Abstract
We have evaluated the effects of three potent immunosuppressive agents, cyclosporin A (CsA), FK506 and rapamycin, on the murine contact sensitivity (CS) reaction to the hapten trinitrochlorobenzene. Development of CS reaction requires participation of three distinct T cell subsets: αβ+, CD4+ T lymphocytes, which are the classical effector cell of the CS reaction, γδ+ T lymphocytes, and αβ+, double-negative (CD4− CD8−) T lymphocytes that express the B220 molecule and produce IL-4. We found that all three drugs inhibit the development of the CS reaction, but they affect different target cells. In fact, rapamycin and FK-506 block both αβ+, CD4+ and γδ+ T lymphocytes, while CsA inhibits only the αβ+, CD4+ T lymphocyte. None of the three drugs exerted any inhibitory activity on the αβ+, double-negative (CD4− CD8−) T lymphocytes. Hapten-immune lymph node cells from mice treated in vivo with CsA or FK506 failed to proliferate and to produce IL-2 when re-exposed to the specific antigen in vitro. In contrast, immune lymph node cells from mice that had been treated in vivo with rapamycin gave optimal antigen-specific proliferation and IL-2 production in vitro. The implications of these observations are discussed in relation to the use of these immunosuppressive agents for prevention of allograft rejection.
Keywords: cyclosporin A, FK506, rapamycin, contact sensitivity, T cell subsets
INTRODUCTION
Cyclosporin A (CsA) is a fungal metabolite that has been in clinical use as an immunosuppressive drug for the treatment of transplant rejection and autoimmune diseases for a number of years. More recently, two novel immunosuppressive agents have been described, FK506 (Tacrolimus) and rapamycin (Sirolimus (SRL)), that have aroused considerable interest because both appear to be 10–100-fold more active than CsA in various models [1,2]. SRL is a macrolide with anti-fungal activity produced by Streptomyces hygroscopicus [3], that has been also found to inhibit immune response and prevent graft rejection [3,4]. Interest in the immunosuppressive activity of this agent has recently been rekindled as a result of its structural resemblance to FK506 [1,2], produced by S. tsukubaensis, which is strongly immunosuppressive in several experimental systems and has now entered clinical trials as an immunosuppressive drug for use in organ transplantation. FK506 and SRL are structurally related, and although both, like CsA, are cyclic microbial metabolites, they have no structural relationship with CsA. However, from a functional point of view, the mechanisms of action of CsA and FK506 are similar and differ from the mechanism of action of SRL. In vitro, CsA and FK506 specifically inhibit the Ca2+ arm of T cell activation signal and the appearance of IL-2 mRNA [5,6]. In contrast, SRL does not prevent accumulation of IL-2 mRNA, at least when induced through a calcium-associated pathway, but impairs the response of T cells to IL-2 and IL-4 [7,8]. This is somewhat surprising in the light of the finding that FK506 and SRL bind to the same intracellular receptor, namely FKBP-12, which is a peptidyl prolyl cis-trans isomerase [9]. The complexes formed between SRL and FK506 with FKBP interact with different effector mechanisms inhibiting T cell proliferation [10]. The precise mechanism is unknown, but as a consequence of SRL activity a cellular p70 S6 protein kinase has been shown to be inhibited [11]. CsA and FK506, although interacting with different binding proteins, have been shown to use an identical effector mechanism. Both compounds complexed with their binding protein inhibit calcineurin, a calmodulin-dependent protein phosphatase 2B [12]. The result is therefore the inhibition of early activation gene expression responsible for the expression of cytokines such as IL-2 [13,14]. Despite these extensive in vitro studies of the immunosuppressive mechanisms of these agents, very few studies have been reported in vivo.
During the last few years, we have been involved in defining the cellular and molecular mechanisms responsible for contact sensitivity (CS) reaction, which is one of the most widely studied in vivo models of cell-mediated immune response in the mouse. We now know that the CS reaction is a particularly complex phenomenon which requires participation of three distinct T cell subsets: (i) αβ+, CD4+ T lymphocytes, which represent the classical effector cell of the CS reaction [15–17]; (ii) γδ+ T lymphocytes, which rearrange the Vγ3 gene region [18]; (iii) αβ+, double-negative (CD4− CD8−) T lymphocytes which rearrange an invariant Vα14 T cell receptor Vα chain and express the B220 molecule [19]. This third T cell subset produces IL-4 that acts on the γδ+ T lymphocytes expressing IL-4 receptors. Once they have bound IL-4, the γδ+ T lymphocytes acquire the ability to leave the lymph nodes and to localize at the site of antigen challenge [20–22]. Figure 1 illustrates this scenario.
Fig. 1.
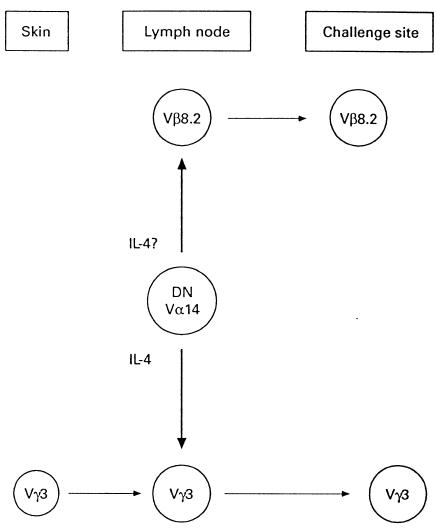
Schematic representation of the T cell subsets that mediate the contact sensitivity reaction (see text for explanation).
The above studies have prompted us to analyse the effects of the three immunosuppressive drugs, CsA, FK506 and SRL in the CS reaction model in vivo. The results reported show that all three drugs were able to inhibit the development of the CS reaction. However, SRL and FK506 blocked both αβ+, CD4+ and γδ+ T lymphocytes, while CsA inhibited only αβ+, CD4+ T lymphocytes. None of the three drugs exerted any inhibitory activity on αβ+, double-negative (CD4− CD8−) T lymphocytes. Further analysis of the immunosuppressive activity of CsA, FK506 and SRL revealed that immune lymph node cells from mice treated in vivo with CsA or FK506 failed to proliferate and to produce IL-2 when re-exposed to the specific antigen in vitro. In contrast, immune lymph node cells from mice that had been treated in vivo with SRL gave optimal antigen-specific proliferation and IL-2 production in vitro.
All together, the present results may provide some explanation for the different immunosuppressive potency of CsA, FK506 and SRL, as well as some basis to explain the synergistic effect of SRL and CsA in inhibiting allograft rejection.
MATERIALS AND METHODS
Mice
Male mice of the strain CBA/J were obtained from Olac Ltd. (Bicester, UK) and bred at the Institute of General Pathology, under facility conditions. Eight to 12-week-old mice were used in each experiment and each experimental group consisted of five to eight mice.
Contact sensitivity
Mice were immunized epicutaneously with 0.1 ml 3% trinitrochlorobenzene (TNCB; Sigma, St Louis, MO) dissolved in acetone:ethanol (1:3) and applied on the shaved skin of the thorax and abdomen. Four to five days later, the mice were challenged by applying 20 μl 0.1% TNCB in olive oil on both sides of both ears. The increase in ear thickness was measured 24 h later with an engineer's micrometer and expressed in units of 10−3 cm ± s.d.
Passive transfer of contact sensitivity
Mice were immunized with TNCB as described above and the draining lymph node cells were harvested 4 days later. The cells were washed three times in medium RPMI 1640 (GIBCO, Grand Island, NY) supplemented with 5% heat-inactivated fetal calf serum (FCS; GIBCO), penicillin, streptomycin, glutamine and 2-mercaptoethanol (2-ME; 2 × 10−5 m) and 5 × 107 cells in 0.5 ml of medium were injected intravenously into naive recipient mice. The mice were challenged immediately with TNCB and increase in ear thickness was measured after 24 h.
Proliferation assay
Four day TNCB-immune lymph node cells (2 × 105 in 100 μl of medium) were cultured at 37°C in the presence of 5% CO2 in flat-bottomed 96-well microtitre plates (Nunc, Roskilde, Denmark) with TNCB-modified syngeneic spleen cells, as antigen-presenting cells (APC; 4 × 105 in 100 μl of medium) according to [23]. Three days later, the wells were pulsed with 1 μCi methyl-3H-thymidine (Amersham, Aylesbury, UK) and harvested 18 h later. Results are expressed as mean ct/min of triplicate wells ± s.d. S.d. were usually < 10% of the means.
IL-2 production and assay
Four day TNCB-immune lymph node cells were cultured with TNCB–APC as described in the previous section. After 24 h, supernatants were collected by centrifugation, filtered and stored at −70°C. IL-2 activity of supernatants was measured by their ability to support the growth of CTLL cells. Briefly, CTLL cells (5 × 104 in 50 μl of medium) were added to 50 μl of serial dilutions of supernatants in flat-bottomed microwell plates and incubated for 24 h at 37°C. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide, 5 mg/ml, 20 μl/well) was added and the reaction was stopped after 4 h with 100 μl 10% SDS. After overnight incubation, the absorbance (570–630 nm) was measured with an ELISA reader. Because also IL-4 allows survival of CTLL cells, the supernatants were absorbed twice in microtray wells coated with the anti-IL-4 MoAb 11B11 (a kind gift of Dr W. E. Paul, NIAID, Bethesda, MD), according to our published method. IL-2 activity of supernatants is expressed in U/ml, i.e. the reciprocal of the dilution giving 50% maximal response [24].
In vivo treatment with drugs
SRL was kindly provided by Dr S.N. Seghal (Wyeth-Ayerst Research Labs, Princeton, NY). CsA was a generous gift of Dr S. Cammisuli (Sandoz Research Labs, Basel, Switzerland). FK506 was provided by Fujisawa Pharmaceutical Co. (Osaka, Japan). All the drugs were dissolved in ethanol to give a 1 mm stock solution and were stored at −20°C. For in vivo use, SRL was injected intraperitoneally at 5 mg/kg body weight; CsA was injected intraperitoneally at 70 mg/kg and FK506 was injected intraperitoneally at 5 mg/kg. These drug concentrations were shown in previous papers [25,26] and in our preliminary experiments to cause maximal inhibition of the CS reaction when injected intraperitoneally. As a control, mice were injected with ethanol.
Magnetic bead separation
Mixtures of T cell subsets involved in the CS reaction were obtained by positive selection [17,20], according to the following sequence. Lymph node cells from TNCB-immune mice were passed over a nylon wool column to obtain a T cell-enriched population. The cells were then first incubated with anti-B220-coated microbeads (Milteny, Sunnyvale, CA) at a 1:5 cell:bead ratio. The mixtures of cells and beads were kept in a flat-walled flask in an ice bath with a flat magnet (Biomag Magnetic Separator) on one side of the flask. After 30 min the media containing non-adherent cells were removed carefully with a pipette and used for subsequent cell subset separation. The adherent cells that were still attached to the beads were recovered. This procedure was repeated twice to give a good enrichment of B220+ cells. The non-adherent population was then incubated with anti-αβ T cell receptor (TCR) MoAb (2 μg/106 cells, H57-597; Pharmingen, San Diego, CA), followed by anti-hamster immunoglobulin-coated beads (Biomag, 1 μm iron magnetic particles; Advanced Magnetics, Cambridge, MA). The adherent cells were recovered and used as a source of αβ+ T lymphocytes, while non-adherent cells were used for further cell separation. In fact, these non-adherent cells were incubated with anti-γδ TCR MoAb (2 μg/106 cells, GL3; Pharmingen) and then with anti-hamster immunoglobulin-coated magnetic beads. The adherent cells were recovered and used as a source of γδ+ T lymphocytes. Using these procedure, the purity of adherent population was always > 90% and their viability exceeded 95%, as assessed by FACS analysis.
For transfer experiments, the mixtures of cells and beads were injected intraperitoneally. When positively selected populations were used for passive transfer, 35 × 106αβ, 1 × 106γδ and 7.5 × 105 B220 cells were injected intraperitoneally.
In these experiments, mice were challenged on the ears 24 h after cell transfer and increase in ear thickness was measured after a further 24 h.
Statistical analysis
The double-tailed Student's t-test was used to analyse the significance of the differences between groups.
RESULTS
Effect of SRL, CsA and FK506 on the CS reaction and its passive transfer
Mice were injected intraperitoneally with SRL (5 mg/kg), FK506 (5 mg/kg) and CsA (70 mg/kg) at the day of immunization with TNCB. These doses of drugs have been found in previous studies [25,26] and in preliminary experiments (data not shown) to cause significant inhibition of the CS reaction. As a control, a group of mice received ethanol. The mice so treated were then tested for their ability to develop CS and transfer systemically CS into naive recipient mice. Figure 2a shows that mice injected with SRL, FK506 and CsA failed to develop CS, while control mice injected with ethanol developed a significant CS reaction. Figure 2b shows the results of a passive transfer experiment. Four day TNCB-immune lymph node cells from untreated mice or from mice injected with ethanol were fully able to transfer CS reaction into naive recipient mice. In contrast, 4 day TNCB-immune lymph node cells from mice that had been injected with SRL, FK506 or CsA failed to transfer significant CS reaction into naive recipient mice.
Fig. 2.
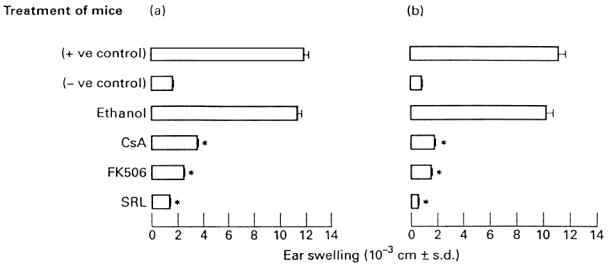
Inhibition of the contact sensitivity (CS) reaction (a) and its passive transfer (b) by Sirolimus (SRL), FK506 and cyclosporin A (CsA). Positive control group refers to mice that were immunized (CS) or received 4 day trinitrochlorobenzene (TNCB)-immune lymph node cells (passive transfer) and were challenged with TNCB. Negative control refers to mice that were only challenged with TNCB (challenge only). *P < 0.001 compared with the positive control group.
Effect of SRL, CsA and FK506 on the different T cell subsets that mediate the CS reaction
We have already reported that efficient transfer of the CS reaction requires participation of three distinct T cell populations: an αβ+, CD4+ T lymphocyte, a γδ+ T lymphocyte, and an αβ+, double-negative cell which expresses the B220 marker.
In the next set of experiments, we aimed to detect which T cell subset was affected by SRL, FK506 and CsA. Four day TNCB-immune lymph node cells from mice that had been treated with the drugs were injected into naive recipient mice together with αβ+, γδ+ or B220+ T lymphocytes that had been positively sorted from TNCB-immune lymph node cells. Note that as the B220+ cell is an αβ+ cell, a sequential sorting was performed in which B220+ cells were initially purified and thereafter αβ+ cells and γδ+ cells were positively purified.
Figure 3 shows that 4 day TNCB-immune lymph node cells from mice that had been injected with SRL or FK506 failed to transfer a significant CS reaction. However, transfer of 4 day TNCB-immune lymph node cells from mice that had been treated with FK506 (group A) or SRL (group B) was not restored by adding one of the positively selected populations, i.e. αβ+, γδ+ or B220+ T lymphocytes. As none of the selected populations was able to restore the passive transfer of the CS reaction, the strong likelihood is that more than a cell subset is affected by SRL and FK506. Figure 3, group C, further confirms the observation that 4 day TNCB-immune lymph node cells from mice that had been treated with CsA also failed to transfer CS. However, coinjection of these cells with positively selected αβ+ cells completely restored the transfer of the CS reaction. Cotransfer of 4 day TNCB-immune lymph node cells from CsA-treated mice with positively selected γδ+ or B220+ T lymphocytes virtually failed to give significant CS reaction. Therefore, we conclude that CsA affects only αβ+ T lymphocytes.
Fig. 3.
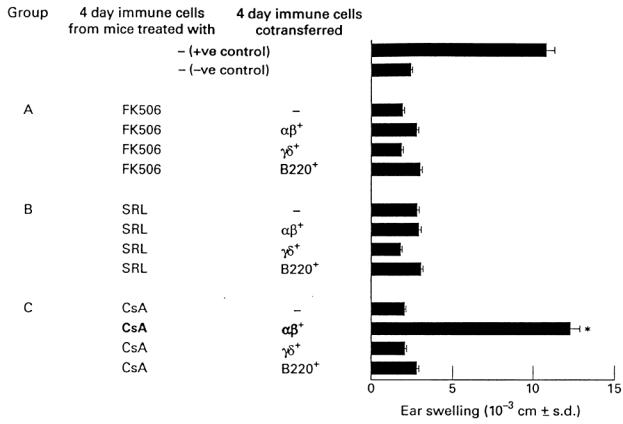
Effect of Sirolimus (SRL), FK506 and cyclosporin A (CsA) on the T cell subsets that mediate the contact sensitivity (CS) reaction. Four day trinitrochlorobenzene (TNCB)-immune lymph node cells from mice that had been treated with SRL, CsA or FK506 were injected into naive recipient mice together with αβ+, γδ+ or B220+ T lymphocytes that had been positively sorted from TNCB-immune lymph node cells. *P < 0.001 compared with the negative control group.
SRL and FK506 inhibit both αβ+and γδ+ T lymphocytes in the CS reaction
The above reported reconstitution experiments show that CsA affects αβ+ T lymphocytes, while SRL and FK506 seem to affect more than one single subset. In order to address this issue, 4 day TNCB-immune lymph node cells from mice that had been treated with SRL or FK506 were transferred into naive recipient mice, together with combinations of two positively selected populations of αβ+, γδ+ or B220+ T lymphocytes.
Figure 4 shows that transfer of 4 day TNCB-immune lymph node cells from SRL- or FK506-treated mice with a combination of αβ+ and γδ+ T lymphocytes from normal donors fully restored the CS reaction. In contrast, transfer of 4 day TNCB-immune lymph node cells from SRL- or FK506-treated mice with a combination of αβ+ and B220+ T lymphocytes, or with a combination of γδ+ and B220+ T lymphocytes, failed to give significant CS into naive recipient mice. From these results we conclude that SRL and FK506 affect both the αβ+ and γδ+ T lymphocytes involved in the CS reaction.
Fig. 4.
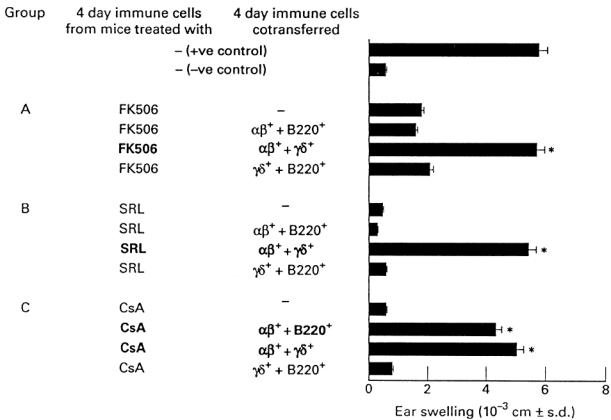
Sirolimus (SRL) and FK506 inhibit both αβ and γδ T lymphocytes. Four day trinitrochlorobenzene (TNCB)-immune lymph node cells from mice that had been treated with SRL or FK506 were transferred into naive recipient mice together with combinations of two positively selected populations of αβ+, γδ+ or B220+ T lymphocytes. *P < 0.001 compared with the negative control group.
These results were confirmed by experiments in which cotransfer of 4 day TNCB-immune lymph node cells from SRL- or FK506-treated mice together with a TNCB-specific T cell line consisting of αβ+ CD4+ and γδ+ T cells, but lacking B220+ cells [20], gave a significant CS reaction into recipient mice (our unpublished results).
Effect of SRL, CsA and FK506 on antigen-specific proliferation and IL-2 production
Mice were injected intraperitoneally with SRL, FK506 and CsA at the day of immunization with TNCB. As a control, a group of mice received ethanol. Lymph node cells were harvested 4 days later and tested for their ability to proliferate and produce IL-2 upon subsequent in vitro exposure to the specific antigen. Figure 5 shows that the lymph node cells of mice that had been immunized with TNCB and injected with CsA or FK506 had impaired ability to proliferate and produce IL-2 upon stimulation with TNCB–APC in vitro. In contrast, the lymph node cells of mice that had been immunized with TNCB and injected with SRL proliferated and produced IL-2 at levels comparable to control groups (i.e. lymph node cells of mice that had been immunized only with TNCB). Injection of mice with ethanol had virtually no inhibitory effect on the ability of lymph node cells to proliferate and produce IL-2.
Fig. 5.
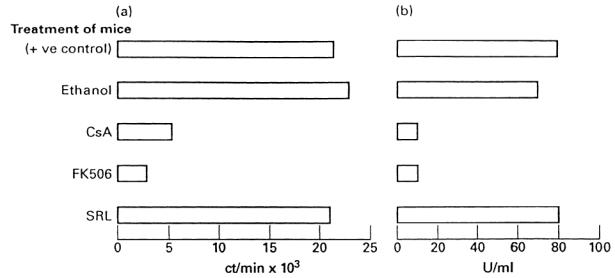
Inhibition of antigen-specific proliferation (a) and IL-2 production (b) by Sirolimus (SRL), FK506 and cyclosporin A (CsA). Positive control group refers to trinitrochlorobenzene (TNCB)-immune lymph node cells taken from mice that did not receive any drug.
DISCUSSION
SRL, FK506 and CsA are three potent immunosuppressive drugs. CsA is actually used in clinical practice to prevent graft rejection and in the treatment of certain autoimmune diseases. SRL and FK506 possess a more potent immunosuppressive activity than CsA, and clinical trials are in course in organ transplantation. Furthermore, recent results have reported a synergistic suppressive effect of SRL and CsA on the prevention of experimental allograft rejection [27].
Despite extensive in vitro studies, the exact mechanisms by which these drugs exert their immunosuppressive activity in vivo are still ill understood, and very little information is available on the cellular and molecular targets during the in vivo outcome of an immune response.
We have recently defined the cellular and molecular mechanisms responsible for the CS reaction, which is one of the most widely studied in vivo models of cell-mediated immune response in the mouse. The CS reaction requires participation of three distinct T cell subsets (see Fig. 1): (i) an αβ+, CD4+ T lymphocyte, which is the classical effector cell of the CS reaction [15–17]; (ii) a γδ+ T lymphocyte, which rearranges the Vγ3 gene region [18]; (iii) an αβ+, double-negative (CD4− CD8−) T lymphocyte which rearranges an invariant Vα14 TCR Vα chain and expresses the B220 molecule [19]. This third T cell subset produces IL-4 [19] that acts on the γδ+ T lymphocytes that express IL-4 receptors. Once they have bound IL-4, the γδ+ T lymphocytes acquire the ability to leave the lymph nodes and to localize at the site of antigen challenge ([20–22] and our unpublished results). This in vivo model therefore provides a useful tool to study the cellular and molecular targets of SRL, FK506 and CsA in vivo.
The results reported in this study clearly show that SRL, FK506 and CsA strongly inhibit the CS reaction when injected in vivo, and immune lymph node cells from drug-treated mice also fail to transfer the CS reaction when injected into naive recipient mice. In these experiments, optimal inhibition of the CS reaction and its passive transfer was obtained with 5 mg/kg SRL and FK506, while 70 mg/kg CsA were necessary to achieve similar inhibitory effects. Our results are in very good agreement with those obtained by Carlsson et al. [25] in bovine serum albumin (BSA)-induced CS reaction in vivo, and by Borel et al. [26] in the CS reaction to the hapten Oxazolone in vivo. A deep analysis of the mechanisms of action of SRL, FK506 and CsA revealed that they display their immunosuppressive activities on quite different target cell populations. By reconstitution experiments, it was in fact observed that transfer of CS by immune lymph node cells of CsA-treated mice was fully reconstituted by providing αβ+ T lymphocytes, but not by γδ+ or B220+ T lymphocytes, suggesting that αβ+ cells were actually lacking or functionally inactive in mice treated with CsA. In contrast, αβ+, γδ+ or B220+ T lymphocyte populations alone failed to restore a passive transfer of the CS reaction carried out by immune lymph node cells of SRL- or FK506-treated mice, thus indicating that more than a single cell subset was affected by these two drugs. Support for this possibility comes from the results reported in Fig. 4. Transfer of 4 day TNCB-immune lymph node cells from SRL- or FK506-treated mice with a combination of αβ+ and γδ+ T lymphocytes from normal donors fully restored the CS reaction. In contrast, transfer of 4 day TNCB-immune lymph node cells from SRL- or FK506-treated mice with a combination of αβ+ and B220+ T lymphocytes, or with a combination of γδ+ and B220+ T lymphocytes, failed to give significant CS into naive recipient mice. From these results, we conclude that CsA, FK506 and SRL affect the CS reaction by a suppressive effect on different T cell subsets: CsA inhibits the αβ+ CD4+ T lymphocytes, while FK506 and SRL inhibit both the αβ+ CD4+ and the γδ+ T lymphocytes. None of the drugs had inhibitory activity on the B220+ T cell population. This is in full agreement with other studies showing that in vivo FK506 alone fails to cause significant inhibition of the development of a B220+, αβ+, double-negative T cell subset which occurs in autoimmune-prone mouse strains [28].
Our in vivo results clearly differ from those obtained in several models in vitro, in which CsA and FK506 act through similar, if not identical, mechanisms, which are different from the mechanisms of action of SRL. This suggests that studies in vitro, in which proliferation and cytokine production are measured, are not sufficient alone to provide a clear picture of the immunosuppressive activities of CsA, FK506 and SRL. Support for this assumption comes from the results reported in Fig. 5, in which we report that mice injected with CsA and FK506 failed to proliferate and produce IL-2 upon re-exposure to the specific antigen in vitro, while mice injected with SRL showed good antigen-specific proliferative and IL-2 response in vitro, although failing to develop CS reaction in vivo.
These results merit some discussion in the light of the mechanisms leading to T lymphocyte activation. Signal transduction through the TCR induces an elevation of intracellular Ca2+ concentration and activation of ras. The sustained rise in intracellular Ca2+ activates calcineurin that, in turn, induces nuclear translocation of the cytosolic components of NF-AT. This pathway finally leads to activation of a number of early genes, amongst which is the IL-2 gene.
CsA binds to its receptor, cyclophilin, and the resulting complex blocks the activity of calcineurin. The net effect of this block is a complete inhibition of the translocation of NF-AT from the cytoplasm to the nucleus and a lack of T cell cytokine gene transcription (reviewed in [29]).
The critical steps affected by SRL lie downstream to these events. SRL does not block cytokine gene transcription at these early stages of T cell activation, but inhibits T cell proliferation in response to several cytokines and growth factors at the G1- to S-phase progression (reviewed in [29]).
Experiments in which CsA, FK506 and SRL have been used in vivo have so far failed to provide a clear explanation of their immunosuppressive activity. Studies on chronic graft-versus-host reaction (GVHR), which is regarded as a Th2-mediated reaction, have shown that SRL exerts a potent inhibitory activity, while CsA and FK506 cause marked potentiation of GVHR at low doses and only exhibit inhibition at higher doses [30]. It is believed that the potentiating effect of CsA and FK506 is a consequence of selective inhibitory activity of the Th1 subset, while at higher doses the selectivity towards Th1 subset is lost and there is a non-specific suppression of both Th subsets. Accordingly, CsA has proved to be more effective in those autoimmune diseases that subsequently have proved to be Th1-mediated [31,32]. In contrast, in another in vivo system, the primary Th2 response to Ascaris suum extracts was inhibited by high-dose FK506, while the secondary Th2 response was potentiated [33]. The explanation is that only primary, but not secondary, IL-4 production is FK506-sensitive [33]. Finally, in rats SRL inhibits production of cytotoxic antibodies while increasing serum levels of non complement-fixing alloantibodies, and this is accompanied by selective activation of Th2 cells that mediate long-term survival of heart allografts [34].
Recent analysis documented synergism between SRL and CsA in blocking rat heart and kidney allograft rejection [27], but the mechanisms are unknown. The synergism may reflect complementary biological activities of these drugs at the single-cell level, for example by facilitating the inhibition of cytokine signal transduction [27]. Alternatively, or in addition, the synergism may reflect complementary inhibitory activity of these drugs on different T cell subsets. This study provides results supporting this possibility, as it shows that CsA inhibits αβ T lymphocytes, while FK506 and SRL inhibit both αβ and γδ T lymphocytes.
These results may be of relevance in the understanding of allograft rejection after transplantation. In fact, it has been reported that γδ T lymphocytes are present in the biopsies of kidneys and hearts undergoing rejection [35,36], although it is not clear if these cells play a role, if any, in the cell-mediated response that causes rejection. In contrast, in mice lacking αβ+ T lymphocytes because of a disruption in the TCRα gene, γδ cells were sufficient to cause a significant percentage of skin graft rejections in certain alloantigen combinations [37]. As γδ cells are mainly located in epithelia (skin, intestine), these cells may play an important role in the graft rejection involving such organs, while their role might be only marginal in the rejection of different organs (kidney, heart). Further studies are, however, required in vivo to better define the mechanisms of the immunosuppressive activity exerted by CsA, FK506 and SRL on αβ and the γδ T lymphocytes: the CS reaction model used here may provide a simple and useful tool to this end.
Acknowledgments
We thank S. N. Sehgal for the generous gift of SRL, S. Cammisuli for the generous gift of CsA and W. E. Paul for the kind gift of the anti-IL-4 MoAb. This work was supported by grants from the Ministry for Education and Scientific and Technologic Research (MURST 40% and 60% to A.S. and F.D.) and from Istituto Superiore di Sanità (Progetto Sostituzioni funzionali, organi artificiali e trapianti d'organo) to A.S.
References
- 1.Morris RE, Wu J, Shorthouse R. Comparative immunopharmacologic effects of FK506 and CyA in in vivo models of organ transplantation. Transplant Proc. 1990;22:110–2. [PubMed] [Google Scholar]
- 2.Morris RE. Rapamycins: antifungal, anti-tumor, anti-proliferative and immunosuppressive molecules. Transplant Rev. 1992;6:39–72. [Google Scholar]
- 3.Schmidt JA, Bundick RV. Immunosuppressants as cytokine inhibitors. In: Bodmer M, Henderson B, editors. Pharmacological modulation of cytokines. New York: CRC Press; 1995. pp. 213–51. [Google Scholar]
- 4.Martel RR, Klicius J, Galer S. Inhibition of the immune response by rapamycin, a new antifungal antibiotic. Can J Physiol Pharmacol. 1977;55:48–51. doi: 10.1139/y77-007. [DOI] [PubMed] [Google Scholar]
- 5.Schreiber SL, Crabtree GR. The mechanisms of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–42. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 6.Ho S, Clipstone N, Timmermann L, et al. The mechanisms of action of Cyclosporin A and FK506. Clin Immunol Immunopathol. 1996;80:S40–45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- 7.Sigal NH, Dumont FJ. Cyclosporin A, FK506 and rapamycin: pharmacological probes of lymphocyte signal transduction. Ann Rev Immunol. 1992;10:519–60. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- 8.Dumont FJ, Staruch MJ, Koprach SL, et al. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK506 and rapamycin. J Immunol. 1990;144:251–8. [PubMed] [Google Scholar]
- 9.Schreiber SL. Chemistry and biology of the immunophillins and their immunosuppressive ligands. Sci. 1991;251:283–7. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 10.Bierer BE, Mattila PS, Standaert RF, et al. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophillin and either FK506 or rapamycin. Proc Natl Acad Sci USA. 1990;87:9231–5. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung J, Kuo CJ, Crabtree GR, et al. Rapamycin-FKBP specifically blocks growth-dependent activation of and signalling by the 70 kD S6 protein kinases. Cell. 1992;69:1227–36. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Farmer JD, Lane WS, et al. Calcineurin is a common target of cyclophillin–cyclosporin A and FKBP–FK506 complexes. Cell. 1991;66:807–15. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 13.Granelli-Piperno A, Nolan P, Inaba K, et al. The effect of immunosuppressive agents on the induction of nuclear factors that bind to sites on the interleukin 2 promoter. J Exp Med. 1990;172:1869–72. doi: 10.1084/jem.172.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson DJ, Naya I, Bundick RV, et al. Comparison of the effects of FK506, cyclosporin A and rapamycin on IL-2 production. Immunol. 1991;73:316–21. [PMC free article] [PubMed] [Google Scholar]
- 15.Dieli F, Asherson GL, Sireci G, et al. Dominant Vβ8 gene usage in response to TNP: failure to use other Vβ chains following removal of Vβ8+ T cells by monoclonal antibodies in vivo. Immunol. 1994;82:99–105. [PMC free article] [PubMed] [Google Scholar]
- 16.Dieli F, Asherson GL, Tomonari K, et al. T cell receptor Vα chain expression influences reactivity to the hapten TNP. Int Immunol. 1996;8:101–8. doi: 10.1093/intimm/9.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Dieli F, Asherson GL, Sireci G, et al. Development of interferon gamma-producing CD8+γδ+ T lymphocytes and interleukin 2-producing CD4+αβ+ T lymphocytes during contact sensitivity. J Immunol. 1997;158:2567–75. [PubMed] [Google Scholar]
- 18.Dieli F, Asherson GL, Sireci G, et al. γδ T lymphocytes involved in contact sensitivity preferentially rearrange the Vγ3 region and require IL-7. Eur J Immunol. 1997;22:404–13. doi: 10.1002/eji.1830270131. [DOI] [PubMed] [Google Scholar]
- 19.Salerno A, Dieli F, Sireci G, et al. Three cell subsets are required for the systemic transfer of delayed-type hypersensitivity reaction by antigen-specific T cell lines. Cell Immunol. 1997;175:1–7. doi: 10.1006/cimm.1996.1034. [DOI] [PubMed] [Google Scholar]
- 20.Dieli F, Asherson GL, Colonna Romano G, et al. IL-4 is essential for the systemic transfer of delayed hypersensitivity by T cell lines. Role of γδ cells. J Immunol. 1994;152:2698–704. [PubMed] [Google Scholar]
- 21.Salerno A, Dieli F, Sireci G, et al. Interleukin-4 is a critical cytokine in contact sensitivity. Immunol. 1995;84:404–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Asherson GL, Dieli F, Sireci G, et al. Role of IL-4 in delayed-type hypersensitivity. Clin Exp Immunol. 1996;103:1–4. doi: 10.1046/j.1365-2249.1996.845537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asherson GL, Dieli F. Immune deviation in the mouse: transfer of selective depression of the contact sensitivity and interleukin-2 response with retention of interferon-gamma production requires CD8+ T cells. Immunol. 1992;76:427–32. [PMC free article] [PubMed] [Google Scholar]
- 24.Dieli F, Asherson GL, Bonanno CT, et al. Major histocompatibility complex control of the class of the immune response to the hapten trinitrophenyl. Immunol. 1995;84:355–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Carlsson R, Calhoun W, Lugay J, et al. Oral effect of rapamycin on T-cell mediated inflammation in rodent models. Faseb J. 1990;4:1021. (Abstr.). [Google Scholar]
- 26.Borel JF, Feurer C, Gubler HU, et al. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976;6:468–75. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- 27.Stepkowsky SM, Tian L, Napoli LK, et al. Synergistic mechanisms by which sirolimus and cyclosporin inhibit rat heart and kidney allograft rejection. Clin Exp Immunol. 1997;108:63–68. doi: 10.1046/j.1365-2249.1997.d01-984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woo J, Wright TM, Lemster B, et al. Combined effects of FK506 (tacrolimus) and cyclophosphamide on atypical B220+ T cells, cytokine gene expression and disease activity in MRL/MpJ-lpr/lpr mice. Clin Exp Immunol. 1995;100:118–25. doi: 10.1111/j.1365-2249.1995.tb03612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho S, Clipstone N, Timmermann L, et al. The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol. 1996;80:S40–45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- 30.Bundick RV, Craggs RI, Holness E. The effect of cyclosporin A, FK506 and Rapamycin on the murine chronic graft-vs.-host response—an in vivo model of Th2-like activity. Clin Exp Immunol. 1995;99:467–72. doi: 10.1111/j.1365-2249.1995.tb05574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon AK, Seipelt E, Wu P, et al. Analysis of cytokine profiles in synovial T cell clones from chlamydial reactive arthritis patients: predominance of the Th1 subset. Clin Exp Immunol. 1993;94:122–6. doi: 10.1111/j.1365-2249.1993.tb05988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersson J, Nagy S, Groth CG, et al. Effects of FK506 and cyclosporin A on cytokine production studied in vitro at a single-cell level. Immunol. 1992;75:136–42. [PMC free article] [PubMed] [Google Scholar]
- 33.Yamashita M, Yamaoka M, Seki N, et al. Non-T cell-derived IL-4 plays an important role in IgE production induced by antigen resensitization and is resistant to FK506. J Immunol. 1996;157:714–9. [PubMed] [Google Scholar]
- 34.Ferraresso M, Tian L, Ghobrial R, et al. Rapamycin inhibits production of cytotoxic but not noncytotoxic antibodies and preferentially activates T helper 2 cells that mediate long-term survival of heart allografts in rats. J Immunol. 1994;153:3307–18. [PubMed] [Google Scholar]
- 35.Kirk DA, Ibrahim S, Dawson DA, et al. Characterization of T cells expressing the γ/δ antigen receptor in human renal allografts. Hum Immunol. 1993;36:11–19. doi: 10.1016/0198-8859(93)90003-j. [DOI] [PubMed] [Google Scholar]
- 36.Vaessen LMB, Schipper F, Knopp C, et al. Inverted Vδ1/Vδ2 ratio within the T cell receptor (TCR)-γδ T cell population in peripheral blood of heart transplant recipient. Clin Exp Immunol. 1996;103:119–24. doi: 10.1046/j.1365-2249.1996.909604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandler P, Frater JA, Douex DC, et al. Immune responsiveness in mutant mice lacking T-cell receptor αβ+ cells. Immunol. 1995;85:531–7. [PMC free article] [PubMed] [Google Scholar]


