Abstract
IL-11, a member of the IL-6 type cytokines, has some biological activity related to the joint destruction in rheumatoid arthritis (RA), such as induction of osteoclast differentiation. However, its expression and regulation in rheumatoid inflamed joints has not been clarified. In the present study we examined the capacity of fresh rheumatoid synovial cells (fresh RSC) to produce IL-11, and the effect of indomethacin, dexamethasone and IFN-γ on IL-11 production. Fresh RSC obtained from eight patients with RA produced large amounts of IL-11, measured by ELISA, and showed strong expression of IL-11 mRNA, determined by Northern blotting. Indomethacin inhibited the production of IL-11 by about 55%. Prostaglandin E2 (PGE2) completely prevented the inhibition, suggesting that IL-11 production by fresh RSC was in part mediated by PGE2. Dexamethasone inhibited the production of IL-11 by more than 80%. Interestingly, the inhibition was not abolished by PGE2. IFN-γ inhibited the production of IL-11 from IL-1α-stimulated cultured rheumatoid synovial fibroblasts, although IFN-γ did not inhibit the production of IL-11 by fresh RSC. These results suggest that the production of IL-11 by rheumatoid synovia was differentially regulated by PGE2 and IFN-γ, and that treatment with indomethacin or dexamethasone decreased the level of IL-11 at inflammatory joints in patients with RA.
Keywords: rheumatoid synovial cells, IL-11, interferon-gamma, indomethacin, dexamethasone
INTRODUCTION
Rheumatoid arthritis (RA) is an inflammatory joint disease in which perpetuation of chronic synovitis leads to bone and cartilage degradation ]1[. Inflammatory cytokines or soluble factors are essential in the pathogenesis of RA, and rheumatoid synovia are known to be rich sources of these mediators [1].
IL-11 is a functionally pleiotropic cytokine that was isolated from a bone mallow-derived stromal cell line by screening of the ability to stimulate the proliferation of IL-6-dependent cells [2]. The IL-11 receptor is a cell surface receptor that consists of two components: a unique ligand-binding 150-kD glycoprotein [3,4] and a non-ligand-binding, signal-transducing 130-kD glycoprotein chain (gp130) [5]. IL-11 is now classified as an IL-6-type cytokine based on functional similarities with IL-6, and the shared use of gp130 molecules in their receptor complexes [6]. IL-11 has the ability to differentiate B cells via a T cell- and macrophage-dependent mechanism [7], to exert multiple effects in haematopoiesis [8–11], and to induce acute-phase reactants [12]. In addition, IL-11 plays an important role in development of osteoclast [13–15]. Interestingly, a recent report [16] shows that rheumatoid synovial cells express large amounts of IL-11 at both mRNA and protein levels. These findings suggest that IL-11 may be involved in the pathogenesis of RA. However, little is known about the regulation of IL-11 expression in rheumatoid synovia. In the present study, we examined the capacity of fresh rheumatoid synovial cells to produce IL-11, and the effects of indomethacin, dexamethasone and IFN-γ on the production.
MATERIALS AND METHODS
Materials
Human recombinant IL-1α and IFN-γ were kindly provided by Dainippon Pharmaceutical Co. (Osaka, Japan) and Shionogi & Co. Ltd. (Osaka, Japan), respectively. Dexamethasone, indomethacin, and Clostridium collagenase were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Deoxyribonuclease 1 was obtained from Sigma Chemical Co. (St Louis, MO).
Patients
Eight patients (six women, two men, age range 54–72 years) with seropositive RA according to American College of Rheumatology criteria [17] seen at the Orthopaedic Department of Saiseikai Takaoka Hospital or Toyama Medical and Pharmaceutical University Hospital were included in this study. All patients were receiving non-steroidal anti-inflammatory drugs. In addition, two patients were treated with bucillamine, and two with gold sodium thiomalate.
Cell and tissue cultures
Fresh rheumatoid synovial cells. Synovial tissues were aseptically obtained from RA patients undergoing synovectomy of knees or total knee replacement. After removing the adipose tissue, the remaining synovial tissue was minced into fragments. The tissue fragments were treated with 1 mg/ml of collagenase and 5 μg/ml of deoxyribonuclease I in serum-free Dulbecco's modified Eagle's medium (DMEM) (Nissui Pharmaceutical Co., Tokyo, Japan) containing penicillin (100 U/ml)/streptomycin (100 μg/ml) (Life Technologies, Grand Island, NY) at 37°C with gentle agitation until dissolution of the fragments. After removal of tissue debris by passing the cell suspension through a cell strainer, the large mononuclear cells were collected, washed, adjusted to a concentration of 2 × 105/ml, and 0.5 ml of this suspension was added to each well in a 24-well plate. These cells were cultured overnight in plastic dishes containing DMEM supplemented with 10% heat-inactivated fetal calf serum (FCS; ICN Biomedicals Japan, Osaka, Japan) (DMEM medium) at 37°C in a humidified atmosphere of 95% air and 5% CO2. Non-adherent cells were removed, and the remaining freshly isolated adherent rheumatoid synovial cells were used as fresh rheumatoid synovial cells (RSC). These cells were incubated in the presence or absence of indomethacin, dexamethasone or IFN-γ for 24 h. In some experiments, the culture supernatants of fresh RSC or a rheumatoid synovial piece were harvested, and replaced with fresh media every 24 h for up to 96 h. The cell-free culture supernatants were stored at −20°C until assay for IL-11.
Cultured rheumatoid synovial fibroblasts. Fresh RSC were further grown to confluence, and were passed to 10-cm culture dishes after trypsin treatment. The cells between the fourth and eighth passages were morphologically fibroblast-like, and all negative for CD14 and HLA-DR antigens on their cell surface. These cells were therefore used as cultured rheumatoid synovial fibroblasts (RSF) in this study. Cultured RSF were seeded in 24-well plate at a concentration of 5 × 104 cells/well with 0.5 ml of DMEM medium, and incubated in the presence or absence of IL-1α with or without the indicated concentrations of indomethacin, dexamethasone, prostaglandin E2 (PGE2) or IFN-γ.
IL-11 measurement
The level of IL-11 in supernatants was quantified by ELISA using a Quantikine Human IL-11 Immunoassay Kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
RNA isolation
After incubation of fresh RSC or cultured RSF with or without indomethacin, dexamethasone or IFN-γ for 6 h, total RNA was isolated according to the method by Chomczynski & Sacchi [18] using Isogen (Wako Pure Chemical Industries) as a lysis buffer. Total RNA was collected according to the manufacturer's protocol.
Northern blot analysis
Total RNA (10 μg) was size-fractionated by electrophoresis on a 1% agarose (FMC BioProducts, Rockland, ME)/17% formamide gel, blotted onto nylon membranes (GeneScreen, DuPont-NEN, Boston, MA), and UV-cross-linked. Blots were prehybridized in hybridization buffer (50% formamide, 5× SSC, 0.1% (w/v) N-laurylsarcosine, 0.02% (w/v) SDS, 2% (w/v) Blocking Reagent (Boehringer Mannheim, Tokyo, Japan), and 100 μg/ml of denatured sheared salmon sperm DNA for 2 h at 42°C, hybridized overnight in hybridization buffer containing a digoxigenin-labelled IL-11 cDNA probe at 42°C, and washed under stringent conditions. A 1250-bp IL-11 cDNA that was Eco RI-excised from clone pHuIL-11/PMT (kindly provided by Dr P. Schendel, Genetics Institute, Cambridge, MA) was labelled with digoxigenin using a random primer method (Boehringer Mannheim). After incubation with an anti-digoxigenin antibody conjugated with alkaline phosphatase (Boehringer Mannheim), membranes were immersed in the chemiluminescent substrate, CSPD (Tropix, Bedford, MA), and exposed to Fuji new RX x-ray film (Fuji Photo Film, Kanagawa, Japan).
Statistical analysis
Values are presented as means ± s.d. Data were analysed by Student's t-test. Differences were considered significant at P < 0.05.
RESULTS
Spontaneous production of IL-11 by fresh RSC
The kinetics of IL-11 production by fresh RSC or a rheumatoid synovial piece was first determined by ELISA. As shown in Fig. 1, both similarly secreted large amounts of IL-11 without any stimuli for 1–2 days, after which the production decreased rapidly, suggesting that these fresh RSC and a rheumatoid synovial piece were spontaneously activated to produce IL-11, but that the culture conditions did not continuously stimulate them to produce it. The over-production of IL-11 by fresh RSC was observed in all patients studied: a mean ± s.e.m. production of IL-11 by RSC for 24 h after the start of culture was 31.2 ± 6.1 ng/ml in the eight patients.
Fig. 1.
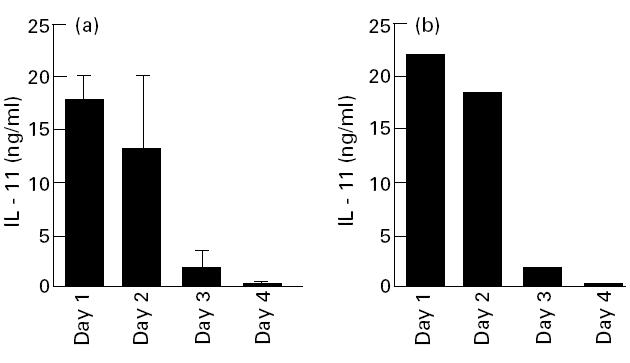
Kinetics of IL-11 production by fresh rheumatoid synovial cells (fresh RSC) and synovial pieces. (a) Cells isolated from rheumatoid synovia were placed in a 24-well plate at a concentration of 1 × 105/well, and cultured with 0.5 ml of Dulbecco's modified Eagle's medium (DMEM). The culture media were collected, and exchanged with fresh media every 24 h for up to 96 h. The amounts of IL-11 in supernatants were measured by ELISA. Each bar represents mean ± s.d. of four different wells. (b) A rheumatoid synovial piece was also cultured with 1 ml of DMEM medium. The culture supernatants were collected, and exchanged with fresh media every 24 h for up to 96 h.
Spontaneous expression of IL-11 mRNA in fresh RSC
We next examined the gene expression of IL-11 in fresh synovial adherent cells obtained from RA and osteoarthritis (OA). Northern blot analysis using a cDNA encoding human IL-11 revealed that two transcripts, 2.5 kb and 1.5 kb long, existed in humans [2]. As shown in Fig. 2, all three fresh RSC strongly expressed IL-11 mRNA. In contrast, the fresh synovial cells from a patient with OA expressed considerably less IL-11 mRNA than those of fresh RSC. Rheumatoid synovial pieces also expressed comparable levels of IL-11 mRNA (data not shown). Rheumatoid synovial cells mainly consist of macrophage-like cells and fibroblast-like cells [19,20]. In the preliminary experiments, we confirmed that CD14+ macrophage-like cells, obtained by a separation method using magnetic beads, had no capacity to express IL-11 at both levels of protein and mRNA, suggesting that the main producers of IL-11 in fresh RSC were fibroblast-like cells (data not shown).
Fig. 2.
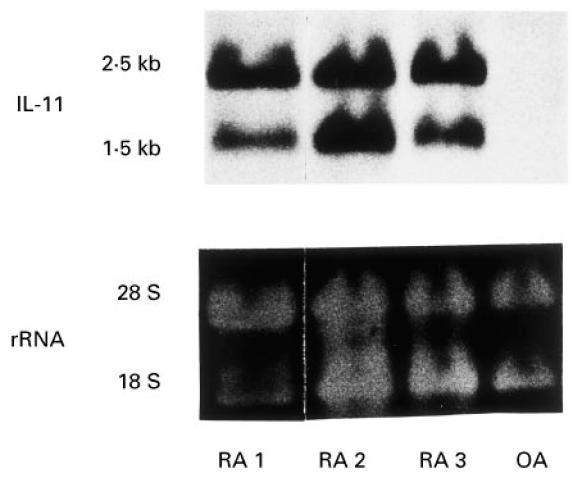
Northern blot analysis for IL-11 in fresh synovial cells from rheumatoid arthritis (RA) and osteoarthritis (OA). Adherent synovial cells enzymatically obtained from three patients with RA and a patient with OA were harvested for Northern blotting. Total RNA (10 μg) was loaded per lane, blotted, and hybridized with digoxigenin-labelled IL-11 cDNA as described in Materials and Methods. The lower part of the panel shows the 18S and 28S rRNA on ethidium bromide-stained gel as a loading control.
Inhibition of spontaneous IL-11 production and mRNA expression in fresh RSC by indomethacin and dexamethasone
Since indomethacin and dexamethasone, inhibitors of PGE2, are widely used in the treatment of RA, it was of interest to clarify whether these drugs could inhibit the ability of fresh RSC, having a pathogenic nature, to produce large amounts of IL-11. As shown in Fig. 3a, the over-production of IL-11 was significantly inhibited by indomethacin and dexamethasone from 51.9 ± 7.7 ng/ml to 23.3 ± 5.1 ng/ml (P < 0.01) and to 10.3 ± 0.9 ng/ml (P < 0.001), respectively. In addition, indomethacin and dexamethasone also inhibited the gene expression of IL-11 in fresh RSC (Fig. 3b). Next, the effect of PGE2, a major arachidonic acid metabolite in rheumatoid synovia [21,22], on IL-11 inhibition by these drugs was examined. Although cultured RSF produced a trace amount of IL-11 without stimulation, addition of IL-1α resulted in increasing IL-11 production (Fig. 4). Indomethacin and dexamethasone significantly inhibited IL-1α-stimulated IL-11 production from 1405.9 ± 163.9 pg/ml to 301.4 ± 11.8 pg/ml (P < 0.0005) and to 45.6 ± 1.2 pg/ml (P = 0.0001), respectively. Exogenous PGE2 (10−5 m) abolished IL-11 inhibition by indomethacin, whereas it had only a marginal effect on that by dexamethasone (Fig. 4).
Fig. 3.
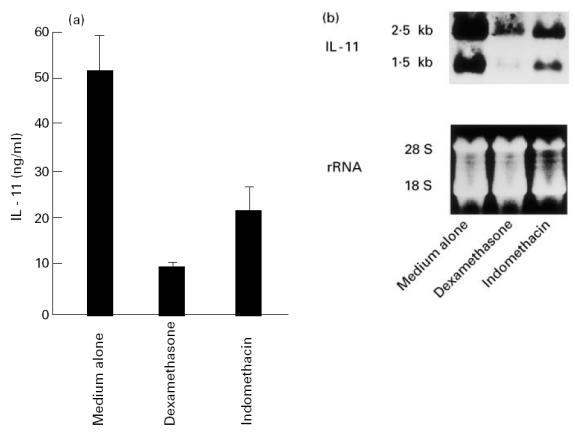
Inhibition of IL-11 expression in fresh rheumatoid synovial cells (fresh RSC) by indomethacin and dexamethasone. (a) Fresh RSC were incubated in the presence or absence of 10−5 m indomethacin (IND) or 10−6 m dexamethasone (DEX) for 24 h. After incubation, the amounts of IL-11 in the supernatants were measured by ELISA. Each bar represents mean ± s.d. of three different wells. (b) Fresh RSC were incubated in the presence or absence of 10−5 m IND or 10−6m DEX for 6 h. After incubation, the treated cells were harvested for Northern blotting. Total RNA (10 μg) was size-fractionated, blotted, and hybridized with digoxigenin-labelled IL-11 cDNA as described in Materials and Methods. The lower part of the panel shows the 18S and 28S rRNA on ethidium bromide-stained gel as a loading control. Data are representative of three separate experiments.
Fig. 4.
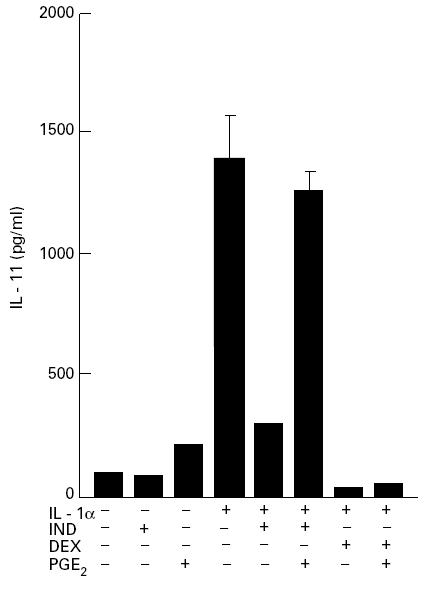
Inhibitory effects of indomethacin and dexamethasone on the production of IL-11 by cultured rheumatoid synovial fibroblasts (RSF): the effects of prostaglandin E2 (PGE2). Rheumatoid synovial cells at four to eight passages were used as cultured RSF. The cells were cultured at a concentration of 5 × 104/well in 24-well plates in the presence or absence of IL-1α (1 ng/ml) with or without 10−5 m indomethacin (IND) or 10−6 m dexamethasone (DEX) for 24 h. PGE2 (10−5 m) was added in combination with IL-1α and IND, or IL-1α and DEX. The amounts of IL-11 in the supernatants were measured by ELISA. Each bar represents mean ± s.d. of three different wells.
Inhibition of IL-11 expression in IL-1α-stimulated cultured RSF by IFN-γ
IL-1 is known to induce IL-11 production in a variety of cells [15,23,24]. Since IFN-γ has been reported to antagonize with IL-1 in biological activity [25], we examined the effect of IFN-γ on the production of IL-11 by fresh RSC. IFN-γ did not inhibit the production of IL-11 by fresh RSC at any concentration from 1 to 1000 U/ml (Fig. 5a). In contrast, IFN-γ inhibited the production of IL-11 by IL-1α-stimulated cultured RSF in a dose-dependent fashion (Fig. 5b). The inhibition was also observed at the mRNA level (Fig. 5c). Next, we examined the effect of delayed addition of IFN-γ, after stimulation with IL-1α, on the production of IL-11. The cultured RSF were activated with IL-1α, and then IFN-γ was added to the culture at different time points. Cultured supernatants were harvested 24 h after stimulation with IL-1α. As shown in Fig. 6, the inhibitory effect of IFN-γ became much less potent with the addition delayed by more than 4 h.
Fig. 5.
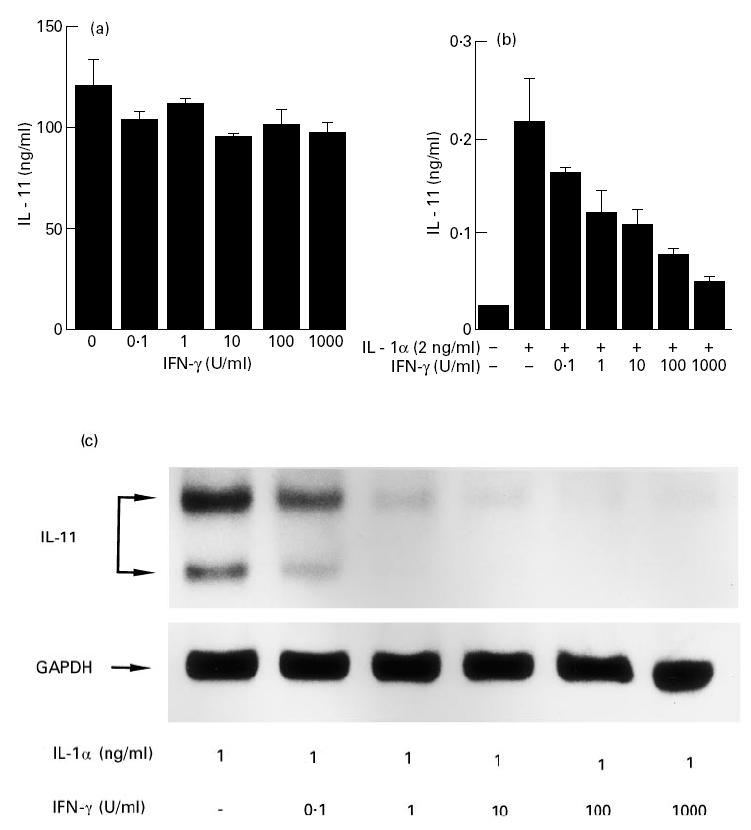
Effect of IFN-γ on the production of IL-11 in fresh rheumatoid synovial cells (fresh RSC) and cultured rheumatoid synovial fibroblasts (cultured RSF). Fresh RSC (a) and cultured RSF (b) were cultured in the presence or absence of increasing concentrations of IFN-γ with or without IL-1α (2 ng/ml) for 24 h. The amounts of IL-11 in supernatants were measured by ELISA. Each bar represents mean ± s.d. of three different wells. (c) Cultured RSF were cultured in the presence of increasing concentrations of IFN-γ with IL-1α (1 ng/ml) for 6 h. After incubation, the treated cells were harvested for Northern blotting. Total RNA (10 μg) was size-fractionated, blotted, and hybridized with digoxigenin-labelled IL-11 cDNA as described in Materials and Methods. The lower part of the panel shows the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) used as a house keeping gene. Data are representative of two separate experiments.
Fig. 6.
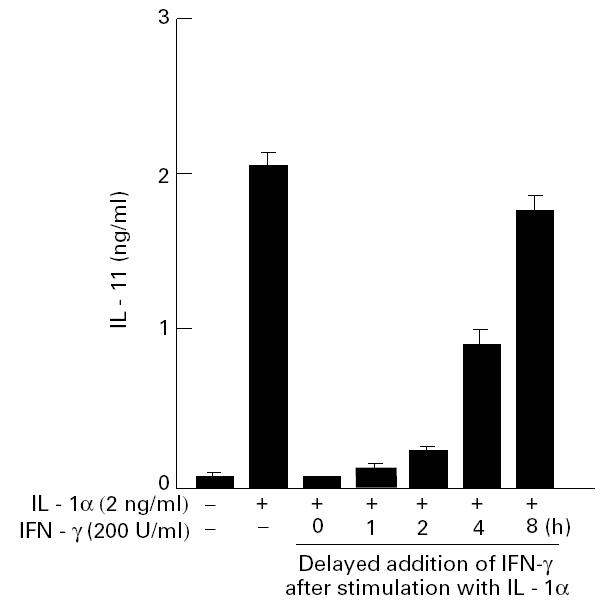
Effect of delayed addition of IFN-γ on IL-11 production by cultured rheumatoid synovial fibroblasts (cultured RSF). Cultured RSF were activated with IL-1α (2 ng/ml), and then IFN-γ (200 U/ml) was added to the culture at the indicated time. Cultured supernatants were harvested for IL-11 measurement 24 h after stimulation with IL-1α. Each bar represents mean ± s.d. of four different wells.
DISCUSSION
IL-11 was widely considered as a stimulator of haematopoiesis because of its multiple effects on haematopoiesis and the haematopoietic microenvironment [8–11]. However, we focused on the pathological roles of IL-11 in rheumatoid joints. IL-11 has several biological activities related to inflammatory events in RA, such as induction of B cell differentiation [7], acute-phase reactants [12], tissue inhibitor of metalloproteinase synthesis [23], and osteoclast differentiation [13–15]. In particular, the effect of IL-11 on osteoclasts is of interest. Girasole et al. [13] showed that neutralizing antibodies to IL-11 can suppress IL-1-, tumour necrosis factor (TNF)-, and 1,25 (OH)2 D3-induced osteoclast development, suggesting that osteoclast differentiation by these cytokines may be in part mediated by IL-11.
It has been reported that IL-1 and PGE2, actively produced by rheumatoid synovia, have the capacity to stimulate IL-11 production in a variety of cells [15,23,24]. Therefore, it is likely that these inducers strongly stimulate rheumatoid synovia to produce IL-11. We demonstrate here that freshly obtained RSC produced large amounts of IL-11, and showed a strong expression of IL-11 mRNA. This result is in agreement with the recent study [16] showing that RSC expressed the mRNA for IL-11 at higher levels than did synovial cells from OA. It is likely that IL-11 actively produced by rheumatoid synovia is involved in bone destruction in rheumatoid joints via osteoclast differentiation. However, regulatory mediators of the expression of IL-11 have not been extensively investigated in fresh rheumatoid synovia.
PGE2 is reported to positively regulate IL-11 production in osteoblasts [15]. To clarify the involvement of PGE2 in over-production of IL-11 by fresh RSC, we examined the effect of two PGE2 inhibitors, dexamethasone and indomethacin. Indomethacin reduced IL-11 production by about 55%, suggesting that over-production of IL-11 in fresh rheumatoid synovia is in part due to PGE2. A recent study demonstrated that dexamethasone inhibited IL-11 production by bone mallow-derived progenitor cells [26]. In the present study, dexamethasone inhibited IL-11 production more potently than did indomethacin. Interestingly, the inhibition by dexamethasone was not cancelled by PGE2. The mechanism of inhibition by dexamethasone remains to be clarified. It has been shown that glucocorticoid receptors inhibit AP-1 activity via binding with c-Jun protein [27]. Since IL-11 gene contains AP-1 site, that is known to be important for IL-11 transcription, in the 5′ flanking lesion [28], dexamethasone may inhibit the production of IL-11 via inhibition of AP-1 activity. Indomethacin and dexamethasone are widely used as anti-rheumatic drugs. Therefore, it is likely that the expression of IL-11 in inflammatory joints may be modulated in patients with RA. It is of interest to clarify further whether inhibition of IL-11 by these drugs would be beneficial or not for patients with RA.
IFN-γ is a promising biological agent for the treatment of RA [29,30]. It has been known that IFN-γ plays important roles in modulating the cytokine network [31]. In particular, it antagonized with IL-1 in cell growth, PGE2 release, and collagenase production [25]. In the present study, we showed that IFN-γ could inhibit IL-11 production by IL-1α-stimulated cultured RSF, although it did not inhibit IL-11 production by fresh RSC. This is the first study showing the inhibitory effect of IFN-γ on IL-11 expression in rheumatoid synovial cells. The mechanism of IFN-γ-mediated inhibition of IL-11 remains to be determined. The effects of IFN-γ were not mediated by change in PGE2 levels (data not shown). The delayed addition experiment indicated that IFN-γ is less effective on activated cells, suggesting that IFN-γ acts on RSF in initial steps in IL-1 signalling for IL-11. Since IL-11 gene contains a IFN-regulatory factor-1 binding site in the 5′-region [2], IFN-γ may inhibit the transcription of IL-11 mRNA via signal molecules such as IFN regulatory factors. Previous reports showed that IFN-γ has inhibitory effects on bone resorption in an in vitro system [32], and on the formation of osteoclast-like multinucleated cells in long-term human bone mallow cultures stimulated with vitamin D [33]. We suggested that the effects of IFN-γ on bone resorption might be mediated by IL-11 suppression.
In view of its pleiotropic effects, it is likely that IL-11 plays important roles in tissue remodelling in physiological conditions. We suggest here the dynamic expression of IL-11 in rheumatoid joints and the differential regulation by anti-rheumatic drugs and IFN-γ. The role of IL-11 in pathological sites like rheumatoid joints should be defined in the near future.
Acknowledgments
The authors gratefully acknowledge Dr Paul Schendel for providing IL-11 cDNA, Dainippon Pharmaceutical for providing recombinant human IL-1α, Drs Hitoshi Yamada, Kouichi Kanekasu and Hiroaki Matsuno for rheumatoid synovial specimens. We also thank Kazuko Taki for preparing the manuscript and Dr Tomohito Hamazaki for critical reading of the manuscript.
References
- 1.Arend PW, Dayer J-M. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990;33:305–15. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- 2.Paul SR, Bennett F, Calvetti JA, et al. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl Acad Sci USA. 1990;87:7512–6. doi: 10.1073/pnas.87.19.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilton DJ, Hilton AA, Raicevic A, et al. Cloning of a murine IL-11 receptor α-chain; requirement for gp130 for high affinity binding and signal transduction. EMBO J. 1994;13:4765–75. doi: 10.1002/j.1460-2075.1994.tb06802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin T, Miyazawa K, Yang YC. Characterization of interleukin-11 receptor and protein tyrosine phosphorylation induced by interleukin-11 in mouse 3T3-L1 cells. J Biol Chem. 1992;267:8347–51. [PubMed] [Google Scholar]
- 5.Yin T, Taga T, Tsang ML, Yasukawa K, Kishimoto T, Yang YC. Involvement of IL-6 signal transducer gp130 in IL-11-mediated signal transduction. J Immunol. 1993;151:2555–61. [PubMed] [Google Scholar]
- 6.Kishimoto T, Taga T, Akira S. Cytokine signal transduction. Cell. 1994;76:253–62. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 7.Anderson KC, Morimoto C, Paul SR, Chauhan D, Williams D, Cochran M, Barut BA. Interleukin-11 promotes accessory cell-dependent B-cell differentiation in humans. Blood. 1992;80:2797–804. [PubMed] [Google Scholar]
- 8.Neben TY, Loebelenz J, Hayes L, McCarthy K, Stoudemire J, Schaub R, Goldman SJ. Recombinant human interleukin-11 stimulates megakaryocytopoiesis and increases peripheral platelets in normal and splenectomized mice. Blood. 1993;81:901–8. [PubMed] [Google Scholar]
- 9.Quesniaux VFJ, Clark SC, Turner K, Fagg B. Interleukin-11 stimulates multiple phases of erythropoiesis in vitro. Blood. 1992;80:1218–23. [PubMed] [Google Scholar]
- 10.Lemoli RM, Fogli M, Fortuna A, Motta MR, Rizzi S, Benini C, Tura S. Interleukin-11 stimulates the proliferation of human hematopoietic CD34+ and CD34+CD33−DR− cells and synergizes with stem cell factor, interleukin-3, and granulocyte-macrophage colony-stimulating factor. Exp Hematol. 1993;21:1668–72. [PubMed] [Google Scholar]
- 11.Keller DC, Du XX, Srour EF, Hoffman R, Williams DA. Interleukin-11 inhibits adipogenesis and stimulates myelopoiesis in human long-term marrow cultures. Blood. 1993;82:1428–35. [PubMed] [Google Scholar]
- 12.Baumann H, Schendel P. Interleukin-11 regulates the hepatic expression of the same plasma protein genes as interleukin-6. J Biol Chem. 1991;266:20424–7. [PubMed] [Google Scholar]
- 13.Girasole G, Passeri G, Jilka RL, Manolagas SC. Interleukin-11: a new cytokine critical for osteoclast development. J Clin Invest. 1994;93:1516–24. doi: 10.1172/JCI117130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes FJ, Howells GL. Interleukin-11 inhibits bone formation in vitro. Calcif Tissue Int. 1993;53:362–4. doi: 10.1007/BF01351844. [DOI] [PubMed] [Google Scholar]
- 15.Romas E, Udagawa N, Zhou H, et al. The role of gp130-mediated signals in osteoclast development: regulation of interleukin 11 production by osteoblasts and distribution of its receptor in bone marrow cultures. J Exp Med. 1996;183:2581–91. doi: 10.1084/jem.183.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamoto H, Yamamura M, Morita Y, Harada S, Makino H, Ota Z. The synovial expression and serum levels of interleukin-6, interleukin-11, leukemia inhibitory factor, and oncostatin M in rheumatoid arthritis. Arthritis Rheum. 1997;40:1096–105. doi: 10.1002/art.1780400614. [DOI] [PubMed] [Google Scholar]
- 17.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 18.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Graabaek PM. Ultrastructural evidence for two distinct types of synoviocytes in rat synovial membrane. J Ultrastruct Res. 1982;78:321–39. doi: 10.1016/s0022-5320(82)80006-3. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson LS, Pitsillides AA, Worrall JG, Edwards JC. Light microscopic characterization of the fibroblast-like synovial intimal cell (synoviocyte) Arthritis Rheum. 1992;35:1179–84. doi: 10.1002/art.1780351010. [DOI] [PubMed] [Google Scholar]
- 21.Robinson DR, Tashjian A, Levine L. Prostaglandin-stimulated bone resorption by rheumatoid synovia. A possible mechanism for bone destruction in rheumatoid arthritis. J Clin Invest. 1975;56:1181–8. doi: 10.1172/JCI108195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dayer JM, Krane SM, Russell RG, Robinson DR. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci USA. 1976;73:945–9. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maier R, Ganu V, Lotz M. Interleukin-11, an inducible cytokine in human articular chondrocytes and synoviocytes, stimulates the production of the tissue inhibitor of metalloproteinases. J Biol Chem. 1993;268:21527–32. [PubMed] [Google Scholar]
- 24.Elias JA, Zheng T, Whiting NL, Trow TK, Merrill WW, Zitnik R, Ray P, Alderman EM. IL-1 and transforming growth factor-β regulation of fibroblast-derived IL-11. J Immunol. 1994;152:2421–9. [PubMed] [Google Scholar]
- 25.Nakajima H, Hiyama Y, Tsukada W, Warabi H, Uchida S, Hirose S. Effects of interferon gamma on cultured synovial cells from patients with rheumatoid arthritis: inhibition of cell growth, prostaglandin E2, and collagenase release. Ann Rheum Dis. 1990;49:512–6. doi: 10.1136/ard.49.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1α. J Cell Physiol. 1996;166:585–92. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Yang-Yen H-F, Chambard J-C, Sun Y-L, Smeal T, Schmidt TJ, Drouin J, Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein–protein interaction. Cell. 1990;62:1205–15. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- 28.Yang L, Yang Y-C. Regulation of interleukin (IL)-11 gene expression in IL-1 induced primate bone marrow stromal cells. J Biol Chem. 1994;269:32732–9. [PubMed] [Google Scholar]
- 29.Lemmel EM, Gaus W, Hofschneider PH. Multicenter double-blind trial of interferon-γ vs. placebo in the treatment of rheumatoid arthritis. Arthritis Rheum. 1991;34:1621–2. [PubMed] [Google Scholar]
- 30.Cannon GW, Emkey RD, Denes A, et al. Prospective 5-year followup of recombinant interferon-γ in rheumatoid arthritis. J Rheumatol. 1993;20:1867–73. [PubMed] [Google Scholar]
- 31.Billiau A. Interferon-γ: biology and role in pathogenesis. Adv Immunol. 1996;62:61–130. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- 32.Gowen M, Mundy GR. Actions of recombinant interleukin 1, interleukin 2, and interferon-γ on bone resorption in vitro. J Immunol. 1986;136:2478–82. [PubMed] [Google Scholar]
- 33.Takahashi N, Mundy GR, Roodman GD. Recombinant human interferon-γ inhibits formation of human osteoclast-like cells. J Immunol. 1986;137:3544–9. [PubMed] [Google Scholar]


