein) and are packaged in even more concentrated form in the granules. Abundant, concentrated granule proteins would be much more likely to fix sufficient antibody for visualization by IIF than more dispersed, dilute cytosolic proteins.
In the systemic vasculitides, ANCA of defined specificities, i.e. antibodies against PR3 and MPO, are important diagnostic and prognostic markers [2]. Specific antibodies are associated with particular clinical syndromes. In IBD, a variety of autoantibodies against granulocyte proteins can be detected, frequently simultaneously in one patient. As we report here, besides antibodies against lactoferrin and BPI, antibodies against catalase and α-enolase are commonly found in patients with both UC and CD. Catalase even appears to be the major antigen in IBD, as has been demonstrated in this study. Further research should address the clinical and pathophysiological roles of these autoantibodies in IBD and other idiopathic inflammatory disorders.
Acknowledgments
This study was financially supported by the Dutch Organisation for Scientific Research NWO and the Dutch Society for Immunology NVVI. We want to thank Dr H. Bak and Dr W. J. Weijer (Eurosequence, Groningen, The Netherlands) for performing amino acid sequence analysis.
References
- 1.Van der Woude FJ, Lobatto S, Permin H, et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985;i:425–9. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]
- 2.Kallenberg CGM, Brouwer E, Weening JJ, Cohen Tervaert JW. Anti-neutrophil cytoplasmic antibodies: current diagnostic and pathophysiological potential. Kidney Int. 1994;46:1–15. doi: 10.1038/ki.1994.239. [DOI] [PubMed] [Google Scholar]
- 3.Kallenberg CGM, Brouwer E, Mulder AHL, et al. ANCA—pathophysiology revisited. Clin Exp Immunol. 1995;100:1–3. doi: 10.1111/j.1365-2249.1995.tb03594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kallenberg CGM, Mulder AHL, Cohen Tervaert JW. Antineutrophil cytoplasmic antibodies: a still-growing class of autoantibodies in inflammatory disorders. Am J Med. 1992;93:675–82. doi: 10.1016/0002-9343(92)90202-m. [DOI] [PubMed] [Google Scholar]
- 5.Peen E, Almer S, Bodemar G, et al. Anti-lactoferrin antibodies and other types of ANCA in ulcerative colitis, primary sclerosing cholangitis, and Crohn's disease. Gut. 1993;34:56–62. doi: 10.1136/gut.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulder AHL, Broekroelofs J, Horst G, et al. Anti-neutrophil cytoplasmic antibodies (ANCA) in inflammatory bowel disease: characterization and clinical correlates. Clin Exp Immunol. 1994;95:490–7. doi: 10.1111/j.1365-2249.1994.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoffel MP, Csernok E, Herzberg C, et al. Anti-neutrophil cytoplasmic antibodies (ANCA) directed against bactericidal/permeability increasing protein (BPI): a new seromarker for inflammatory bowel disease and associated disorders. Clin Exp Immunol. 1996;104:54–59. doi: 10.1046/j.1365-2249.1996.d01-654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walmsley RS, Zhao MH, Hamilton ML, et al. Anti-neutrophil cytoplasm autoantibodies against bactericidal/permeability-increasing protein in inflammatory bowel disease. Gut. 1997;40:105–9. doi: 10.1136/gut.40.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayet WJ, Hermann E, Finsterwalder J, et al. Antibodies to cathepsin G in Crohn's disease. Eur J Clin Invest. 1992;22:427–33. doi: 10.1111/j.1365-2362.1992.tb01485.x. [DOI] [PubMed] [Google Scholar]
- 10.Lesavre P, Nusbaum P, Halbwachs-Mecarelli L. Methods of detection of anticathepsin G autoantibodies in human. Adv Exp Med Biol. 1993;336:257–61. [PubMed] [Google Scholar]
- 11.Sobajima J, Ozaki S, Okazaki T, et al. Anti-neutrophil cytoplasmic antibodies (ANCA) in ulcerative colitis: anti-cathepsin G and a novel antibody correlate with a refractory type. Clin Exp Immunol. 1996;105:120–4. doi: 10.1046/j.1365-2249.1996.d01-724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kossa K, Coulthart A, Ives CT, et al. Antigen specificity of circulating anti-neutrophil cytoplasmic antibodies in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1995;7:783–9. [PubMed] [Google Scholar]
- 13.Schmitt WH, Csernok E, Flesch BK, et al. Autoantibodies directed against lysozyme: a new target antigen for anti-neutrophil cytoplasmic antibodies (ANCA) Adv Exp Med Biol. 1993;336:267–72. doi: 10.1007/978-1-4757-9182-2_40. [DOI] [PubMed] [Google Scholar]
- 14.Nässberger L, Ljungh A, Schumacher G, Kollberg B. β-Glucoronidase antibodies in ulcerative colitis. Lancet. 1992;340:734–5. doi: 10.1016/0140-6736(92)92279-o. [DOI] [PubMed] [Google Scholar]
- 15.Sobajima J, Ozaki S, Osakada F, et al. Novel autoantigens of perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCA) in ulcerative colitis: non-histone chromosomal proteins, HMG1 and HMG2. Clin Exp Immunol. 1997;107:135–40. doi: 10.1046/j.1365-2249.1997.d01-907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Morain C, Tobin A, Leen E, et al. Criteria of case definition in Crohn's disease and ulcerative colitis. Scand J Gastroenterol. 1989;24:7–11. doi: 10.3109/00365528909091340. [DOI] [PubMed] [Google Scholar]
- 17.Wiik A. Delineation of a standard procedure for indirect immunofluorescence detection of ANCA. APMIS. 1989;97(Suppl. 6):12–13. [PubMed] [Google Scholar]
- 18.Van der Giessen M, Huitema MG, Cohen Tervaert JW, Kallenberg CGM. A technical note on the routinely performed ANCA detection. APMIS. 1989;97(Suppl. 6):37. [Google Scholar]
- 19.Mulder AHL, Horst G, Haagsma EB, et al. Prevalence and characterization of neutrophil cytoplasmic antibodies in autoimmune liver diseases. Hepatol. 1993;17:411–7. [PubMed] [Google Scholar]
- 20.Monajemi H, Meenan J, Lamping R, et al. Inflammatory bowel disease is associated with increased mucosal levels of bactericidal/permeability-increasing protein. Gastroenterol. 1996;110:733–9. doi: 10.1053/gast.1996.v110.pm8608882. [DOI] [PubMed] [Google Scholar]
- 21.Dentener MA, Francot GJM, Smit FT, et al. Presence of bactericidal/permeability-increasing protein in disease: detection by ELISA. J Infect Dis. 1995;171:739–43. doi: 10.1093/infdis/171.3.739. [DOI] [PubMed] [Google Scholar]
- 22.Olofsson T, Olsson I. Purification of human granulocyte catalase in chronic myeloid leukemia. Biochim Biophys Acta. 1977;482:301–8. doi: 10.1016/0005-2744(77)90243-1. [DOI] [PubMed] [Google Scholar]
- 23.Redlitz A, Fowler BJ, Plow EF, Miles LA. The role of an enolase-related molecule in plasminogen binding to cells. Eur J Biochem. 1995;227:407–15. doi: 10.1111/j.1432-1033.1995.tb20403.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhao MH, Jones SJ, Lockwood CM. Bactericidal/permeability-increasing protein (BPI) is an important antigen for anti-neutrophil cytoplasmic autoantibodies (ANCA) in vasculitis. Clin Exp Immunol. 1995;99:49–56. doi: 10.1111/j.1365-2249.1995.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–8. [PubMed] [Google Scholar]
- 26.Hewick RM, Hunkapiller MW, Hood LE, Dreyer WJ. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981;356:7990–7. [PubMed] [Google Scholar]
- 27.Rosenfeld J, Capdevielle J, Guillemot JC, Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem. 1992;203:173–9. doi: 10.1016/0003-2697(92)90061-b. [DOI] [PubMed] [Google Scholar]
- 28.Quan F, Korneluk RG, Tropak MB, Gravel RA. Isolation and characterization of the human catalase gene. Nucl Acids Res. 1986;14:5321–35. doi: 10.1093/nar/14.13.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giallongo A, Feo A, Moore R, et al. Molecular cloning and nucleotide sequence of a full-length cDNA for human α enolase. Proc Natl Acad Sci USA. 1986;83:6741–5. doi: 10.1073/pnas.83.18.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shanahan F. Neutrophil autoantibodies in inflammatory bowel disease: are they important? Gastroenterol. 1994;107:586–9. doi: 10.1016/0016-5085(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 31.Kaneko K, Suzuki Y, Yamashiro Y, Yabuta K. Is p-ANCA in ulcerative colitis directed against β-glucuronidase? Lancet. 1993;341:320. doi: 10.1016/0140-6736(93)92686-n. [DOI] [PubMed] [Google Scholar]
- 32.Mulder AHL, Horst G, Van Leeuwen MA, et al. Anti-neutrophil cytoplasmic antibodies in rheumatoid arthritis: characterization and clinical correlates. Arthritis Rheum. 1993;36:1054–60. doi: 10.1002/art.1780360805. [DOI] [PubMed] [Google Scholar]
- 33.Moodie FDL, Leaker B, Cambridge G, et al. Alpha-enolase: a novel cytosolic autoantigen in ANCA positive vasculitis. Kidney Int. 1993;43:675–81. doi: 10.1038/ki.1993.97. [DOI] [PubMed] [Google Scholar]
- 34.Deisseroth A, Dounce AL. Catalase: physical and chemical properties, mechanism of catalysis, and physiological role. Physiol Rev. 1970;50:319–75. doi: 10.1152/physrev.1970.50.3.319. [DOI] [PubMed] [Google Scholar]
- 35.Schonbaum GR, Chance B. Catalase. In: Boyer P, editor. The enzymes. 3. XIII. New York: Academic Press; 1976. pp. 363–408. [Google Scholar]
- 36.Ballinger CA, Mendis-Handagama SMLC, Kalmar JR, et al. Changes in the localization of catalase during differentiation of neutrophilic granulocytes. Blood. 1994;83:2654–68. [PubMed] [Google Scholar]
- 37.Wold F. Enolase. In: Boyer P, editor. The enzymes. 3. V. New York: Academic Press; 1971. pp. 499–537. [Google Scholar]
- 38.Falk RJ, Terrell RS, Charles LA, Jennette C. Anti-neutrophil cytoplasmic antibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–9. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulder AHL, Heeringa P, Brouwer E, et al. Activation of granulocytes by anti-neutrophil cytoplasmic antibodies: a FcγRII-dependent process. Clin Exp Immunol. 1994;98:270–8. doi: 10.1111/j.1365-2249.1994.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulder AHL, Stegeman CA, Kallenberg CGM. Activation of granulocytes by anti-neutrophil cytoplasmic antibodies (ANCA) in Wegener's granulomatosis: a predominant role for the IgG3 subclass of ANCA. Clin Exp Immunol. 1995;101:227–32. doi: 10.1111/j.1365-2249.1995.tb08343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van de Wiel BA, Dolman KM, Van der Meer-Gerritsen CH, et al. Interference of Wegener's granulomatosis autoantibodies with neutrophil proteinase 3 activity. Clin Exp Immunol. 1992;90:409–14. doi: 10.1111/j.1365-2249.1992.tb05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffin SV, Lockwood CM. Anti-myeloperoxidase (MPO) antibodies interfere with the inhibition of MPO by caeruloplasmin. The Immunologist. 1997;5(Suppl. 1):94. (Abstr.). [Google Scholar]
- 43.Heeringa P, Brouwer E, Cohen Tervaert JW, et al. Animal models of anti-neutrophil cytoplasmic antibody associated vasculitis. Kidney Int. in press. [DOI] [PubMed]