Abstract
A number of cell types situated along interfaces of various tissues and organs such as the peritoneum and the intestine have been shown to secrete inflammatory cytokines in a polarized fashion. Retinal pigment epithelial (RPE) cells are positioned at the interface between the vascularized choroid and the avascular retina, forming part of the blood–retina barrier. These cells are potent producers of inflammatory cytokines and are therefore considered to play an important role in the pathogenesis of ocular inflammation. Whether cytokine secretion by these cells also follows a vectorial pattern is not yet known, and was therefore the subject of this study. Monolayers of human RPE cells (primary cultures and the ARPE-19 cell line) cultured on transwell filters were stimulated to produce IL-6 and IL-8 by adding IL-1β (100 U/ml) to either the upper or the lower compartment. After stimulation, the human RPE cell lines showed polarized secretion of IL-6 and IL-8 towards the basal side, irrespective of the side of stimulation. The ARPE-19 cell line also secreted IL-6 and IL-8 in a polarized fashion towards the basal side after basal stimulation; polarized secretion was, however, not apparent after apical stimulation. The observation that human RPE cells secrete IL-6 and IL-8 in a polarized fashion towards the choroid may represent a mechanism to prevent damage to the adjacent fragile retinal tissue.
Keywords: retinal pigment epithelium, polarized secretion, cytokine, IL-6, IL-8
INTRODUCTION
The retinal pigment epithelium (RPE), strategically located between the photoreceptor layer and the vascularized choroid, plays an important role in maintaining homeostasis in the outer retina. It regulates the transport of nutrients to the photoreceptors, phagocytoses old rod outer segments and absorbs stray light. As part of the blood–retina barrier, i.e. the outer blood–retina barrier [1], the RPE cell monolayer limits access of blood components to the retina. In addition to these functions, the RPE is thought to play an important role in immune responses. This is suggested by the ability of RPE cells to express cytokines [2], HLA class II antigens [3,4], adhesion molecules [5,6] and FasL [7]. In vitro, RPE cells have been shown to produce IL-1 [8,9], IL-6 [10–13], IL-8 [13,14], monocyte chemotactic protein-1 (MCP-1) [15], granulocyte-macrophage colony-stimulating factor (GM-CSF) [9] and transforming growth factor-beta 2 (TGF-β2) [16] when simulated with IL-1β, which is a proinflammatory cytokine produced early in the immune response [17], or other cytokines.
The eye is an immune privileged site where, in view of its essential function and fragility, immunological processes must be strictly regulated. Although leucocyte recruitment is essential for host defence to clear infectious agents, a vigorous inflammatory response can lead to local damage of host tissues and even to impaired organ function. One way to achieve the immune privileged status is by the intraocular production of immunosuppressive or anti-inflammatory cytokines, such as TGF-β2 [18,19]. Furthermore, the eye is separated from the rest of the body by the blood–retina barrier forming a physical barrier for immune cells. Another mechanism by which the RPE could contribute to such a highly regulated immune response could be the secretion of (pro)inflammatory cytokines, preferentially in the direction of the choroid, thereby reducing the potential damage of the neurosensory retina during inflammation. Although numerous investigators have shown that cultured RPE cells can readily be induced to secrete proinflammatory cytokines [2,8–15], a possible polarized production of these factors has not been reported and therefore was the subject of our study. As a model we investigated the secretion of two different cytokines, IL-6 and IL-8. IL-8 is a chemoattractant for neutrophils and stimulates diapedesis of T cells [20]. Recent reports show polarized secretion of IL-8 by human intestinal [21,22] and human mesothelium [23] cells. IL-6 is an important proinflammatory cytokine known to be involved in intraocular inflammation [24–26]. Polarized secretion of IL-6 has been reported for mouse uterine epithelial cells [27].
Polarized secretion of the inflammatory cytokines IL-6 and IL-8 was investigated with monolayers of human RPE cells on transwell filters. RPE cells were stimulated at the apical or the basal side with IL-1β and the secretion of IL-6 and IL-8 was measured. The data presented in this study show that RPE cells are indeed capable of polarized secretion of cytokines after stimulation with IL-1β. Furthermore, we show that the ARPE-19 cell line reacts differently compared with the primary cultures of RPE cells.
MATERIALS AND METHODS
RPE cell cultures
Human donor eyes (age of the donors 12–23 years) obtained from the eye bank were used as a source of primary RPE cells. These RPE cells (further designated as ‘RPE cell lines’) were isolated within 24 h post mortem [28]. After removal of the cornea (for transplantation) and the anterior segment, the optic nerve was cut and vitreous and neural retina were washed out of the eye cup with Hanks' balanced salt solution (HBSS; Gibco BRL, Breda, The Netherlands), allowing trypsin dissociation of the RPE cells by two subsequent incubations at 37°C. The first incubation was performed with 0.025% trypsin and 0.01% ethylene diamine tetraacetic acid (EDTA) in Puck's saline solution (Gibco BRL) for 15 min, and the second incubation with 0.25% trypsin and 0.1% EDTA in Puck's saline solution for 45 min. Cells obtained from the second incubation were plated in 24-well plates (Costar, Cambridge, MA) at 105 cells/well in Iscove's modified Dulbecco's medium (IMDM; Gibco BRL) supplemented with 20% fetal calf serum (FCS; Gibco BRL), penicillin 100 U/ml (Gibco BRL) and streptomycin 100 μg/ml (Gibco BRL). Non-adherent cells were removed after 2 days by washing and refreshing the culture medium. At confluency, cells were detached by trypsin treatment and passed to culture flasks at ≈ 4 × 104 cells/cm2. For stimulation experiments RPE cells were used between passages 2 and 8.
To investigate whether the RPE cell cultures were contaminated with, for instance, fibroblasts, the cell lines were analysed morphologically and by immunohistochemistry. Phase contrast microscopy revealed pigmentation of the RPE cells during the first few passages and the cells displayed characteristic epithelial morphology throughout the culture. For immunohistochemistry the cells were cultured on tissue chamber slides (Lab-Tek; Nunc Inc., Naperville, IL) and stained with either antibodies specific to cytokeratin 8/18 (CAM 5:2; Becton Dickinson, San Jose, CA) or specific to a glucose transporter protein (a-GLUT-1 stains barrier epithelium in the eye; a kind gift from L. Andersson, University of Uppsala, Uppsala, Sweden) [29,30]. Both antibodies react with RPE cells and not with fibroblasts. As a negative control an antibody against an irrelevant bacterial protein was used (mouse negative control immunoglobulins; Dako). The cultures tested revealed positive staining of all cells with anti-cytokeratin 8/18 and a-GLUT-1, indicating that the RPE cells were not contaminated with other cell types. This indicates that in agreement with earlier studies [28] the isolation procedure whereby vitreous and neural retina are removed from the eye, followed by a dissociation of the RPE cells with trypsin, is an efficient procedure to obtain pure RPE cells.
The ARPE-19 cell line [31] (a kind gift from Professor L.M. Hjelmeland, University of California, Davis, CA) was grown in Dulbecco's modified essential medium and F12 (DMEM/F12; 1:1; Gibco BRL) supplemented with 10% FCS, penicillin 100 U/ml and streptomycin 100 μg/ml. All cells were cultured at 37°C and 5% CO2. Medium was changed twice a week.
RPE cell monolayers on transwell filters
RPE cells were cultured on transwell filters (Costar; 12 mm diameter, 0.4 μm pore size) according to the method of Dunn et al. [31] with minor modifications. Briefly, filters were coated with 160 μl of a 1:40 dilution of Matrigel (Collaborative Biomedical Products, Bedford, MA) in medium and air-dried overnight. Earlier experiments with laminin-coated or non-coated filters showed multilayering of the RPE cells (data not shown). The RPE cell lines as well as ARPE-19 cells were seeded at 1.6 × 105 cells/cm2 in a volume of 200 μl/filter, in the appropriate medium supplemented with 1% FCS. In the lower compartment 1000 μl medium were added, thereby levelling the height of the liquid levels to prevent hydrostatic pressure. Transepithelial resistance (TER) was measured once a week using an Endohm chamber and an ohmmeter (World Precision Instruments, Sarasota, USA). TER measurements were corrected for background by subtracting the value of a matrigel-coated filter without cells. Filters with RPE monolayers were used for experiments 19 days after plating and when the TER was at least 20 Ω/cm2.
Microscopy of RPE cell monolayers on transwell filters
The possible formation of multilayers of RPE cells was checked by light microscopy of cryostat sections of the filters. The filters were washed with PBS, frozen in liquid N2, embedded in Tissue-Tek (Miles Inc., Elkhart, IN), cut in 10 μm thick sections, fixed in acetone during 5 min and air-dried. The sections were stained with haematoxylin for 1 min, followed by staining with eosin for 1 min. Under the conditions employed we did not observe the formation of multiple layers of RPE cells.
The integrity of the RPE cell monolayers was further analysed by electron microscopy (EM) and immunohistochemistry. For scanning EM, filters were washed with HBSS, after which the cells were fixed in a 0.08 m cacodylate-buffered solution containing 1% glutaraldehyde and 1.25% paraformaldehyde pH 7.3. After fixation for several days, the filters were dehydrated with ethanol, critical-point dried with CO2 as the intermediate, and coated with a few nanometres of platinum. The filters were inspected and micrographed with a Philips SEM 505 scanning electron microscope (Philips Industries, Eindhoven, The Netherlands) with a secondary emission detector.
For transmission EM, filters were post-fixed in OsO4, dehydrated in a graded series of ethanol, and embedded in epon. Ultrathin sections were cut, stained with uranyl acetate and lead citrate, and inspected and photographed in a Philips EM 400 electron microscope (Philips Industries) equipped with an EDAX PV 9800 system and an ultrathin window detector (EDAX PV 9760/53).
The formation of tight junctions was determined by immunofluorescence. Filters were washed with PBS and fixed with 5% paraformaldehyde, washed again with PBS, permeabilized by incubation with 0.2% Triton X-100 for 15 min, blocked with normal goat serum and incubated for 1 h with rabbit antibodies specific to the occludin-associated protein ZO-1 (Zymed Labs Inc, San Francisco, CA). After subsequent washing with PBS, filters were incubated for 1 h in the dark with TRITC-conjugated swine anti-rabbit immunoglobulins (Dako), rinsed in PBS and observed with a fluorescence microscope (Leica, Rijswijk, The Netherlands).
Barrier properties of RPE cell monolayers on transwell filters
The barrier properties of the RPE cell monolayers were tested by investigating whether IL-8 could pass the RPE cell monolayer. Recombinant human IL-8 (1 μg/ml; Medgenix Diagnostics S.A., Fleurus, Belgium) was added in either the upper or lower compartment and the medium of the opposite compartment was collected and assayed for the presence of IL-8 (see below) at different time points.
Stimulation of the RPE cell monolayers
To induce cytokine (IL-6 and IL-8) secretion, the RPE cell monolayers were stimulated with 100 U/ml IL-1β. This concentration has been found to strongly stimulate RPE cells to secrete IL-6 and IL-8, as shown by others [11,14] and ourselves (data not shown). Nineteen days after seeding and when the culture had reached a TER of at least 20 Ω·cm2, the RPE cell monolayers were washed with HBSS and cultured for an additional 24 h under serum-free conditions. At this time ≈ 2 × 105 cells were present on the filters. The filters were washed and medium containing 0.1% FCS was added. Cells were then stimulated with 100 U/ml IL-1β (specific activity 109 U/mg; Genzyme, Cambridge, MA) in the upper compartment (apical side) or in the lower compartment (basal side). At different time points, the medium of both the upper and lower compartment was collected, snap frozen, and stored at −20°C until assay.
Measurement of IL-6 and IL-8 was performed using a commercially available ELISA. The IL-6 ELISA was a sandwich ELISA with a detection limit of 2 pg/ml (Pelikine Compact human IL-6 ELISA; CLB, Amsterdam, The Netherlands). The IL-8 ELISA was a sandwich ELISA with a detection limit of 0.5 pg/ml (ScreeningLine; Medgenix Diagnostics).
Statistical analysis
Statistical testing was performed after log transformation of the data and analysis of homogeneity of variance was performed according to the Bonferroni method (SPSS software; SPSS Inc., USA). Difference between direction of cytokine secretion, time and experiment were tested by the anova method. To test for a significant difference between direction of cytokine secretion at the different time points, the Newmann–Keuls test was used (SPSS). Differences were considered significant at P < 0.05.
RESULTS
Barrier properties of RPE cell monolayers on transwell filters
After seeding, the ARPE-19 cell and the RPE cell line monolayers showed a gradual increase in the TER, whereby a maximal TER was reached after ≈ 14 days. Maximal TER for the ARPE-19 cells was 29.2 ± 6.8 Ω·cm2 (mean ± s.d.) and for the RPE cell lines 26.5 ± 5.3 Ω·cm2. All the TER given are net TER; the TER of coated filters without cells was subtracted from the TER of the filters with cells. The TER of the coated filters without cells was 14.4 ± 1.5 Ω·cm2.
To study the morphology of the cultured cells, filters with ARPE-19 cells as well as RPE cell lines were subjected to light and electron microscopy. Light microscopy of filter sections revealed a confluent monolayer without evidence of multilayering of the culture (Fig. 1). Scanning EM of filters which were not yet confluently overgrown with cells (indicated by low TER values) showed the uncovered filter surface with its characteristic pores (Fig. 2a). However, when the filters were confluently overgrown by RPE cells these pores could no longer be observed (Fig. 2b).
Fig. 1.

Light micrograph of a section of an ARPE-19 cell monolayer on filter after haematoxylin–eosin staining (× 550).
Fig. 2.
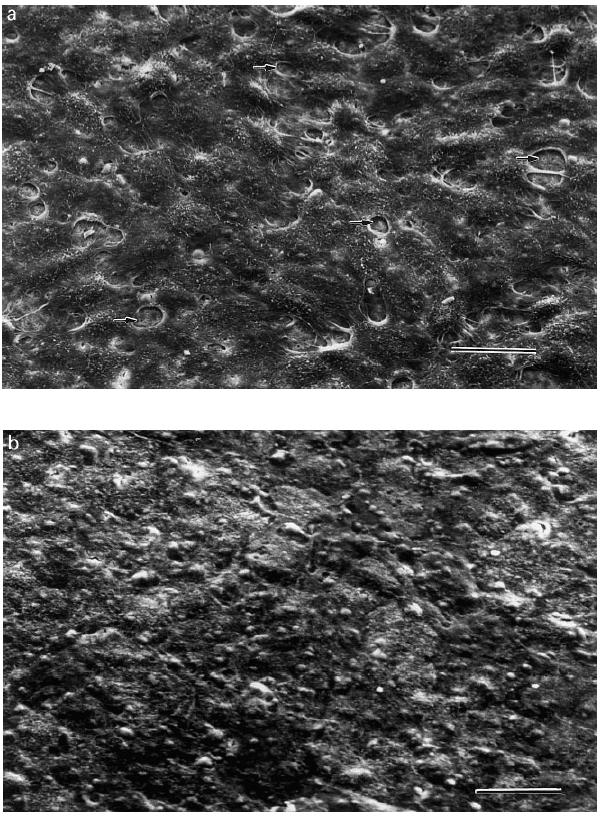
Scanning electron micrograph of an ARPE-19 cell monolayer on filter. (a) Filter with a preconfluent culture of ARPE-19 cells. Arrow indicates pores of the filter. (b) Confluently overgrown filter with ARPE-19 cells. Bar = 50 μm.
Transmission EM of the filters suggested the presence of a junctional complex (Fig. 3). Transmission EM also revealed microvilli at the RPE membranes at the side of the upper compartment (Fig. 3). This and the observation that the tight junctions are situated at the apical side of the lateral membrane (data not shown) indicate that the upper compartment represents the apical side of the monolayer and the lower compartment represents the basal side (choroidal side) of the RPE cell monolayer.
Fig. 3.
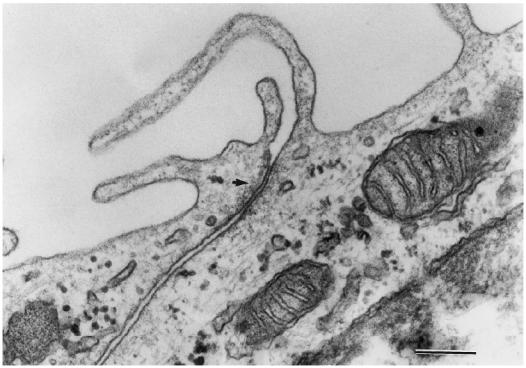
Transmission electron micrograph of a section of an ARPE-19 cell monolayer on filter. Arrow indicates tight junction complex. Bar = 0.2 μm.
Immunofluorescence staining of the cell layers for the occludin-associated protein ZO-1 indicated the presence of tight junctions around every cell (Fig. 4). The fact that the ZO-1 staining was only seen in one plane of the filters also supported the observation that multilayering of the cells had not occurred. The experiments described above were repeated using RPE cell line monolayers and revealed similar results (data not shown). Together, this strongly suggests that the RPE cells grew in a monolayer with barrier properties very similar to the in vivo situation.
Fig. 4.
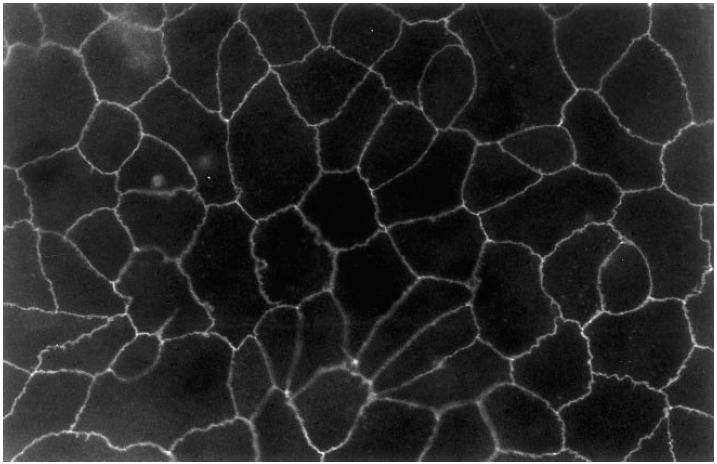
Fluorescence micrograph of an ARPE-19 cell monolayer on filter stained with ZO-1 antibody (× 500).
To assess the barrier properties for proteins we measured diffusion of IL-8 across the cell-covered filters. Diffusion of exogenous IL-8 was very low: < 20% of the exogenous IL-8 added to the lower compartment and < 15% of the exogenous IL-8 added to the upper compartment could be detected at the opposite side of the RPE cell monolayer after 8 h (Fig. 5). This indicates that IL-8 secreted in the medium at one side of the filter will not significantly contribute to the amount detected on the other side of the RPE monolayer.
Fig. 5.
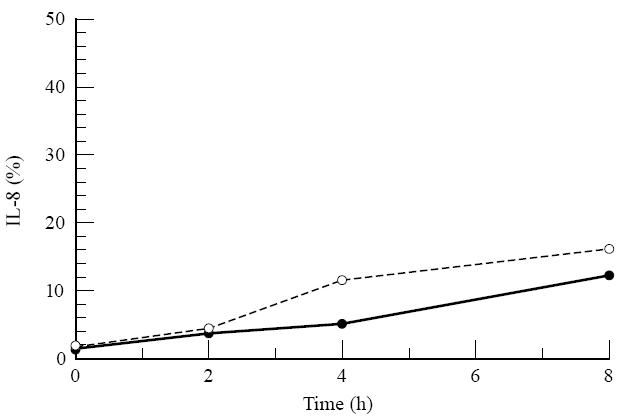
Diffusion of IL-8 through a retinal pigment epithelial (RPE) cell monolayer on transwell filter. In the upper or lower compartment of the RPE monolayer, 1 μg/ml of recombinant IL-8 was added. At different time points the amount of IL-8 was measured in the opposite compartment. Data are expressed as the mean of two filters and plotted as percentage of the added amount of IL-8. •, IL-8 added to the upper compartment; ○, IL-8 added to the lower compartment.
Polarized IL-6 and IL-8 secretion by the human ARPE-19 cell monolayer
Analysis of the IL-6 and IL-8 production by the ARPE-19 cell line was investigated after IL-1 stimulation. A slight secretion of IL-6 and IL-8 at both apical and basal side was found at 48 h with ARPE-19 monolayers (n = 10) that were not stimulated with IL-1β (Figs 6 and 7); 5 pg IL-6 and 2 pg IL-8 at the apical side and 2 pg IL-6 and 3 pg IL-8 at the basal side per filter.
Fig. 6.
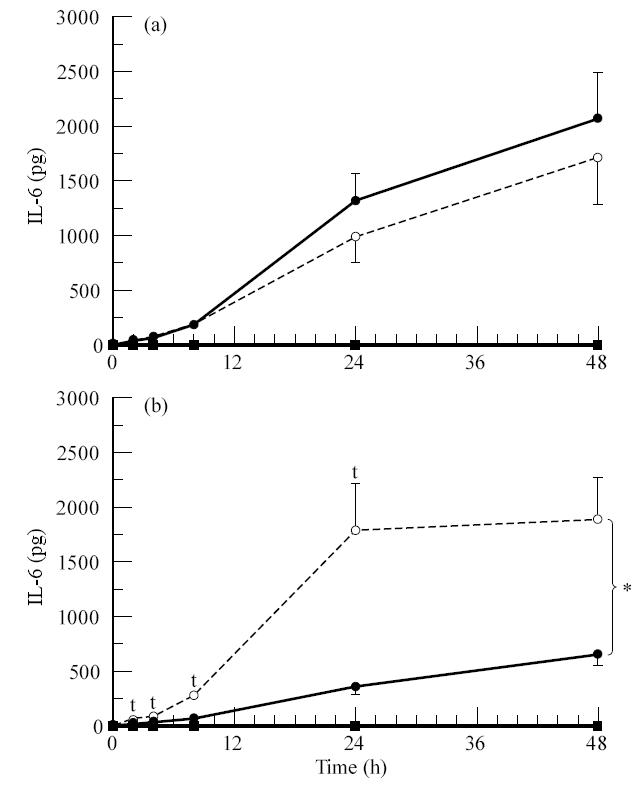
Secretion of IL-6 by the ARPE-19 cell line after stimulation with IL-1β from the apical side (a) or the basal side (b). ARPE-19 cells were seeded on transwell filters at a concentration of 1.6 × 105 cells/cm2 and cultured for at least 19 days in Dulbecco's modified essential medium (DMEM)/F12 supplemented with 1% fetal calf serum (FCS). Filters were used when the net trans-epithelial resistance (TER) was at least 20 Ω·cm2. Before stimulation monolayers were cultured for 24 h in serum-free medium. Filters were stimulated from either the upper or lower compartment with IL-1β at a final concentration of 100 U/ml. Data are expressed as absolute amounts of IL-6 per filter and are expressed as the means of 10 filters ± s.e.m. Statistical analysis of the log transformed data revealed a significant difference between the upper and the lower secretion after basal stimulation when tested by anova method (*) and a significant difference at the indicated time points (†) by Newman–Keuls method (P < 0.05). Measured in the upper • and lower ○ compartment after IL-1β stimulation, measured in the upper ▪ and lower □ compartment without IL-1β stimulation.
Fig. 7.
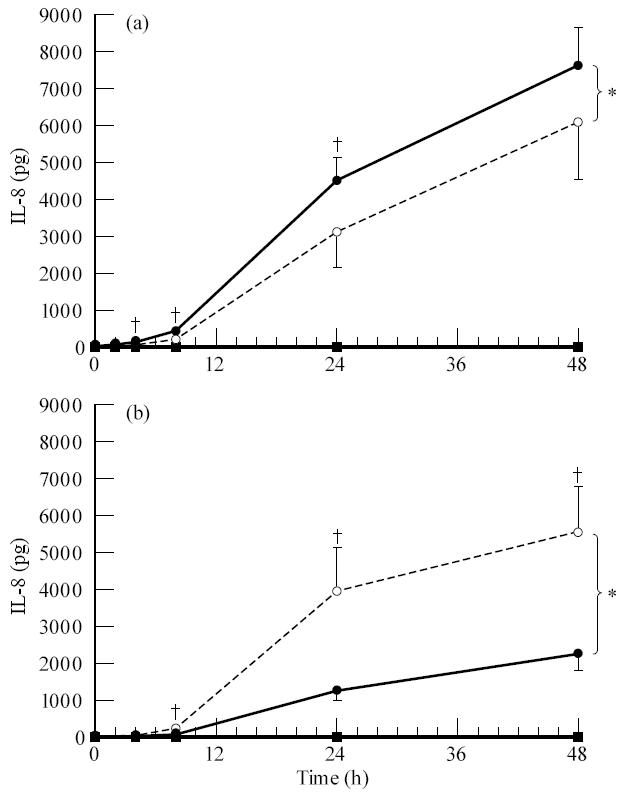
Secretion of IL-8 by the ARPE-19 cell line after stimulation with IL-1β from the apical side (a) or the basal side (b). ARPE-19 cells were seeded on transwell filters at a concentration of 1.6 × 105 cells/cm2 and cultured for at least 19 days in Dulbecco's modified essential medium (DMEM)/F12 supplemented with 1% fetal calf serum (FCS). Filters were used when the net trans-epithelial resistance (TER) was at least 20 Ω·cm2. Before stimulation monolayers were cultured for 24 h in serum-free medium. Filters were stimulated from either the upper or lower compartment with IL-1β at a final concentration of 100 U/ml. Data are expressed as absolute amounts of IL-8 per filter. Data are expressed as the means of 10 filters ± s.e.m. Statistical analysis of the log transformed data revealed a significant difference between the upper and the lower secretion after apical and basal stimulation when tested by anova (*) and a significant difference at the indicated time points (†) by Newman–Keuls method (P < 0.05). Measured in the upper • and lower ○ compartment after IL-1β stimulation, measured in the upper ▪ and lower □ compartment without IL-1β stimulation.
Secretion of IL-6 was markedly induced by IL-1β stimulation (Fig. 6). Apical stimulation revealed maximal secretion of IL-6 at 48 h and was 2091 ± 415 pg in the upper compartment and 1718 ± 436 pg in the lower compartment per filter. Basal stimulation also revealed maximal secretion at 48 h and was 678 ± 111 pg in the upper compartment and 1929 ± 420 pg in the lower compartment per filter. Statistical analysis revealed a significant difference between the apical and the basal secretion of IL-6 after basal stimulation. Basal secretion was higher and thus the polarized secretion of IL-6 after basal stimulation of the ARPE-19 cells was directed towards the basal side. After apical stimulation no statistical difference between the apical and basal secretion of IL-6 could be detected.
IL-8 secretion after apical stimulation was maximal after 48 h; 7650 ± 1030 pg in the upper compartment and 6130 ± 1529 pg in the lower compartment (Fig. 7). When stimulated from the basal side IL-8 secretion was again maximal after 48 h and was 2336 ± 502 pg in the upper compartment and 5567 ± 1323 pg in the lower compartment. Statistical analysis revealed that the ARPE-19 cells secreted IL-8 in a polarized fashion and that this polarized secretion after basal stimulation was directed towards the basal side.
These experiments indicate that the ARPE-19 cell line is capable of secreting both IL-6 and IL-8 in a polarized fashion towards the basal side. This is evident after stimulation from the basal side, but was not apparent after apical stimulation.
Polarized IL-6 and IL-8 secretion by human RPE cell line monolayers
Further experiments to investigate the polarized secretion of cytokines was performed using RPE cell monolayers from three different donors (two filters per time point per donor) (Figs 8 and 9). Levels of IL-6 and IL-8 produced by these RPE cells without stimulation were low, 3–153 pg IL-6 and < 0.4–106 pg IL-8 at the apical side and 44–182 pg IL-6 and < 0.8–123 pg IL-8 at the basal side per filter.
Fig. 8.
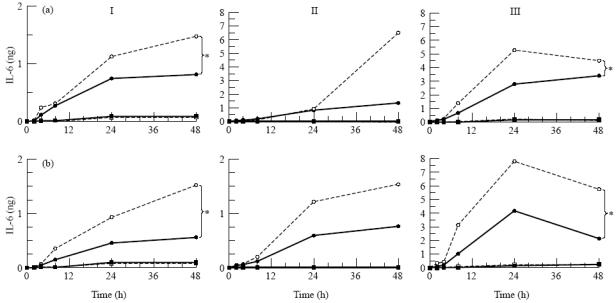
Secretion of IL-6 by three donor retinal pigment epithelial (RPE) cell cultures (I, II, III) after stimulation with IL-1β from the apical side (a) or the basal side (b). RPE cell lines were seeded on transwell filters at a concentration of 1.6 × 105 cells/cm2 and cultured for at least 19 days in Iscove's modified Dulbecco's medium (IMDM) supplemented with 1% fetal calf serum (FCS) and were used when net trans-epithelial resistance (TER) was at least 20 Ω·cm2. Before stimulation monolayers were cultured for 24 h in serum-free medium. Filters were stimulated from either the upper or lower compartment with IL-1β at a final concentration of 100 U/ml. Data are expressed as absolute amounts of IL-6 per filter and are expressed as the mean of two filters. Statistical analysis of the log transformed data revealed a significant difference between the upper and the lower secretion after both apical and basal stimulation when all donors are accounted for and individual donors as indicated (*) when tested by anova. Measured in the upper • and lower ○ compartment after IL-1β stimulation, measured in the upper ▪ and □ lower compartment without IL-1β stimulation.
Fig. 9.
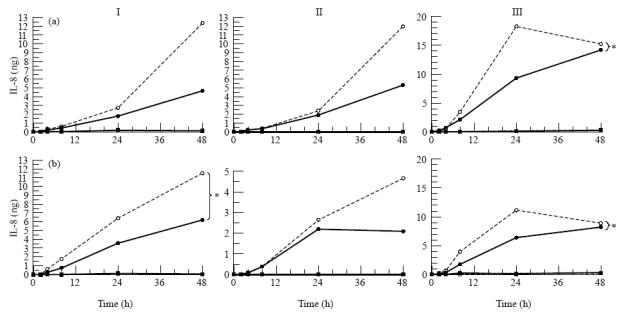
Secretion of IL-8 by three donor retinal pigment epithelial (RPE) cell cultures (I, II, III) after stimulation with IL-1β from the apical side (a) or the basal side (b). RPE cell lines were seeded on transwell filters at a concentration of 1.6 × 105 cells/cm2 and cultured for at least 19 days in Iscove's modified Dulbecco's medium (IMDM) supplemented with 1% fetal calf serum (FCS) and were used when net trans-epithelial resistance (TER) was at least 20 Ω·cm2. Before stimulation monolayers were cultured for 24 h in serum-free medium. Filters were stimulated from either the upper or lower compartment with IL-1β at a final concentration of 100 U/ml. Data are expressed as absolute amounts of IL-8 per filter and are expressed as the mean of two filters. Statistical analysis of the log transformed data revealed a significant difference between the upper and the lower secretion after both apical and basal stimulation when all donors are accounted for and individual donors as indicated (*) when tested by anova. Measured in the upper • and lower ○ compartment after IL-1β stimulation, measured in the upper ▪ and lower □ compartment without IL-1β stimulation.
The amount of IL-6 and IL-8 secreted by RPE cell lines markedly increased after stimulation with IL-1β, but varied considerably between the RPE monolayers of the three different donors (Figs 8 and 9) and ranged from 500 pg to 8000 pg of IL-6 and from 1500 pg to 18 000 pg IL-8 per filter.
IL-6 was secreted by RPE cell lines preferentially towards the basal side, independent of the side of stimulation (Fig. 8). The difference between apical and basal directed secretion became apparent 24 h after stimulation with IL-1β. The difference between apical and basal secretion of IL-6 was statistically significant when all donors were accounted for. For donor I and donor III this difference was significant both after apical and basal stimulation (Fig. 8).
IL-8 secretion by the RPE cell lines was also directed towards the basal compartment, independent of the side of stimulation, and also became apparent after at least 24 h following stimulation of the cells with IL-1β (Fig. 9). Donor III showed significant polarized secretion of IL-8 after both apical and basal stimulation with IL-1β, donor I, however, showed polarized secretion of IL-8 after basal stimulation. When all donors where accounted for, statistical analysis showed a significant difference between the apical and basal secretion of IL-8 after both apical and basal stimulation.
DISCUSSION
Although various groups have shown that RPE cells are an important source of cytokines in the posterior segment of the eye, this is, as far as we know, the first study demonstrating polarized production of cytokines by RPE cells. In this study we show that human RPE cells secrete IL-6 and IL-8 in a polarized fashion, following stimulation with IL-1β. IL-1β was used as a stimulus since this is a cytokine produced early in the inflammatory response and has been shown to optimally stimulate RPE cells to produce IL-6 [10–13] and IL-8 [13,14] at concentrations comparable to the concentration used in this study. Whether the levels of IL-1β used are physiological is not known. Analysis of vitreous samples in uveitis or proliferative diabetic retinopathy revealed levels of IL-1 up to 30 pg/ml [32], which is approx. six-fold lower than the concentrations we used to stimulate RPE cells in vitro. Diffusion of IL-1 into the vitreous may, however, have resulted in a reduction of the initial local concentration present at the RPE cell layer. Whether other stimuli than IL-1 also result in polarized cytokine secretion is the subject of further studies.
Polarized cytokine secretion was observed in primary cultures of RPE cells obtained from three different donors and was also studied in the immortalized ARPE-19 cell line. This latter cell line has been extensively investigated by others, is highly differentiated and has been shown to have specific polar properties such as formation of tight junctions, formation of apical microvilli and the presence of Na+/K+-ATPase at the apical membrane [31,33]. Only one other spontaneously immortalized human RPE cell line has been studied extensively and has less well defined cytoskeletal properties [34].
The polarized secretion of IL-6 and IL-8 by the RPE cell lines was directed towards the basal side. The ARPE-19 cell line also revealed basal directed polarized secretion after basal stimulation, whereas this was not observed after apical stimulation. The reason for the observed difference between the ARPE-19 cell line and the primary RPE cultures is not clear at the present time, but emphasizes the importance of using primary cultures for these experiments.
To investigate the barrier properties of the cells on the porous filter support, TER measurements were performed. The background values can differ compared with background values reported by other authors due to different medium components and distance between the electrodes. This possibly explains the higher TER found by others [35]. On the other hand, our TER values are similar to TER measured by others using the same ARPE-19 cell line, when these latter values were corrected for the background [31,33]. Treatment of RPE cell monolayers with 0.5 mm EDTA for 15 min to detach the RPE cells resulted in a drop of TER values to background levels (data not shown). This indicates that the TER measured was only due to the resistance of the RPE cell monolayer on filter and was also not caused by a possible accumulation of extracellular matrix.
The presence of an intact barrier was also investigated in diffusion experiments after the addition of IL-8 to either the upper or lower compartment. In view of the diffusion that occurred (< 20% in 8 h), the measured polarized secretion of cytokines would even be an underestimation of the true polarized secretion.
RPE cells are capable of secreting extracellular matrix proteins which will, due to refreshing of the medium, mainly be deposited between the RPE cell monolayer and the filter. It is possible that secreted cytokines stick to extracellular matrix proteins, suggesting that not all basal secreted cytokine will be measured. This will also result in an underestimation of the polarized secretion of cytokine towards the basal side of the RPE cell lines, and may explain the fact that polarized secretion could only be seen with the ARPE-19 cells under certain conditions. Further experiments are needed to address this issue.
Recent reports have shown that human mesothelial cells and human intestinal cells secrete IL-8 in a polarized fashion to attract neutrophils [21–23]. The polarized secretion of IL-8 created a chemotactic gradient and facilitated neutrophils to migrate across the cell layer. It has also been shown that the migration over the monolayer of mesothelial cells could be blocked by MoAbs against IL-8, indicating that IL-8 is responsible for the migration of neutrophils across the cell monolayer to the compartment with the highest IL-8 levels [23]. In our experiments we found a preferential secretion of IL-8 towards the basal side of the RPE cell monolayers. This will attract neutrophils from the choroidal vessels to the RPE, but may prevent the neutrophils from crossing the RPE barrier (due to a lower concentration of IL-8 at the opposite side of the RPE). Thus in case of an ongoing immune reaction, polarized secretion of IL-8 attracts neutrophils but could prevent them from migrating into the eye.
Taken together, our experiments suggest that the polarized production of inflammatory cytokines by RPE cells towards the choroid may be a mechanism to prevent damage to the vulnerable neurosensory retina.
Acknowledgments
The human tissue used in this study is tissue donated for transplantation purposes, which could not be used for implantation in a patient. The authors wish to thank the BIS foundation (Leiden, The Netherlands) for its assistance in obtaining the tissue, as well as Dr E. Pels (Cornea Bank, Amsterdam, The Netherlands). The authors wish to thank Professor L.M. Hjelmeland (University of California, Davis, CA) for generously providing the human ARPE-19 cell line. The authors also thank J. Klooster and J. Ruijter for excellent technical assistance.
References
- 1.Zinn KM, Benjamin-Henkind JV. Anatomy of the human retinal pigment epithelium. In: Zinn KM, Marmor M, editors. The retinal pigment epithelium. Cambridge: Harvard University Press; 1979. pp. 3–31. [Google Scholar]
- 2.Campochiaro PA. Cytokine production by retinal pigmented epithelial cells. Int Rev Cytol. 1993;146:75–82. doi: 10.1016/s0074-7696(08)60380-0. [DOI] [PubMed] [Google Scholar]
- 3.Detrick B, Newsome DA, Percopo CM, et al. Class II antigen expression and gamma interferon modulation of monocytes and retinal pigment epithelial cells from patients with retinitis pigmentosa. Clin Immunol Immunopathol. 1985;36:201–11. doi: 10.1016/0090-1229(85)90121-7. [DOI] [PubMed] [Google Scholar]
- 4.Liversidge JM, Sewell HF, Forrester JV. Human retinal pigment epithelial cells differentially express MHC class II (HLA, DP, DR and DQ) antigens in response to in vitro stimulation with lymphokine or purified IFN-gamma. Clin Exp Immunol. 1988;73:489–94. [PMC free article] [PubMed] [Google Scholar]
- 5.Elner SG, Elner VM, Pavilack MA, et al. Modulation and function of intercellular adhesion molecule-1 (CD54) on human retinal pigment epithelial cells. Lab Invest. 1992;66:200–11. [PubMed] [Google Scholar]
- 6.Platts KE, Benson MT, Rennie IG, et al. Cytokine modulation of adhesion molecule expression on human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1995;36:2262–9. [PubMed] [Google Scholar]
- 7.Griffith TS, Brunner T, Fletcher SM, et al. Fas ligand-induced apoptosis as a mechanism of immune privilege. Sci. 1995;270:1189–92. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe GJ, Van Le L, Valea F, et al. Expression of interleukin-1 alpha, interleukin-1 beta, and an interleukin-1 receptor antagonist in human retinal pigment epithelial cells. Exp Eye Res. 1992;55:325–35. doi: 10.1016/0014-4835(92)90197-z. [DOI] [PubMed] [Google Scholar]
- 9.Planck SR, Huang XN, Robertson JE, et al. Retinal pigment epithelial cells produce interleukin-1 beta and granulocyte-macrophage colony-stimulating factor in response to interleukin-1 alpha. Curr Eye Res. 1993;12:205–12. doi: 10.3109/02713689308999465. [DOI] [PubMed] [Google Scholar]
- 10.Planck SR, Dang TT, Graves D, et al. Retinal pigment epithelial cells secrete interleukin-6 in response to interleukin-1. Invest Ophthalmol Vis Sci. 1992;33:78–82. [PubMed] [Google Scholar]
- 11.Elner VM, Scales W, Elner SG, et al. Interleukin-6 (IL-6) gene expression and secretion by cytokine-stimulated human retinal pigment epithelial cells. Exp Eye Res. 1992;54:361–8. doi: 10.1016/0014-4835(92)90048-w. [DOI] [PubMed] [Google Scholar]
- 12.Benson MT, Shepherd L, Rees RC, et al. Production of interleukin-6 by human retinal pigment epithelium in vitro and its regulation by other cytokines. Curr Eye Res. 1992;11(Suppl.):173–9. doi: 10.3109/02713689208999529. [DOI] [PubMed] [Google Scholar]
- 13.Kuppner MC, McKillop-Smith S, Forrester JV. TGF-beta and IL-1 beta act in synergy to enhance IL-6 and IL-8 mRNA levels and IL-6 production by human retinal pigment epithelial cells. Immunol. 1995;84:265–71. [PMC free article] [PubMed] [Google Scholar]
- 14.Elner VM, Strieter RM, Elner SG, et al. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol. 1990;136:745–50. [PMC free article] [PubMed] [Google Scholar]
- 15.Elner SG, Strieter RM, Elner VM, et al. Monocyte chemotactic protein gene expression by cytokine-treated human retinal pigment epithelial cells. Lab Invest. 1991;64:819–25. [PubMed] [Google Scholar]
- 16.Jaffe GJ, Roberts WL, Wong HL, et al. Monocyte-induced cytokine expression in cultured human retinal pigment epithelial cells. Exp Eye Res. 1995;60:533–43. doi: 10.1016/s0014-4835(05)80068-5. [DOI] [PubMed] [Google Scholar]
- 17.di Giovine FS, Duff GW. Interleukin 1: the first interleukin. Immunol Today. 1990;11:13–20. doi: 10.1016/0167-5699(90)90005-t. [DOI] [PubMed] [Google Scholar]
- 18.Cousins SW, Trattler WB, Streilein JW. Immune privilege and suppression of immunogenic inflammation in the anterior chamber of the eye. Curr Eye Res. 1991;10:287–97. doi: 10.3109/02713689108996334. [DOI] [PubMed] [Google Scholar]
- 19.Cousins SW, McCabe MM, Danielpour D, et al. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991;32:2201–11. [PubMed] [Google Scholar]
- 20.Baggiolini M, Dahinden CA. CC chemokines in allergic inflammation. Immunol Today. 1994;15:127–33. doi: 10.1016/0167-5699(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 21.McCormick BA, Colgan SP, Delp-Archer C, et al. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckmann L, Jung HC, Schurer-Maly C, et al. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterol. 1993;105:1689–97. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 23.Zeillemaker AM, Mul FP, Hoynck van Papendrecht AA, et al. Polarized secretion of interleukin-8 by human mesothelial cells: a role in neutrophil migration. Immunol. 1995;84:227–32. [PMC free article] [PubMed] [Google Scholar]
- 24.de Vos AF, Hoekzema R, Kijlstra A. Cytokines and uveitis, a review. Curr Eye Res. 1992;11:581–97. doi: 10.3109/02713689209001814. [DOI] [PubMed] [Google Scholar]
- 25.de Boer JH, Van Haren MA, de Vries-Knoppert WA, et al. Analysis of IL-6 levels in human vitreous fluid obtained from uveitis patients, patients with proliferative intraocular disorders and eye bank eyes. Curr Eye Res. 1992;11(Suppl.):181–6. doi: 10.3109/02713689208999530. [DOI] [PubMed] [Google Scholar]
- 26.Murray PI, Hoekzema R, Van Haren MA, et al. Aqueous humor interleukin-6 levels in uveitis. Invest Ophthalmol Vis Sci. 1990;31:917–20. [PubMed] [Google Scholar]
- 27.Jacobs AL, Sehgal PB, Julian J, et al. Secretion and hormonal regulation of interleukin-6 production by mouse uterine stromal and polarized epithelial cells cultured in vitro. Endocrinol. 1992;131:1037–46. doi: 10.1210/endo.131.3.1505448. [DOI] [PubMed] [Google Scholar]
- 28.Mannagh J, Arya DV, Irvine AR. Tissue culture of human retinal pigment epithelium. Invest Ophthalmol. 1973;12:52–64. [PubMed] [Google Scholar]
- 29.Andersson L, Lundahl P. C-terminal-specific monoclonal antibodies against the human red cell glucose transporter. Epitope localization with synthetic peptides. J Biol Chem. 1988;263:11414–20. [PubMed] [Google Scholar]
- 30.Harik SI, Kalaria RN, Whitney PM, et al. Glucose transporters are abundant in cells with ‘occluding’ junctions at the blood–eye barriers. Proc Natl Acad Sci USA. 1990;87:4261–4. doi: 10.1073/pnas.87.11.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn KC, Aotaki-Keen AE, Putkey FR, et al. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–69. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 32.Franks WA, Limb GA, Stanford MR, et al. Cytokines in human intraocular inflammation. Curr Eye Res. 1992;11(Suppl.):187–91. doi: 10.3109/02713689208999531. [DOI] [PubMed] [Google Scholar]
- 33.Tugizov S, Maidji E, Pereira L. Role of apical and basolateral membranes in replication of human cytomegalovirus in polarized retinal pigment epithelial cells. J Gen Virol. 1996;77:61–74. doi: 10.1099/0022-1317-77-1-61. [DOI] [PubMed] [Google Scholar]
- 34.Davis AA, Bernstein PS, Bok D, et al. A human retinal pigment epithelial cell line that retains epithelial characteristics after prolonged culture. Invest Ophthalmol Vis Sci. 1995;36:955–64. [PubMed] [Google Scholar]
- 35.Hernandez EV, Hu JG, Frambach DA, et al. Potassium conductances in cultured bovine and human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1995;36:113–22. [PubMed] [Google Scholar]


