Abstract
As previous studies have indicated that genital tract mucosal T cell function may be impaired in HIV infection, we investigated the T cell cytokine mRNA in the genital tract mucosa of HIV-infected women to determine if there are alterations in the cytokine profile which may explain the T cell impairment. The in situ hybridization technique was used to investigate the T helper-1 (Th1: IL-2, interferon-gamma (IFN-γ)) and Th2 cytokine (IL-4, IL-5, IL-10) mRNA profile in cervical biopsies from 10 HIV+ and 10 HIV− subjects. Cervical intraepithelial neoplasia (CIN) and genital infection had previously been excluded and the distribution of immunocompetent cells within the cervical mucosa was known for each subject. Non-parametric tests were used to compare the optical density (OD) of cytokine mRNA in the HIV+ and HIV− groups. Comparisons were also made between peripheral CD4 lymphocyte counts, cervical CD4/CD8 T lymphocyte ratios and cytokine mRNA OD in HIV+ subjects. The HIV+ women had significantly higher mRNA OD for the Th2 cytokines IL-4, IL-5 and IL-10 than HIV− women. There was also significantly lower IL-2 mRNA OD in the former group. HIV+ women had lower IFN-γ mRNA than HIV− women, but the difference was not statistically significant. There was no correlation between cytokine mRNA OD and peripheral CD4 count or cervical CD4/CD8 ratio. The predominance of Th2 cytokines, which are immuno-inhibitory, in the cervical mucosa of HIV+ women may underlie the impaired cytotoxic potential observed in the CD8+ T lymphocytes and may contribute to the susceptibility of HIV-infected women to recurrent genital tract infections and cervical neoplasia.
Keywords: HIV, female, immunology, cytokines, uterine cervix
INTRODUCTION
The existence of an immune system in the female lower genital tract has been acknowledged for several years [1–3], but its role in the prevention of infection and protection against cervical neoplasia is poorly understood. The rising prevalence of HIV infection in women [4] and the risks of transmission to the offspring [5] have recently focused the attention of researchers on the mechanisms of mucosal transmission of HIV. Epidemiological studies have been successful in identifying some of the factors which predispose to the horizontal [6] and vertical [7,8] transmission of HIV, but there is now a recognition that an understanding of local immune function may be the key to elucidating the mechanism of HIV transmission.
Work undertaken in this Department [9] has shown that HIV infection is associated with alterations in the distribution of immunocompetent cells in the lower genital tract. In particular, there is an increase in absolute cytotoxic (CD8+) lymphocyte proportions in the cervical mucosa when compared with HIV− women. These CD8+ cells, although primed (CD45RO+), appeared to be functionally impaired, in that they showed little or no expression of cytolytic granules (perforinlow, TIA-1−). These changes occurred before there was evidence of systemic immune dysfunction. It was postulated that systemic HIV infection may occur because of the inability of the inept T cells to overcome sexually acquired HIV at a local level. It was also suggested that the impaired cytotoxic potential of these cells may underlie the susceptibility of HIV-infected women to recurrent viral and fungal infections of the genital tract and to cervical intraepithelial neoplasia (CIN).
It is, as yet, unclear why these changes in T cell subset distribution occur. It has been shown that HIV infection is associated with changes in the pattern of cytokine production in the peripheral blood [10,11]. However, there have been few attempts to investigate cytokine production in the lower genital tract in HIV-infected females. Belec et al. [12] showed that levels of proinflammatory cytokines were raised in cervicovaginal secretions of HIV-infected women. It may be that changes also occur in T cell cytokine production in the genital tract which may underlie the alterations in immunocompetent cell distribution observed in the cervical mucosa of HIV+ women.
Two distinct patterns of cytokine were originally defined among a panel of mouse T cell clones [13]. T helper-1 (Th1) cells produce IL-2 and interferon-gamma (IFN-γ), while Th2 cells express IL-4, IL-5 and IL-10. Evidence from strong immune responses in both mice and humans suggests that Th1 and Th2 cell patterns both occur and are important in vivo [14,15]. Th1 responses are usually immunostimulatory, while Th2 responses are inhibitory. The response pattern induced by a particular pathogen is usually predictable and appropriate [16], but there are severe consequences if an incorrect pattern is induced. HIV establishes a close interaction with the immune system that can result in increased or decreased production of various cytokines [10]. It has been suggested that T lymphocytes in the peripheral blood of HIV-infected individuals switch from producing Th1 cytokines to producing Th2 cytokines. This switch from a Th1 to Th2 cytokine production pattern is said to favour progression to AIDS [17,18]. Altered patterns of cytokine production have been described at other mucosal surfaces in HIV infection [19], but there are few studies investigating cytokine production in the lower genital tract in HIV-infected women. We therefore decided, using the technique of in situ hybridization, to study the Th1, Th2 cytokine mRNA profile in cervical biopsies from HIV-infected women and to compare these with cervical biopsies from HIV− women, with a view to elucidating further the immune mechanisms of the lower genital tract and changes associated with HIV infection.
SUBJECTS AND METHODS
Subjects
A group of 10 HIV+ women at varying stages of disease and a group of 10 HIV− volunteers with documented negative HIV antibody tests were recruited to the study. The HIV+ women were out-patient attenders of the Ian Charleson Centre, the dedicated HIV unit of the Royal Free Hospital. The HIV− women were recruited from the same-day HIV antibody testing clinic, also based at the Ian Charleson Day Centre. The study had received prior approval from the Hospital Ethics Committee and informed consent was obtained in all cases. Demographic details including social, sexual and menstrual history were available for all subjects, and genital infection and CIN had previously been excluded. As previously described [9], a biopsy of the squamo-columnar junction of the cervix was obtained from each subject during the proliferative phase of the menstrual cycle. Peripheral blood was collected by venepuncture from all HIV+ subjects on the same day as the cervical biopsy was taken.
Preparation of biopsy specimens
The biopsy specimens were frozen in liquid nitrogen, cut and fixed as previously described [9]. The cut sections, mounted on poly-l-lysine coated slides, were wrapped back to back in cling film and stored at −20°C until required. Prior staining of sections from each biopsy with haematoxylin and eosin established that there was no histological abnormality in biopsies from both groups. The distribution of immunocompetent cells, including the CD4/CD8 T cell ratios, had been similarly established for biopsy sections from each group [9].
In situ hybridization
mRNA for IL-2, IFN-γ, IL-4, IL-5 and IL-10 were studied. Tissue fixation was performed by immersing the slides in 4% paraformaldehyde for 10 min. The sections were then pretreated by immersing them in 40 ml 10 × PBS + 2 ml 1 m MgCl2 + 358 ml DEPC-H2O (PBS–M) for 10 min and in PBS–M + 0.25% Triton X-100 + 0.25% Nonidet P40 twice for 5 min each time. Acetylation of the specimens was performed by dipping the slides in 20% acetic acid at 4°C for 15 s. The sections were then incubated in 20% glycerol for 1 h. Hybridization was performed by placing the slides in a moist chamber and covering each section with 30 μl of the probe at an appropriate dilution in prehybridization solution. They were incubated for 20 min at 70°C for 20 min and at 37°C overnight. In order to demonstrate that the target nucleic acid was RNA, controls were prepared in which biopsy sections were pretreated with RNase before application of the specific oligonucleotide probe. Additionally, positive controls in which sections were incubated with poly-dT oligonucleotide probes which detects all animal mRNAs were prepared. Simultaneous negative controls in which the specific oligonucleotide probe was omitted were also used.
The slides were washed after hybridization to remove non-specifically bound RNA before detection. Digoxigenin (DIG)-labelled synthetic single-stranded DNA oligonucleotide probes (R&D Systems, Abingdon, UK) were used to identify mRNA for the cytokines of interest and detection was carried out with anti-DIG antibody (Boehringer, East Sussex, UK). The slides were developed with 5-bromo-4-choloro-3-indolyl phosphate (BCIP)/NBT (Sigma, Poole, UK). After developing, the slides were washed in de-ionized water to remove the coverslip. They were wiped carefully and mounted in PBS glycerol.
mRNA expression was quantified on each section by measurement of optical density (OD) using a monochrome video camera attached to a microscope with a × 40 objective. The relative density of the reaction product was recorded using a computerized image analyser (Seescan, Cambridge, UK). Frames were drawn around positively staining cells as previously described [9], selecting three areas per slide. The software automatically calculates the area within the frame and the OD per unit area is generated by the software. The OD of all positively staining cells within each frame was measured and the average OD per biopsy section calculated. Each section was independently analysed by two observers (A.O., L.W.P.). Comparisons were made between the HIV+ and the HIV− group for each cytokine studied.
Statistical analysis
Unless otherwise stated, the median values, with the range in parentheses, are given. The Mann–Whitney U-test was used to compare values from the HIV+ and HIV− samples. A probability value (P) of ≤ 0.05 was taken as indicating statistical significance. Spearman rank correlation was used to assess the correlation between cytokine mRNA expression in cervical biopsies and other immune parameters in the cervix and peripheral blood. All tests of significance were two-sided.
RESULTS
Demography of study population
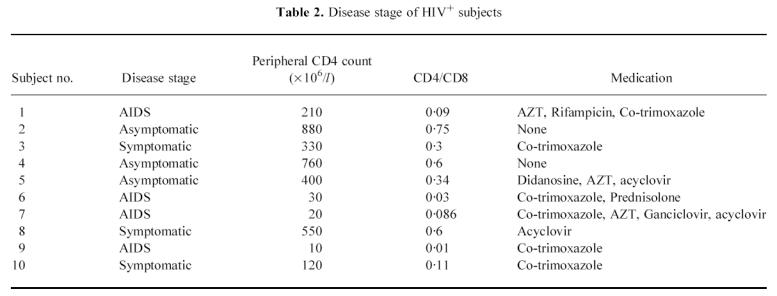
The HIV+ and HIV− women were similar in terms of age, sexual and menstrual history (Table 1). All the HIV+ women acquired HIV through heterosexual intercourse. Table 2 shows the disease stage in the HIV+ women. All HIV+ women used condoms for contraception, but two women used the combined oral contraceptive pill (cocp) in addition. Two of the HIV− women used the cocp, while the others relied on condoms. One woman from each group smoked cigarettes, each smoking over 20 cigarettes a day.
Table 1.
Demographic and lifestyle details of HIV+ and HIV− subjects
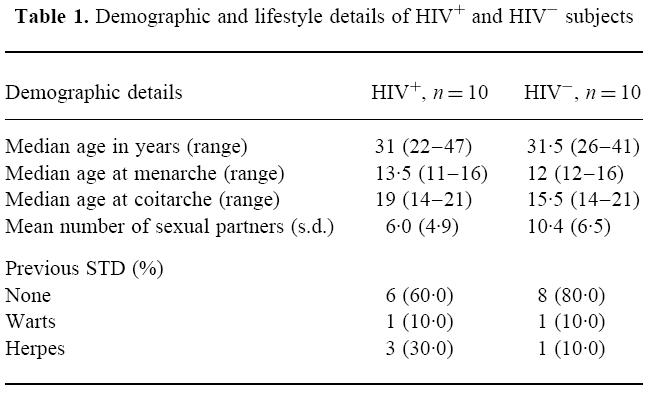
Table 2.
Disease stage of HIV+ subjects
Cytokine mRNA expression
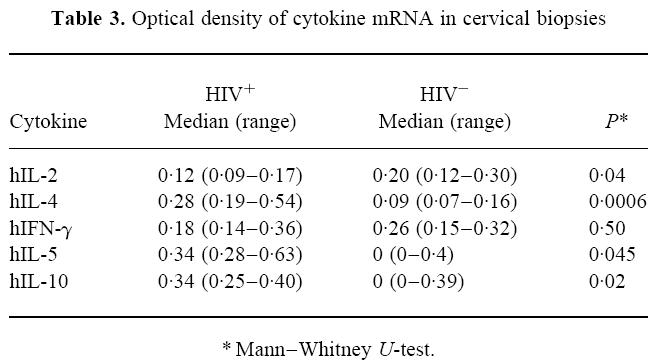
The median OD measurements for each of the cytokines under study are shown in Table 3. mRNA for the Th1 cytokines IL-2 and IFN-γ and the Th2 cytokines IL-4, IL-5 and IL-10 was present in biopsy sections from both the HIV+ and HIV− groups. However, there was significantly less mRNA for IL-2 detected in cervical biopsies from the HIV+ subjects compared with the HIV− subjects. Although there was less IFN-γ mRNA in biopsies from the HIV+ group compared with the HIV− group, this difference was not statistically significant. However, there was significantly more mRNA for the Th2 cytokines, IL-4, IL-5 and IL-10 in the cervical biopsies from HIV+ compared with HIV− subjects.
Table 3.
Optical density of cytokine mRNA in cervical biopsies
Correlates with peripheral blood
No relationship was detected between peripheral CD4 lymphocyte count and cervical cytokine mRNA OD in HIV+ women. The number of women who smoked and the number of women who used the combined oral contraceptive in each group was too small for subgroup analysis, assessing the effects of these factors, to be performed.
Correlates with cervical immune parameters
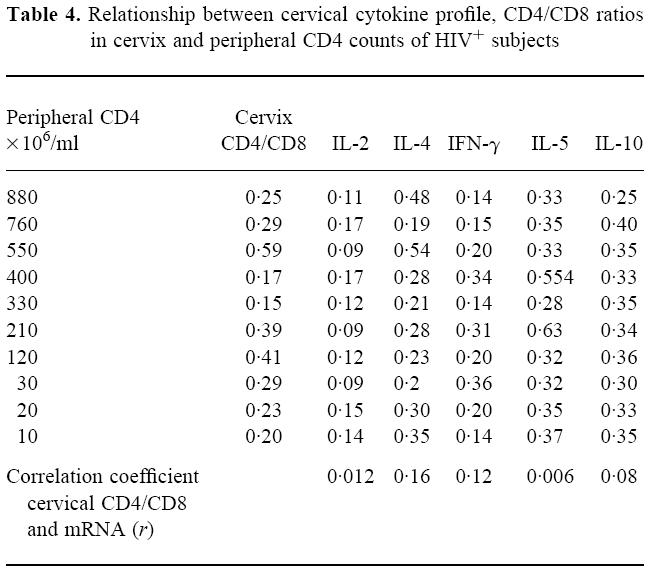
Table 4 shows the peripheral CD4 lymphocyte counts, the cervical CD4/CD8 ratios and the OD for the mRNA of the cytokines under study. As previously demonstrated [9], no relationship was detected between peripheral CD4 counts and cervical CD4/CD8 ratios. In addition, there was no apparent relationship between the cervical CD4/CD8 ratios and the mRNA OD for any of the cytokines under study. The correlation coefficients for cervical CD4/CD8 ratios and cytokine mRNA OD are stated in Table 4.
Table 4.
Relationship between cervical cytokine profile, CD4/CD8 ratios in cervix and peripheral CD4 counts of HIV+ subjects
DISCUSSION
This study demonstrates that there is a predominance of mRNA for Th2 cytokines in the cervix of HIV+ women. This observation reflects the Th1 to Th2 shift described in the literature as being associated with HIV disease and which is said to predispose to HIV disease progression [20].
Th1 and Th2 responses occur naturally, and may be an immunologically appropriate response to invasion by certain pathogens. For instance, there is experimental evidence to show sequential production of Th1 and Th2 cytokines in response to live bacille Calmette–Guérin (BCG) [14]. The initial response was dominated by macrophage cytokines (monokines), followed by a marked production of Th1 cytokines (IL-2, IFN-γ) at days 4–5. Later in the reaction (days 10–12) a Th2 response, with IL-4, IL-5 and IL-10 production, was observed. There is substantial evidence of cross-regulation between the Th1 and Th2 Th cell subsets [16]. For example, IL-10 inhibits synthesis of cytokines by Th1 cells which can result in decreased proliferation. IFN-γ, which is itself inhibited by IL-10, inhibits the growth of Th2 cells and IL-4 preferentially stimulates the growth of Th2 cells. Sander et al. [14] therefore concluded that the production of Th2 cytokines late in the response to BCG may reflect a dynamic in vivo balance, preventing over-expression of inflammatory, potentially tissue-damaging Th1 cytokines.
However, HIV infection is chronic and the presence of infectious virus has been demonstrated in female genital tract secretions at all stages of disease [21–23]. The observation of a predominance of Th2 cytokines may therefore not necessarily reflect an HIV-induced pathological shift in cytokine production, but may be an exaggeration of a normal process. The chronicity of HIV, with the constant background antigenic stimulation, may lead to an accumulation of virally stimulated cells which produce Th2 cytokines. This theory is consistent with the observation in a previous publication that there is an accumulation of ‘primed’ lymphocytes in the cervix of HIV+ women [9]. Paradoxically, the accumulation of Th2 cytokines may predispose to the chronicity of the viral infection. IL-10 inhibits Th1 cytokine synthesis, and as murine cytotoxic T lymphocytes synthesize the Th1 pattern of lymphocytes [24], and the same may occur in humans, IL-10 may have a suppressive effect on their ability to kill the virus. Other conditions, such as IgA nephropathy, have been described where an excess of Th2 cytokine production is associated with a disease process [15,25]. No correlation was observed between cytokine levels and HIV disease stage as defined by peripheral CD4 lymphocyte counts, but there may be other confounding and regulating factors (such as the use of anti-retroviral therapy) which were not directly studied. In addition, the small subject numbers mean that non-linear relationships may not be apparent.
Finally, the susceptibility of HIV+ women to cervical neoplasia and recurrent genital tract infections may, in part, be explained by this cytokine pattern. Th1 cytokines are immunostimulatory and are thus capable of limiting tumour growth and arresting viral and fungal infection, while Th2 cytokines are immuno-inhibitory and are capable of stimulating tumour growth [26]. Thus the predominance of Th2 cytokines in the cervical tissues of HIV+ women may permit neoplastic change, either alone or in conjunction with other cofactors such as human papillomavirus (HPV). Clerici et al. [26] demonstrated that peripheral blood mononuclear cells (PBMC) from women with extensive CIN showed reduced production of IL-2 when stimulated by mitogens when compared with PMBC from women with localized CIN or healthy controls. PMBC from the group with extensive disease showed an increased production of IL-4 and IL-10 compared with the healthy group or those with localized disease. These findings lend weight to the theory that a predominance of Th2 cytokines, either locally or in the peripheral circulation, permits neoplastic change and predisposes women to CIN.
The caveat must be made, however, that the observation of increased mRNA for a particular cytokine may not necessarily be reflected in increased production of that cytokine. It is important, therefore, to study cytokine production in the cervix of HIV+ women and to relate this to the cytokine mRNA. In addition, it is not clear whether the mRNA cytokine changes observed are HIV-induced or if some other cofactor, such as HPV, is involved. Studies have shown that the prevalence of HPV16 and 18, which are considered to be of high oncogenic potential, is increased in the cervix of HIV+ women with or without CIN [27,28]. Further studies, involving larger subject numbers, relating the presence of HPV to local immune dysfunction may help to elucidate further the mechanisms of genital tract immune function.
In conclusion, therefore, the observation of an altered cytokine profile in the cervix of HIV-infected women is consistent with the previously reported finding of functionally impaired, virally stimulated cytotoxic T lymphocytes within the cervical mucosa. The observed alterations in cytokine profile and cytotoxic T cell phenotype may explain why HIV-infected women are susceptible to recurrent viral and fungal infection of the genital tract and CIN.
References
- 1.Rebello R, Green FH. A study of the secretory system of the female genital tract. Br J Obstet Gynaecol. 1975;82:812–6. [PubMed] [Google Scholar]
- 2.Morris HHB, Gatter KC, Stein H, Mason DY. Langerhans cells in human cervical epithelium: an immunohistochemical study. Br J Obstet Gynaecol. 1983;90:400–11. doi: 10.1111/j.1471-0528.1983.tb08935.x. [DOI] [PubMed] [Google Scholar]
- 3.Kutteh WH, Hatch KD, Blackwell RE, Metescy J. Secretory immune system of the female reproductive tract: I. Immunoglobulin and secretory component-containing cells. Obstet Gynecol. 1988;71:56–60. [PubMed] [Google Scholar]
- 4.Nicoll A, Hutchinson E, Soldan K, et al. Survey of human immunodeficiency virus infection among pregnant women in England and Wales: 1990–93. Communicable Dis Report. 1994;4:R115–R120. [PubMed] [Google Scholar]
- 5.Chin J. Current and future dimensions of the HIV/AIDS pandemic in women and children. Lancet. 1990;3336:221–4. doi: 10.1016/0140-6736(90)91743-t. [DOI] [PubMed] [Google Scholar]
- 6.European Study Group on Heterosexual Trransmission of HIV. Comparison of female to male to female transmission of HIV in 563 stable couples. Br Med J. 1992;304:809–13. doi: 10.1136/bmj.304.6830.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Collaborative Study. Risk factors for mother-to-child transmission of HIV-1. Lancet. 1992;343:1464–7. [Google Scholar]
- 8.European Collaborative Study. Caesarean section and the risk of vertical transmission of HIV-1. Lancet. 1994;339:1007–12. [PubMed] [Google Scholar]
- 9.Olaitan A, Johnson MA, Maclean AB, Poulter LW. The distribution of immunocompetent cells in the genital tract mucosa of HIV-positive women. AIDS. 1996;10:759–64. doi: 10.1097/00002030-199606001-00010. [DOI] [PubMed] [Google Scholar]
- 10.Lai PK, Tamura Y, Bradley WG, Donovan J, Tanaka A, Nonoyama M. Cytokine regulation of the human immunodeficiency virus. Int J Immunopharmacol. 1991;13(Suppl. 1):55–61. doi: 10.1016/0192-0561(91)90125-q. [DOI] [PubMed] [Google Scholar]
- 11.Poli G, Fauci AS. The effect of cytokines and pharmacological agents on chronic HIV infection. AIDS Res Human Retrovir. 1992;8:191–7. doi: 10.1089/aid.1992.8.191. [DOI] [PubMed] [Google Scholar]
- 12.Belec L, Gherardi R, Payan C, Prazuck T, Malkin JE, Tevi-Benissan C, Pillot J. HIV-enhancing pro-inflammatory cytokines in cervico-vaginal secretions of normal and HIV-infected women. Cytokine. 1995;7:568–74. doi: 10.1006/cyto.1995.0077. [DOI] [PubMed] [Google Scholar]
- 13.Mossman TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. Definitions according to profiles of cytokine activities, and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 14.Sander B, Skansen-Saphir U, Damm O, Hakansson L, Andersson J, Andersson U. Sequential production of Th1 and Th2 cytokines in response to live bacillus Calmette–Guerin. Immunol. 1995;82:512–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Ichinose H, Miyazaki M, Koji T, et al. Detection of cytokine mRNA-expressing cells in peripheral blood of patients with IgA nephropathy using non-radioactive in situ hybridisation. Clin Exp Immunol. 1996;103:125–32. [PubMed] [Google Scholar]
- 16.Mossman TR, Moore KW. The role of IL-10 in cross regulation of Th-1 and Th-2 responses. Immunol Today. 1991;12:A48–A53. [Google Scholar]
- 17.Clerici M, Sarin A, Coffman RL, et al. Type1/type 2 cytokine modulation of T-cell programmed cell death as a model for human immunodeficiency virus pathogenesis. Proc Natl Acad Sci USA. 1994;91:11811–5. doi: 10.1073/pnas.91.25.11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Prete G, Maggi E, Pizzolo G, Romagnani S. CD30, Th-2 cytokines and HIV infection: a complex and fascinating link. Immunol Today. 1995;16:76–80. doi: 10.1016/0167-5699(95)80092-1. [DOI] [PubMed] [Google Scholar]
- 19.McGowan I, Radford-Smith G, Jewell DP. Cytokine gene expression in HIV-infected intestinal mucosa. AIDS. 1994;8:1569–75. doi: 10.1097/00002030-199411000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Clerici M, Shearer GM. A Th-1 to Th-2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;17:256–8. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 21.Wofsy CB, Cohen JB, Hauer LB, Pandian NS, Michaelis BA, Evans LA, Levy JA. Isolation of AIDS-associated retrovirus from genital tract secretions of women with antibodies to the virus. Lancet. 1986. pp. 527–9. [DOI] [PubMed]
- 22.Voght MW, Witt DJ, Craven DE, Byington Y, Crawford DF, Schooley RT, Hirsch MS. Isolation of HTLVIII/LAV from cervical secretions of women at risk of AIDS. Lancet. 1986. pp. 525–7. [DOI] [PubMed]
- 23.Clemetson BD, Moss GB, Willerford DM, et al. Detection of HIV DNA in cervical and vaginal secretions. Prevalence and correlates among women in Nairobi, Kenya. JAMA. 1993;269:2860–4. [PubMed] [Google Scholar]
- 24.Fong TAT Mossman. Alloreactive murine CD8+ cell clones secrete Th-1 pattern of cytokines. J Immunol. 1990;144:1744–52. [PubMed] [Google Scholar]
- 25.Field E, Noelle RJ, Rousse T, Goeken J, Waldsmchmidt T. Evidence for excessive Th-2 CD4+ activity in vivo. J Immunol. 1993;151:48–90. [PubMed] [Google Scholar]
- 26.Clerici M, Merola M, Ferrario E, et al. Cytokine production patterns in cervical intraepithelial neoplasia: association with human papillomavirus infection. J Natl Cancer Inst. 1997;89:245–50. doi: 10.1093/jnci/89.3.245. [DOI] [PubMed] [Google Scholar]
- 27.Agarossi A, Muggiasca M, Ravasi L, et al. HIV infection and CIN. VIII international conference on AIDS, Milan. Abstract book no. 3045; 1992; p. B95. [Google Scholar]
- 28.Van Doornum GJ, Van den Hoek JA, Van Ameijden EJ, Van Haastrecht HJ, Roos MT, Henquet CJ, Quint WG, Coutinho RA. Cervical HPV infection among HIV-infected prostitutes addicted to hard drugs. J Med Virol. 1993;41:185–90. doi: 10.1002/jmv.1890410303. [DOI] [PubMed] [Google Scholar]


