Abstract
Most thymic epithelial tumours that associate with MG express an epitope that resembles the sequence α373–380 from the cytoplasmic loop of the acetylcholine receptor (AChR). It has been proposed that sensitization to this linear epitope initiates autoimmunity to the AChR in thymoma-associated MG. We therefore tested whether MG/thymoma patients have T cell responses or antibodies to this region of the AChR. We found no significant recognition of the α309–417 region by their thymoma or peripheral blood T cells, or by their serum anti-AChR antibodies. Instead, the T cell epitopes that were recognized, like the previously characterized B cell epitopes, were in the extracellular AChR domain.
Keywords: paraneoplastic autoimmunity, thymoma, acetylcholine receptor, myasthenia gravis
INTRODUCTION
About one third of patients with a thymoma develop MG [1], a classic autoantibody-mediated disorder. The 10% of MG patients with a thymoma invariably have pathogenic antibodies to the highly folded extracellular domain of the acetylcholine receptor (AChR) that are responsible for their muscle weakness [2]. The AChR comprises α, β, γ and δ subunits in fetal muscle; in the adult, the γ is replaced by an ε. These evolutionarily related subunits all have about 210 extracellular amino acids, a cytoplasmic loop from positions 300–400 (approximately) and a short external C terminal tail [2]. In each isoform, the α subunit occurs twice, and is believed to be immunodominant [2]. It is possible that specific T cells, whether developing in these cortical thymomas or immigrating from the circulation, are sensitized to AChR epitopes expressed in situ [2]. However, only low levels of AChR α subunit mRNA have been detected by polymerase chain reaction (PCR) in thymoma tissue [3,4], and RNase protection assays for AChR subunit expression are often negative (MacLennan et al., in preparation). Moreover, the intact AChR has never been detected in thymomas by using conformation-specific MoAbs against its extracellular domain [5], even though these compete with most MG serum antibodies. By contrast, other MoAbs against a linear epitope, α373–380 [6] of the cytoplasmic loop (α299–408) of the AChR α subunit, do bind to thymoma epithelium [5,7,8], but this is apparently a cross-reaction with an AChR-like epitope on a distinct 153-kD polypeptide [8] rather than with any known AChR subunit. Nevertheless, its expression is strongly MG-related, since it is seen in about 80% of thymomas from MG patients, and in few from non-myasthenics [7].
Possibly, therefore, this or other T cell epitopes on the 153-kD polypeptide induce helper T cell and antibody responses that subsequently lead, by a process of determinant spreading, to the production of antibodies directed against the whole AChR [5,7]. If so, one would expect responses to these initiating epitopes to persist in some patients. Accordingly, we have tested for T cell responses to this and other regions of the AChR α subunit in 23 patients with thymoma-associated MG and searched for antibodies binding to the same region in 17 sera.
MATERIALS AND METHODS
Antigens
Recombinant AChR α subunit polypeptides of varying lengths (r1–437, etc.) were expressed in Escherichia coli and purified by preparative SDS–PAGE [9,10]. Peptides were synthesized using F-moc chemistry on LKB Biolynx apparatus [10]; they were coupled to activated Sepharose 4B according to the manufacturer's instructions (Pharmacia, St Albans, UK).
Patients and cells
Fresh samples of thymomas were obtained with informed consent from 23 patients, and peripheral blood lymphocytes (PBL) from 17 of them; they had raised serum anti-AChR titres and generalized MG of mild (n = 7), moderate (n = 12) or severe (n = 1) grades. There were 11 males and 12 females; their onset ages ranged from 17 to 70 years, and durations of symptoms from 3 to 45 months. Thymoma cell suspensions were prepared by mechanical disruption; in 10 cases, pretreatment with corticosteroids had clearly enriched the mature/activated T cells and class II+ cells [11]. From 11 of the 13 non-treated cases, a low density fraction, prepared on 3% Ficoll/10% sodium metrizoate, was enriched similarly [12]; in five cases, these were cocultured with an equal number of fresh irradiated PBL (PBLx) as antigen-presenting cells (APC). In 16 cases, the cells were tested on the day of thymectomy, but, with seven others, cryopreserved thymoma cells were used together with fresh PBLx as above.
To assay responses, fresh PBL (2 × 105), thymus or thymoma cells (1–2 × 105) were cultured in RPMI/5% A+ human serum in triplicate in round-bottomed microwells (Nunclon, Life Technologies, Paisley, UK) with the indicated antigens (≈ 0.5 and 0.1 μg/ml) for 72 h, when 1 μCi 3H-thymidine was added. After a further 18 h, the plates were harvested and counted on a Betaplate flat-bed liquid scintillation counter (Wallac, Turku, Finland). In the eight most recent cases, the cells were cultured in bulk (2–3 × 106/ml) with the same antigens (except that r3–181 replaced r37–181); on day 4 (where possible) and day 7, triplicate samples of 105 cells were pulsed for 18 h with 3H-thymidine as above. Stimulation indices (SI) were calculated as ct/min in the presence of antigen ÷ ct/min with medium only. With thymoma cells, backgrounds ranged from 112 to 25 820 ct/min (median 1098, mean 3855 ± 1433 s.e.m.); for PBL, the range was 242–2893 ct/min (median 839, mean 1050 ± 185 s.e.m.).
Assaying for antibodies to cytoplasmic AChR epitopes
Seventeen thymoma-associated MG sera were tested; their antibody titres to 125I-α-bungarotoxin (α-BT)-labelled human AChR ranged from 3.7 to 25.1 nm. Sub-saturating amounts of each serum were preincubated overnight with a pool of peptides, α309–345, 337–369, 361–377, 364–389, 383–410 and 390–417 (1 mg/ml each), before addition of 125I-α-BT-labelled AChR for 2 h and subsequent precipitation with anti-human immunoglobulin. Results are expressed as:
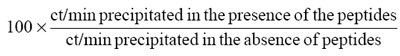 |
after subtraction of ct/min precipitated by control human serum. Rabbit antibodies raised against α309–368 were used as a positive control, together with anti-rabbit immunoglobulin to precipitate. With 12 MG/thymoma sera, we also attempted to affinity-purify antibodies using Sepharose 4B coupled with the same peptide pool, followed by washing and elution with 3 m KCNS and dialysis against PBS.
RESULTS
Screening for T cell responses to cytoplasmic epitopes
On initial testing, we found responses (SI > 2.5) to full-length recombinant human α subunit in seven of 23 thymomas (Fig. 1a), and five of 17 PBL samples (Fig. 1b); in most individuals, the former were higher. However, responses of T cells from both sources were broadly similar whether they were tested against the full-length α subunit or the α37–181 polypeptide that covers most of the extracellular domain (α1–210). With one exception, they were weaker to α87–437, which includes all of the α299–408 cytoplasmic loop (Fig. 1a). Thus we found no clear evidence of any T cell response specific for cytoplasmic sequences; the epitopes we subsequently mapped with synthetic peptides proved to be in the extracellular domain (see Discussion). We also saw no clear correlation between responsiveness and MG severity (not shown).
Fig. 1.
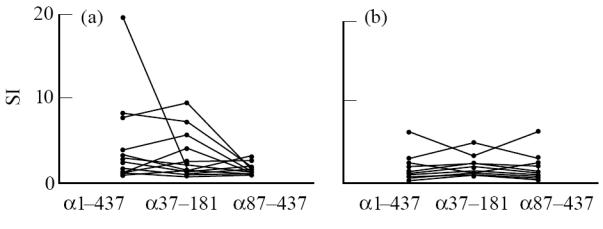
Responses of T cells from MG thymomas (a) and peripheral blood lymphocytes (PBL) (b) to full-length acetylcholine receptor (AChR) α1–437, to r37–181 that includes most of its extracellular α1–211 sequence, and to r87–437 that includes all of the cytoplasmic α299–408 loop. Results are from cell samples on which all three antigen preparations were tested in parallel at ≈ 0.5 μg/ml; in several cases, responses were similar at 0.1 μg/ml but, in others, they declined sharply. In nine of the more recent cases, r3–181 was used instead of r37–181. SI, Stimulation index.
Antibody recognition of cytoplasmic AChR epitopes
Because α373–380 forms a linear epitope that persists in short synthetic peptides [6,13], we next tested sera from thymoma/MG patients for antibody reactivity to a pool of peptides representing α309–417. We found no evidence of specific binding to the peptides on dot blots (data not shown). We next tried to block the binding of anti-AChR antibodies to labelled AChR by preincubation with pooled synthetic α309–417 peptides; in the event, the mean precipitation of AChR by each serum proved to be 98–118% of that in their absence. By contrast, rabbit antibodies to α309–368 were inhibited by 94 ± 6% (mean ± s.d., n = 3) (Fig. 2a). Finally, we tried to affinity-purify antibodies on immobilized α309–417 peptides. In each case, most of the antibody was recovered in the pass-through fractions, representing from 66% to 112% of that applied (Fig. 2b). Very little was detected in the eluates (Fig. 2c), and that which did elute was not specific for the peptides, since it was not inhibitable by preincubation with the pooled peptides (not shown). By contrast, the eluted rabbit antibodies were inhibited by 100%. Thus we found no sign of antibodies to the cytoplasmic domain of the AChR α subunit in these patients.
Fig. 2.
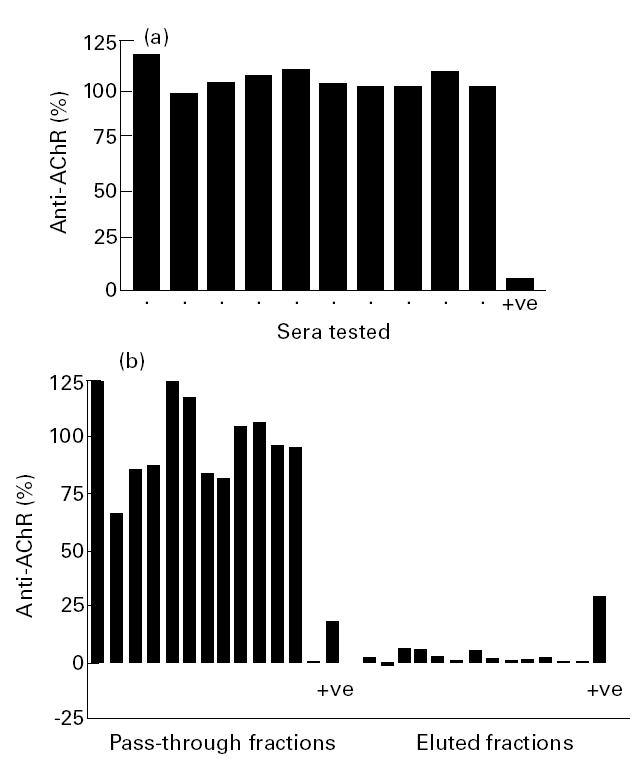
(a) Inhibition of anti-acetylcholine receptor (AChR) antibodies from 10 MG/thymoma patients by preincubation with pooled α309–417 peptides representing the cytoplasmic domain of the AChR α subunit. (b) Attempted purification of antibodies from 12 sera to α309–417 by affinity chromatography, using the same peptide pool immobilized on Sepharose 4B. Anti-AChR antibody levels in the pass-through fractions and the eluates are expressed as a percentage of the total loaded. The rabbit antiserum against α309–368 pool was included as a positive control (+ ve).
DISCUSSION
The association with thymoma in MG patients strongly suggests that their autoimmune response is provoked by these tumours. In particular, the AChR α373–380 epitope is implicated by its frequent expression by the neoplastic epithelial cells [5,7,8]. In spite of that, we found no convincing recognition of this AChR region by T cells from either these patients' thymomas or their peripheral blood. Nor did we find evidence of antibody responses to this region.
It is already well established that most anti-AChR antibodies in MG patients, including those with thymoma [14–16], bind to the extracellular domain, including the main immunogenic region (MIR) near α67–76 [17,18]. These epitopes are so highly conformational that the patients' antibodies almost never cross-react with linear AChR peptides or recombinant α subunit [19]. Now it appears that the specific T cells also recognize epitopes from the extracellular domain. We have subsequently been able to select T cell lines and clones from two MG thymomas, using recombinant human α subunit. Both responded to extracellular sequences which we have now mapped (with synthetic peptides) to the 75–90 and 149–160 regions (Nagvekar et al., in preparation). Despite the fact that anti-MIR antibodies do not bind detectably to thymoma epithelium [5], several groups have detected AChR α subunit mRNA in thymomas by PCR [3,4], and we have recently found significant levels of ε subunit mRNA with the less sensitive RNase protection assay (MacLennan et al., in preparation). If thymomas express individual AChR subunit polypeptides in isolation, these would be very unlikely to form the conformational epitopes that are detected by most MoAbs, though they might well generate linear epitopes that can be recognized by T cells.
Although most of these patients' anti-AChR antibodies clearly recognize the extracellular domain [15,16], we nevertheless checked for a minor subset of antibodies to the putatively autosensitizing α373–380 epitope. This epitope is highly immunogenic in denatured AChR α subunit [6,13], evoking antibodies that cross-react strongly not only with native AChR, but also with short synthetic peptides from the cytoplasmic region. Thus, any such antibodies in MG/thymoma patients' sera should have been readily detectable by inhibition with synthetic peptides or by purification on peptide-affinity columns. Our failure to find either T cell responses or antibody binding to this cytoplasmic region strongly suggests that it is not a major immunogen in these thymomas. However, we cannot absolutely exclude an early initiating role that is subsequently overtaken as other epitopes begin to dominate [20,21]. It seems likelier that other determinants shared by the 153-kD polypeptides, the AChR (including its ε subunit) and/or striational muscle antigens, activate T cells which then initiate a pathogenic autoantibody response on re-encountering these antigens elsewhere. Finally, whereas native AChR readily evokes antibodies specific for its extracellular conformation, it does not elicit antibodies to the α373–380 sequence [22], although this is a highly immunogenic epitope in denatured α subunit [6,13]. The absence of antibodies to this epitope, even in thymoma patients, therefore implies that their autoantibodies are also produced in response to the native structure rather than to unfolded sequences or fragments.
Acknowledgments
This work was supported by the Sir Jules Thorn Charitable Trust, the Muscular Dystrophy Group of Great Britain, and the Medical Research Council. We thank Professor J. Newsom-Davis and other colleagues for generous access to patient material, and Sister E. Goodger and Ms N. Pantic for expert help.
References
- 1.Souadjian JV, Enriquez P, Silverstein MN, Pepin J-M. The spectrum of diseases associated with thymoma. Arch Intern Med. 1974;134:374–9. [PubMed] [Google Scholar]
- 2.Willcox N. Thymic tumours with myasthenia gravis or bone-marrow dyscrasias. In: Peckham M, Pinedo H, Veronesi U, editors. Oxford textbook of oncology. Vol. 1. Oxford: Oxford University Press; 1995. pp. 1562–8. [Google Scholar]
- 3.Hara Y, Ueno S, Uemichi T, et al. Neoplastic epithelial cells express α-subunit of muscle nicotinic acetylcholine receptor in thymomas from patients with myasthenia gravis. FEBS Letters. 1991;279:137–40. doi: 10.1016/0014-5793(91)80268-8. [DOI] [PubMed] [Google Scholar]
- 4.Wheatley LM, Urso D, Tumas K, et al. Molecular evidence for the expression of nicotinic acetylcholine receptor α-chain in mouse thymus. J Immunol. 1992;148:3105–9. [PubMed] [Google Scholar]
- 5.Kirchner T, Tzartos S, Hoppe F, et al. Pathogenesis of myasthenia gravis: acetylcholine receptor-related antigenic determinants in tumour-free thymuses and thymic epithelial tumours. Am J Pathol. 1988;130:268–80. [PMC free article] [PubMed] [Google Scholar]
- 6.Tzartos SJ, Remoundos MS. Precise epitope mapping of monoclonal antibodies to the cytoplasmic side of the acetylcholine receptor α subunit: dissecting a potentially myasthenogenic epitope. Eur J Biochem. 1992;207:915–22. doi: 10.1111/j.1432-1033.1992.tb17124.x. [DOI] [PubMed] [Google Scholar]
- 7.Marx A, Wilisch A, Schultz A, et al. Pathogenesis of myasthenia gravis. Virchows Arch. 1997;430:355–64. doi: 10.1007/s004280050044. [DOI] [PubMed] [Google Scholar]
- 8.Marx A, Wilisch A, Schultz A, et al. Expression of neurofilaments and of a titin epitope in thymic epithelial tumours; implications for the pathogenesis of myasthenia gravis. Am J Pathol. 1996;148:1839–50. [PMC free article] [PubMed] [Google Scholar]
- 9.Beeson D, Brydson M, Wood H, et al. Human muscle acetylcholine receptor: cloning and expression in E. coli of cDNA for the α-subunit. Biochem Soc Trans. 1989;17:219–20. [Google Scholar]
- 10.Matsuo H, Batocchi A-P, Hawke S, et al. Recognition of unnatural epitopes by peptide-selected T cell lines in myasthenia gravis patients and controls. J Immunol. 1995;155:3683–92. [PubMed] [Google Scholar]
- 11.Willcox N, Schluep M, Ritter MA, et al. Myasthenic and nonmyasthenic thymoma. An expansion of a minor cortical epithelial cell subset? Am J Pathol. 1987;127:447–60. [PMC free article] [PubMed] [Google Scholar]
- 12.Sommer N, Willcox N, Harcourt GC, Newsom-Davis J. Myasthenic thymus and thymoma are selectively enriched in acetylcholine receptor-reactive T cells. Ann Neurol. 1990;28:312–9. doi: 10.1002/ana.410280303. [DOI] [PubMed] [Google Scholar]
- 13.Palace J, Vincent A, Beeson D, Newsom-Davis J. Immunogenicity of human recombinant acetylcholine receptor α subunit: cytoplasmic epitopes dominate the antibody response in four mouse strains. Autoimmunity. 1994;18:113–9. doi: 10.3109/08916939409007984. [DOI] [PubMed] [Google Scholar]
- 14.Tzartos SJ, Seybold ME, Lindstrom JM. Specificities of antibodies to acetylcholine receptors in sera from myasthenia gravis patients measured by monoclonal antibodies. Proc Natl Acad Sci USA. 1982;79:188–92. doi: 10.1073/pnas.79.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whiting PJ, Vincent A, Newsom-Davis J. Myasthenia gravis: monoclonal antihuman acetylcholine receptor antibodies used to analyze antibody specificities and responses to treatment. Neurology. 1986;36:612–7. doi: 10.1212/wnl.36.5.612. [DOI] [PubMed] [Google Scholar]
- 16.Vincent A, Whiting PJ, Schluep M, et al. Antibody heterogeneity and specificity in myasthenia gravis. Ann NY Acad Sci. 1987;505:106–20. doi: 10.1111/j.1749-6632.1987.tb51286.x. [DOI] [PubMed] [Google Scholar]
- 17.Barkas T, Mauron A, Roth B, et al. Mapping the main immunogenic region and toxin binding site of the nicotinic acetylcholine receptor. Science. 1987;235:77–80. doi: 10.1126/science.2432658. [DOI] [PubMed] [Google Scholar]
- 18.Tzartos SJ, Cung MT, Demange P, et al. The main immunogenic region (MIR) of the nicotinic acetylcholine receptor and the anti-MIR antibodies. Mol Neurobiol. 1991;5:1–29. doi: 10.1007/BF02935610. [DOI] [PubMed] [Google Scholar]
- 19.Ralston S, Sarin V, Thanh HL, et al. Synthetic peptides used to locate the α bungarotoxin-binding site and immunogenic regions on the α subunits of the nicotinic acetylcholine receptor. Biochemistry. 1987;26:3261–6. doi: 10.1021/bi00386a004. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann PV, Sercarz EE, Forsthuber T, et al. Determinant spreading and the dynamics of the autoimmune T cell repertoire. Immunol Today. 1993;14:203–8. doi: 10.1016/0167-5699(93)90163-F. [DOI] [PubMed] [Google Scholar]
- 21.Vincent A, Jacobson L, Shillito P. Response to human acetylcholine receptor α 138–199: determinant spreading initiates autoimmunity to self-antigen in rabbits. Immunol Letters. 1994;39:269–75. doi: 10.1016/0165-2478(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 22.Ratnam M, Sargent P, Sarin V, et al. Location of antigenic determinants on primary sequences of subunits of nicotinic acetylcholine receptors by peptide mapping. Biochemistry. 1986;25:2621–32. doi: 10.1021/bi00357a051. [DOI] [PubMed] [Google Scholar]


