Abstract
Penicillium marneffei is an important opportunistic fungal pathogen. Host defence mechanisms against P. marneffei are not fully understood. We investigated the fungicidal activity of murine peritoneal macrophages against two forms of P. marneffei, conidia and yeast cells, and the involvement of the NO-mediated killing system. Peritoneal macrophages suppressed the intracellular growth of P. marneffei yeast cells and conidia. The number of live yeast cells within macrophages was significantly reduced by activation of macrophages by interferon-gamma (IFN-γ), while a similar response was not observed with conidia. IFN-γ-induced macrophage fungicidal activity against yeast cells was mediated by NO and was almost completely inhibited by NG-monomethyl-l-arginine (l-NMMA), a competitive inhibitor of NO synthesis, while NG-monomethyl-d-arginine (d-NMMA), an optical isomer of l-NMMA, did not show any influence. NO production by macrophages stimulated with IFN-γ was significantly enhanced when these macrophages were cultured with P. marneffei yeast cells, while conidia did not enhance macrophage NO production. Furthermore, yeast cells were more susceptible to the killing effect of chemically generated NO than conidia. Our results indicate that the yeast form of P. marneffei is more sensitive to the fungicidal activity of IFN-γ-stimulated macrophages than conidia, and suggest that the different effects of two forms of P. marneffei on macrophage NO production and their different susceptibilities to NO may be reasons for the present findings.
Keywords: Penicillium marneffei, conidia, yeast, macrophages, nitric oxide
INTRODUCTION
Disseminated penicilliosis caused by Penicillium marneffei is an endemic deep-seated systemic fungal disease in South-east Asian countries [1,2]. Almost all cases occur in patients who live in [1,3] or travel to [4–6] these countries. The fungal agent also causes an important life-threatening mycotic infection in patients with AIDS [1,7–10]. The number of patients with systemic penicilliosis is increasing rapidly in Thailand, in parallel with that of AIDS patients [1]. Although many epidemiological and clinical studies have been performed, the underlying immune mechanisms responsible for protecting the host against infection with P. marneffei are poorly understood. Using a new experimental murine model of penicilliosis, we have recently demonstrated that cell-mediated immunity plays a central role in host resistance to infections caused by P. marneffei [11]. Although a number of clinical studies in AIDS patients infected with P. marneffei have detected the fungal elements phagocytosed by neutrophils [12] and monocytes [4,13], the exact form of phagocytes responsible for effective host defence and the principal mediator used to exert their fungicidal activity are still poorly understood.
Penicillium marneffei is a dimorphic fungus [14] that grows in a hyphal form at 25°C. This form bears conidiophores consisting of lateral and terminal vericils with three to seven or more phialides and chains of conidia (spores). The fungus converts to a yeast-like form at 37°C [14]. Clinically, only the yeast form is found in tissues [4,13] and peripheral blood [12]. In addition, in our animal model, the yeast but not the hyphal form of P. marneffei was detected in the lungs of mice infected intratracheally with conidia. These observations suggest that host defence targets the yeast form. Therefore, to understand host resistance mechanisms against P. marneffei infection it is necessary to examine the fungicidal activity of macrophages against the yeast form of P. marneffei. In this study, we directly evaluate the fungicidal activity of interferon-gamma (IFN-γ)-activated macrophages by counting the live microorganisms. This method contrasts to that used in the study by Cogliati et al. [15], where the anti-fungal activity of macrophages was estimated by electron microscopic observation.
After appropriate stimulation, macrophages produce NO and its metabolites (reactive nitrogen intermediates (RNI)) [16], and express their antimicrobial capabilities [17] by inhibiting cytochrome P450 activity [18] and causing changes in DNA [19]. Several recent studies have demonstrated that RNI play an important role in host defence against infections caused by various pathogens, including parasites [20–23], fungi [15,24–26], bacteria [27–31], chlamydia [32] and mycobacteria [33–35]. In the present study, we examine the role of RNI in the anti-fungal activity of IFN-γ-stimulated murine peritoneal macrophages. Our results demonstrate that RNI play a central role in macrophage-mediated growth inhibition of P. marneffei, and that the yeast and conidia forms show different susceptibilities to killing by RNI and macrophages.
MATERIALS AND METHODS
Animals
Specific pathogen-free, male BALB/c mice, weighing 20–24 g, were purchased from SLC Japan (Hamamatsu, Japan) and used when 6–8 weeks old. We have recently shown that BALB/c mice can eliminate P. marneffei after intratracheal inoculation of 4 × 105 colony-forming units (CFU) of conidia [11] and that this strain effectively resists infection. All mice were housed in a pathogen-free environment and received sterilized food and water ad libitum at the Laboratory Animal Centre for Biomedical Sciences, University of the Ryukyus. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation at our university.
Microorganisms and culture conditions
A clinical strain of P. marneffei, designated H1140 [36], was recently registered by American Type Culture Collection (ATCC 201013). The method of preparation of conidial suspensions was described in our previous study [36]. For preparing inocula of yeast cells, conidia were incubated in brain heart infusion broth (BHI; Eiken) at 37°C for 7–14 days, as described in a previous study [37]. After incubation, broth cultures were filtered through a 10-μm nylon mesh to remove mixed mycelial fragments and centrifuged at 15 000 g at 4°C for 10 min to separate yeast cells from the culture broth. The yeast cell and conidial preparations contained < 3% and 5%, respectively, of hyphae when viewed by light microscopy.
Culture medium and reagents
RPMI 1640 medium was purchased from Gibco BRL (Grand Island, NY), fetal calf serum (FCS) from Whittaker (Walkersville, MD), NG-monomethyl-l-arginine (l-NMMA) and NG-monomethyl-d-arginine (d-NMMA) from Calbiochem Novabiochem (Tokyo, Japan), and murine recombinant IFN-γ (specific activity 5 × 106 U/mg) from Genzyme Diagnostics (Cambridge, MA).
Preparation of peritoneal macrophages
Mice were injected intraperitoneally with 1.5 ml of 3% thioglycolate. Peritoneal exudate cells were harvested 4 days later by two injection cycles of 5 ml cold RPMI 1640 supplemented with 10% FCS and 10 mm HEPES. The obtained cells were cultured at 1.5–2.0 × 106/ml in glass dishes precoated with FCS for 1 h in a 5% CO2 incubator. After removal of non-adherent cells, the remaining adherent cells were collected by dislodgement using a rubber policeman.
Intracellular killing of P. marneffei by macrophages
Conidia and yeast cells were opsonized with 1 μg/ml of MoAb EBA1 (a kind gift from Sanofi Diagnostics, Pasteur, France), directed against Aspergillus fumigatus galactomannan, because this antibody was reported to cross-react also to P. marneffei and to be previously adopted for the immunohistochemical identification of P. marneffei [38], by incubating in RPMI medium containing 1% FCS on ice for 30 min. After three washes, the number of fungal components was counted using a haemocytometer and confirmed the real inoculum size of conidia or yeasts after counting the number of viable colonies as described below. Macrophages were incubated at 1 × 106/well with opsonized conidia or yeast cells and phagocytosis was allowed to continue over a 30-min period. This was followed by triplicate washing with the culture medium to remove non-phagocytosed free microorganisms. The following cultures were prepared containing 0.05 μg/ml of amphotericin B (AmB) to inhibit extracellular growth of P. marneffei. Twenty-four or 48 h later, the culture supernatants were collected for measurement of nitrite, and the microorganisms were harvested by washing each well twice with 1.0 ml of distilled water. The samples were appropriately diluted with distilled water and inoculated at 50 μl on PDA plates and cultured at 30°C for 2 days. The number of viable colonies was counted and multiplied by the dilution factor. The results were expressed as mean CFU ± s.d.
NO assay
The concentration of NO synthesized by macrophages was estimated from the amount of nitrite that accumulated in the culture using the method described by Stuehr & Nathan [16]. Briefly, 100 μl of supernatant were mixed with the same volume of Griess reagent and absorbance was read at 550 nm using an automated microplate reader. The concentration of nitrite was calculated from a NaNO2 standard curve.
Culture of P. marneffei in NO generating media
Penicillium marneffei conidia or yeast cells were directly exposed to chemically generated NO in a cell-free system, as previously described by Alspaugh & Granger [24]. Briefly, the microorganisms, washed three times with normal saline, were suspended at 2.5 × 104/ml in defined minimal medium, consisting of 15 mmd-glucose, 10 mm MgSO4, 29.4 mm KH2PO4, 13 mm glycine, 3.0 μm vitamin B1, in the presence or absence of 1 mm sodium nitrite (Wako Chemical Co., Tokyo, Japan) at an acidic (pH = 4.0) or neutral condition (pH = 7.0), and then cultured at 37°C without CO2 for 24 h. The organisms were harvested and appropriately diluted with distilled water, and then inoculated at 50 μl on PDA plates, cultured for 2 days, followed by counting the number of colonies.
An alternative method for generating NO in a test tube was also used. For this purpose, we used n-ethyl-2- (1-ethyl-2-hydroxy-2-nitrosohydrazino)-ethanamine (NOC12; Dojindo Labs, Kumamoto, Japan), which is a new NO-releasing compound with a mol. wt of 176.22. One mole of this reagent spontaneously releases 0.5–1 m of NO with a half-life of 100 min in acidic or neutral media at 37°C (manufacturer's instructions and [39]). NOC12 dissolved in 0.1 m NaOH was added at 100 μm every 2 h during the 12 h culture of P. marneffei yeast cells or conidia (104 CFU/ml) in minimal medium (pH 5.5). After reaction, the number of viable colonies was counted and the NO assay was performed as described above.
Statistical analysis
Data were expressed as mean ± s.d. The unpaired Student's t-test was used to compare differences between groups. P < 0.05 was considered significant.
RESULTS
Anti- P. marneffei fungicidal activity of IFN-γ-stimulated macrophages
To obtain a yeast form of P. marneffei, conidia were incubated in BHI broth at 37°C for more than 7 days. The conidia and yeast forms of P. marneffei were clearly distinguishable on microscopic examination. Conidia were round with a diameter of about 5 μm (Fig. 1a, arrows), while yeast cells were oval or sausage-like and contained segmented cytoplasm (Fig. 1b, arrow).
Fig. 1.

Microscopic identification of two forms of Penicillium marneffei. Lactophenol cotton blue staining. (a) Hyphal form bearing conidia (× 200) and (b) yeast form (× 400). Arrows indicate conidia (a) and segmented cytoplasm of yeast cells (b).
The susceptibility of the yeast form of P. marneffei to the fungicidal activity of IFN-γ-stimulated macrophages was examined and compared with that of conidia. Inoculum suspensions of both yeasts and conidia were adjusted to 1 × 105/ml by visual counting, but they resulted in 5 × 104 and 1 × 105/ml by counting of viable CFU, respectively. As shown in Fig. 2a, yeast cells did not grow within unstimulated macrophages, and IFN-γ-stimulated macrophages expressed significant fungicidal activity against yeast forms of P. marneffei at 48 h after culture. In contrast, the number of conidia in cultures containing unstimulated macrophages was slightly reduced 48 h after culture. More importantly, IFN-γ failed to enhance the fungicidal activity of macrophages against conidia (Fig. 2b). Macrophages stimulated with IFN-γ for 24 h before culturing with conidia also could not express the enhancement of fungicidal activity (data not shown).
Fig. 2.
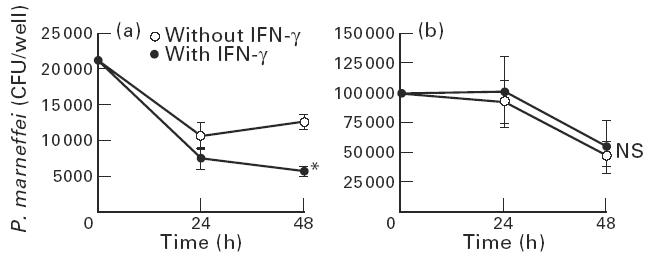
Different susceptibilities of yeasts and conidia of Penicillium marneffei to IFN-γ-activated macrophages. Macrophages (1 × 106/well) were allowed to phagocytose the opsonized yeasts (5 × 104/well) or conidia (1 × 105/well) of P. marneffei. After removing the unphagocytosed fungal elements, the cells were cultured with or without IFN-γ (300 U/ml) in the presence of amphotericin B (AmB; 0.05 μg/ml) for 24 h or 48 h. The number of live microorganisms was counted. Each data point represents the mean ± s.d. of triplicate cultures. Experiments were repeated three times with similar results. (a) Yeast. (b) Conidia. *P < 0.05. CFU, Colony-forming units; NS, not significant.
Involvement of NO in fungicidal activity of macrophages against P. marneffei
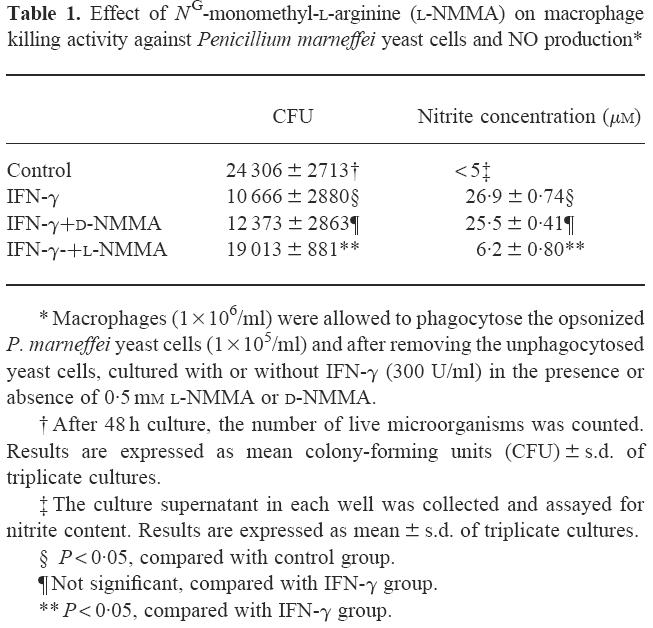
Since NO is a major microbicidal agent produced by murine macrophages, we examined the role of NO produced by IFN-γ-activated macrophages in killing P. marneffei. As shown in Table 1, the number of live intramacrophage yeast cells was significantly reduced following activation by IFN-γ. This enhancement in macrophage killing was significantly inhibited by l-NMMA, a competitive inhibitor of NO synthesis, while d-NMMA, an optical isomer of l-NMMA, did not have any influence. Activation of macrophages by IFN-γ was associated with a marked increase in macrophage production of NO, which was almost completely inhibited by l-NMMA, but not by d-NMMA (Table 1). Importantly, these reagents did not show any effect on the growth of yeast cells in a macrophage-free culture (data not shown).
Table 1.
Effect of NG-monomethyl-l-arginine (l-NMMA) on macrophage killing activity against Penicillium marneffei yeast cells and NO production*
Penicillium marneffei enhances NO production by macrophages
To investigate the underlying mechanisms of different susceptibilities between yeast cells and conidia to the fungicidal activity of IFN-γ-stimulated macrophages, we examined whether these two forms of P. marneffei exert a differential effect on NO production by macrophages. As shown in Table 2, NO was not generated when yeast cells or conidia were incubated for up to 24 h with non-activated or IFN-γ-activated macrophages. However, at 48 h, IFN-γ-stimulated macrophages incubated with opsonized yeast cells produced significantly higher amounts of nitrite compared with conidia or cells only. This augmenting effect was dependent on the number of yeast cells incubated with macrophages (Fig. 3). Thus, the enhanced production of NO in the presence of yeast cells may explain the higher susceptibility of yeast cells to macrophage killing.
Table 2.
Effect of IFN-γ on nitrite production by macrophages phagocytosing Penicillium marneffei yeasts or conidia
Fig. 3.
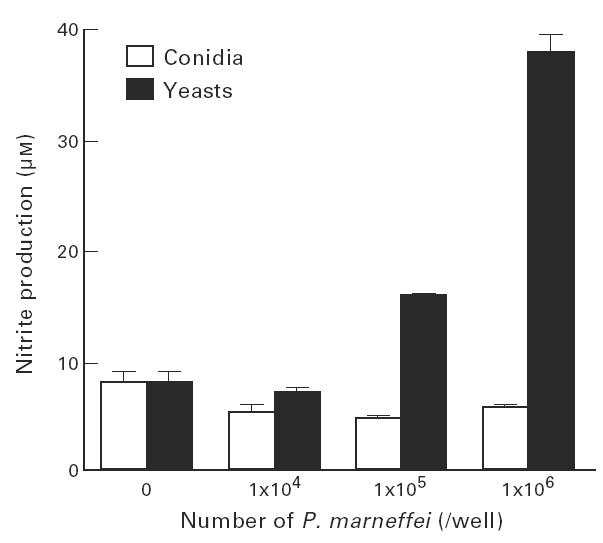
Different effect of yeasts and conidia of Penicillium marneffei on macrophage NO production. Macrophages (1 × 106/well) were allowed to phagocytose the various numbers of opsonized yeasts or conidia of P. marneffei. After removing the unphagocytosed fungal elements, the cells were cultured with IFN-γ (300 U/ml) in the presence of amphotericin B (AmB; 0.05 μg/ml) for 48 h. The culture supernatants were collected and assayed for nitrite content. Experiments were repeated three times with similar results.
Susceptibility of P. marneffei to chemically generated NO
We also examined the susceptibility of yeast cells and conidia to NO produced by macrophages. For this purpose, yeast cells and conidia were exposed to externally produced NO. In these experiments, NO was generated chemically by two different methods. As shown in Table 3, the number of live yeast cells diminished by more than 100 times when they were cultured with 1 mm sodium nitrite under acidic pH, compared with cultures not containing sodium nitrite. Under a neutral pH, no reduction in the number of live yeast cells was observed. In contrast, conidia resisted the killing effect of chemically generated NO at acidic pH. These results were confirmed when we used another NO-generating compound, NOC12. As shown in Table 4, the number of live yeast cells was significantly reduced after exposure to NOC12, while its vehicle, NaOH, did not exert any fungicidal effect. In contrast, conidia were resistant to NOC12. We confirmed that the amount of nitrite generated in cultures of yeast cells (991.3 ± 48.8 μm) was similar to that in cultures of conidia (1025.1 ± 115.7 μm). Thus, the different susceptibilities to NO-mediated killing of yeast cells and conidia may be another mechanism that explains the different sensitivity to macrophage killing.
Table 3.
Effect of chemically generated NO on the number of yeasts and conidia of Penicillium marneffei

Table 4.
Effect of exposure of N-ethyl-2-(1-ethyl-2-hydroxy-2-nitrosohydrazino)-ethanamine (NOC12) on the number of yeasts and conidia of Penicillium marneffei

DISCUSSION
The major finding of the present study was the different in vitro susceptibilities of yeasts and conidia of P. marneffei to NO produced by activated macrophages, suggesting that as conidia transform into yeasts in vivo they become liable to killing by macrophages. Different susceptibilities of conidia and yeasts to NO was confirmed utilizing NO produced by IFN-γ-stimulated macrophages, chemically generated sodium nitrite in acidic medium and NOC12.
In a recent study by Cogliati et al. [15], conidia of P. marneffei were found to be susceptible to NO. Almost all conidia were killed by NO generated chemically from 0.5 mm of sodium nitrite after 72 h. In contrast, our results showed that conidia were not killed by NO generated by even 1 mm of sodium nitrite. Several factors may influence fungal susceptibility tests, such as differences in the methods used and the use of different strains. Among the former factors, the composition of the medium used in the test, pH, duration of incubation, the growth phase of the fungus, strain and inoculum size may influence the results [40]. In the above study, the inoculum size was 106/ml, the medium contained 0.17% yeast nitrogen base without amino acids or ammonium sulfate and the reaction was allowed to continue for 72 h. In contrast, we used the minimal medium and the reaction continued for 24 h only, based on the methods used by Alspaugh & Granger [24] and Wang & Casadevall [41]. Similar differences have also been reported in other species. For example, different strains of Mycobacterium avium complex (MAC) have different susceptibilities to chemically generated NO, and the intracellular growth of NO-susceptible MAC is inhibited, whereas other NO-resistant strains continue to grow inside the macrophages [35]. It should be noted that the results of Cogliati et al. [15] and those of the present study are similar regarding the preferential inhibitory effect of activated macrophages on the yeast form of P. marneffei compared with conidia. However, in contrast to their study, our results directly indicated that opsonized yeasts, but not conidia, were killed by activated macrophages. Thus, in the present study we found an absolute, rather than a relative, difference between two forms of P. marneffei in susceptibility to the fungicidal activity of NO.
Our results also showed that opsonized yeasts of P. marneffei enhanced the production of NO by activated macrophages in vitro. There are only a few reports that have examined the influence of the fungus itself on NO production. Pneumocystis carinii induces NO production from murine and human pulmonary alveolar macrophages [42]. A recent study by Kawakami et al. [43] has shown that Cryptococcus neoformans suppresses NO production by stimulated murine macrophages. The exact fungus-related mechanisms that enhance the production of NO are unknown at present, but are currently investigated in our laboratory.
In the present study, AmB was added at 0.05 μg/ml to the cell culture medium to inhibit the extracellular growth of P. marneffei. This procedure was essential to estimate the intracellular killing of P. marneffei by macrophages. In our preliminary experiments, the extracellular P. marneffei developed in a hyphal form in the absence of AmB and, importantly, harmed macrophage during the cultures, which prevented us from estimating their anti-fungal activity. Importantly, the addition of AmB in culture medium raises the possibility that the intracellular killing of microorganisms by macrophages is not due to their biological effect, but due to the anti-fungal effect of the amphopathic reagent distributed throughout intracellular membranes and other cellular lipid sites. This possibility, however, may not apply in this case, for the following three reasons: (i) IFN-γ-activated macrophages showed a growth-inhibitory effect against the yeast cells, but unstimulated macrophages did not, although both cultures contained the same dose of AmB; (ii) only yeast cells were susceptible to the growth-inhibitory effect of macrophages, although both conidia and yeast cells exerted the same minimal inhibitory concentration (0.25 μg/ml) against AmB as estimated by the microdilution method using RPMI 1640 [44]; and (iii) IFN-γ-activated macrophages showed the fungicidal effect against P. marneffei later than 24 h, although ≈ 50% and 75% of anti-fungal activity of AmB was lost 24 h and 48 h after initiation of the culture, respectively (data not shown). Consistently, Rex et al. [40] showed that the anti-fungal activity of AmB was reduced with prolongation of the incubation time.
On the other hand, AmB is also reported to have various immunomodulating activities on neutrophils, macrophages, natural killer cells, T and B cells [45]. The microbial and tumouricidal activities of macrophages are potentiated by AmB through the induction of production of tumour necrosis factor-alpha (TNF-α) and IL-1 and the generation of respiratory burst [46,47]. Also in our recent study [48], comparative doses of AmB enhanced the anti-cryptococcal effect of IFN-γ-stimulated macrophages through inducing the production of NO. At present, it is difficult to determine whether the fungicidal activity of macrophages was induced by IFN-γ alone or a combined effect with AmB.
In conclusion, the susceptibility of the yeast form of P. marneffei to killing by IFN-γ-activated macrophages was different from that of the conidia form, and their fungicidal activity was mostly mediated by NO. This difference is possibly due to the different susceptibilities of the two forms to NO-mediated killing and their different effects on NO production by macrophages.
Acknowledgments
This work was supported in part by a grant (09670292) from the Ministry of Education, Science and Culture, Japan.
References
- 1.Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirithanthana T. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344:110–3. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 2.McGinnis MR. Penicillium marneffei dimorphic fungus of increasing importance. Clin Microbiol Newslett. 1994;16:29–31. [Google Scholar]
- 3.Sirisanthana V, Sirithanthana T. Penicillium marneffei infection in children infected with human immunodeficiency virus. Pediatr Infect Dis J. 1993;12:1021–5. doi: 10.1097/00006454-199312000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Heath TCB, Patel A, Fisher D, Bowden FJ, Currie B. Disseminated Penicillium marneffei: presenting illness of advanced HIV infection; a clinicopathological review, illustrated by a case report. Pathol. 1995;27:101–5. doi: 10.1080/00313029500169582. [DOI] [PubMed] [Google Scholar]
- 5.Remadi S, Lotfi C, Finci V, Ismail A, Rogiano D, Vassilakos P, Seemayer TA. Penicillium marneffei infection in patients infected with the immunodeficiency virus. A report of two cases. Acta Cytologica. 1995;39:798–802. [PubMed] [Google Scholar]
- 6.Sekhon AS, Stein L, Garg AK, Black WA, Glezos JD, Wong C. Pulmonary Penicillium marneffei: report of the first imported case in Canada. Mycopathologia. 1994;128:3–7. doi: 10.1007/BF01104271. [DOI] [PubMed] [Google Scholar]
- 7.Dixon DM, McNeil MM, Cohen ML, Gellin BG, La Montagne JR. Fungal infections: a growing threat. Public Health Report. 1996;111:226–35. [PMC free article] [PubMed] [Google Scholar]
- 8.Dupont B, Denning DW, Marriott D, Sugar A, Viviani MA, Sirisanthana T. Mycoses in AIDS patients. J Med Vet Mycol. 1994;32(Suppl. 1):65–77. doi: 10.1080/02681219480000731. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan JE, Hu DJ, Holmes KK, Jaffe HW, Masur H, De Cock KM. Preventing opportunistic infections in human immuno-deficiency virus-infected persons: implications for the developing world. Am J Trop Med Hyg. 1996;55:1–11. [PubMed] [Google Scholar]
- 10.Hilmarsdottir I, Meynard JL, Rogeaux O, et al. Disseminated Penicillium marneffei infection associated with human immunodeficiency virus: a report of two cases and review of 35 published cases. J Acquir Immune Defic Syndr. 1993;6:466–71. [PubMed] [Google Scholar]
- 11.Kudeken N, Kawakami K, Kusano N, Saito A. Cell-mediated immunity in host resistance against infection caused by Penicillium marneffei. J Med Vet Mycol. 1996;34:371–8. doi: 10.1080/02681219680000671. [DOI] [PubMed] [Google Scholar]
- 12.Supparatpinyo K, Sirithanthana T. Disseminated Penicillium marneffei infection diagnosed on examination of a peripheral blood smear of a patient with human immunodeficiency virus infection. Clin Infect Dis. 1994;18:246–7. doi: 10.1093/clinids/18.2.246. [DOI] [PubMed] [Google Scholar]
- 13.Deng ZL, Connor DH. Progressive disseminated penicilliosis caused by Penicillium marneffei. Am J Clin Pathol. 1985;84:323–7. doi: 10.1093/ajcp/84.3.323. [DOI] [PubMed] [Google Scholar]
- 14.Garrison RG, Boyd KS. Dimorphism of Penicillium marneffei as observed by electron microscopy. Can J Microbiol. 1973;9:1305–9. doi: 10.1139/m73-209. [DOI] [PubMed] [Google Scholar]
- 15.Cogliati M, Roverselli A, Boelaert JR, Taramelii D, Lombardi L, Viviani MA. Development of an in vitro macrophage system to assess Penicillium marneffei growth and susceptibility to nitric oxide. Infect Immun. 1997;65:279–84. doi: 10.1128/iai.65.1.279-284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stuehr DJ, Nathan CF. Nitric oxide: a macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989;169:1543–55. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granger DL, Hibbs JB, Jr, Perfect JR, Durock DT. Metabolic fate of L-arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest. 1990;85:264–7. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wink DA, Osawa Y, Arbyshire JFD, Jones CR, Eshenaur SC, Nims RW. Inhibition of cytochrome P450 by nitric oxide and an oxide-releasing agent. Arch Biochem Biophys. 1993;300:115–23. doi: 10.1006/abbi.1993.1016. [DOI] [PubMed] [Google Scholar]
- 19.Wink DA, Kasprzak KS, Maragos CM, et al. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Sci. 1991;254:1001–3. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- 20.James SL, Glaven J. Macrophage cytotoxicity against schistosomula Schistosoma mansoni involves arginine- dependent production of reactive nitrogen intermediates. J Immunol. 1989;143:4208–12. [PubMed] [Google Scholar]
- 21.Liew FY, Millott S, Parkinson C, Palmer RMJ, Mocada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990;144:4794–7. [PubMed] [Google Scholar]
- 22.Rockett KA, Awburn MM, Cowden WB, Clark IA. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun. 1991;59:3280–3. doi: 10.1128/iai.59.9.3280-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sternberg J, Mabbott N, Sutherland I, Liew FY. Inhibition of nitric oxide synthesis leads to reduced parasitemia in murine Trypanosoma brucei infection. Infect Immun. 1994;62:2135–7. doi: 10.1128/iai.62.5.2135-2137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alspaugh JA, Granger DL. Inhibition of Cryptococcus neoformans replication by nitrogen oxides supports the role of these molecules as effectors of macrophage-mediated cytostasis. Infect Immun. 1991;59:2291–6. doi: 10.1128/iai.59.7.2291-2296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blasi E, Pitzurra L, Puliti M, Chimienti AR, Mazzolla R, Barluzzi R, Bistoni F. Differential susceptibility of yeast and hyphal forms of Candida albicans to macrophage-derived nitrogen-containing compounds. Infect Immun. 1995;63:1806–9. doi: 10.1128/iai.63.5.1806-1809.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura LT, Wu-Hsieh BA, Howard H. Recombinant murine gamma interferon stimulates macrophages of the RAW cell line to inhibit intracellular growth of Histoplasma capsulatum. Infect Immun. 1994;62:680–4. doi: 10.1128/iai.62.2.680-684.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anthony LSD, Morrissey PJ, Nano FE. Growth inhibition of Francisella ularensis live vaccine strain by IFN-γ activated macrophages is mediated by reactive nitrogen intermediates derived from L-arginine metabolism. J Immunol. 1992;148:1829–34. [PubMed] [Google Scholar]
- 28.Beckerman KP, Rogers HW, Corbett JA. Release of nitric oxide during the T cell-independent pathway of macrophage activation: its role in resistance to Listeria monocytogenes. J Immunol. 1993;150:888–95. [PubMed] [Google Scholar]
- 29.Gebran SJ, Yamamoto Y, Newton C, Klein TW, Friedman H. Inhibition of Legionella pneumophila growth by gamma interferon in permissive A/J mouse macrophages; role of reactive oxygen species, nitric oxide tryptophan, and iron (III) Infect Immun. 1994;62:3917–25. doi: 10.1128/iai.62.8.3197-3205.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang X, Leonard B, Benson R, Baldwin CI. Macrophage of Brucella abortus: role of reactive oxygen intermediates and nitric oxide. Cell Immunol. 1993;151:309–19. doi: 10.1006/cimm.1993.1241. [DOI] [PubMed] [Google Scholar]
- 31.Malawista SE, Montgomery RR, Van Blaricon G. Evidence for reactive nitrogen intermediates in killing of staphylococci by human neutrophil cytoplasts. J Clin Invest. 1992;90:631–6. doi: 10.1172/JCI115903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer J, Woods ML, Vavrin Z, Hibbs JB. Gamma interferon- induced nitric oxide production reduces Chlamydia trachomatis infectivity in McCoy cells. Infect Immun. 1993;61:491–7. doi: 10.1128/iai.61.2.491-497.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams LB, Franzbau SG, Vavrin Z, Hibbs JB, Jr, Krahenbuhl IL. L-arginine-dependent macrophage effector functions inhibit metabolic activity Mycobacterium leprae. J Immunol. 1991;147:1642–6. [PubMed] [Google Scholar]
- 34.Denis M. Interferon-gamma-treated macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991;132:150–7. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- 35.Doi T, Ando M, Akaike T, Suga M, Sato K, Maeda H. Resistance to nitric oxide in Mycobacterium avium complex and its implication in pathogenesis. Infect Immun. 1993;61:1980–9. doi: 10.1128/iai.61.5.1980-1989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kudeken N, Kawakami K, Saito A. CD4+ T cell-mediated fatal hyperinflammatory reactions in mice infected with Penicillium marneffei. Clin Exp Immunol. 1997;107:468–73. doi: 10.1046/j.1365-2249.1997.d01-945.x. [DOI] [PubMed] [Google Scholar]
- 37.Sekhon AS, Garg AK, Padhye AA, Hamir Z. In vitro susceptibility of mycelial and yeast forms of Penicillium marneffei to amphotericin B, fluconazole, 5-fluorocytosine and itraconazole. Eur J Epidemiol. 1993;9:553–8. doi: 10.1007/BF00209535. [DOI] [PubMed] [Google Scholar]
- 38.Estrada JA, Stynen D, Cutsem JV, Pierard-Franchimont C, Pierard GE. Immunohistochemical identification of Penicillium marneffei by monoclonal antibody. Int J Dermat. 1992;31:410–2. doi: 10.1111/j.1365-4362.1992.tb02670.x. [DOI] [PubMed] [Google Scholar]
- 39.Shibuta S, Mashimo T, Zhang P, Ohara A, Yoshiya I. A new nitric oxide donor, NOC-18, exhibits a nociceptive effect in the rat formalin model. J Neurol Sci. 1996;141:1–5. doi: 10.1016/0022-510x(96)00102-5. [DOI] [PubMed] [Google Scholar]
- 40.Rex JH, Pfaller MA, Rinaldi MG, Polak A, Galgiani JN. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–81. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Casadevall A. Susceptibility of melanized and non melanized Cryptococcus neoformans to nitrogen- and oxygen-derived oxidants. Infect Immun. 1994;62:3004–7. doi: 10.1128/iai.62.7.3004-3007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherman MP, Loro ML, Wong VZ, Tashikin DP. Cytokine- and Pneumocystis carinii-induced L-arginine oxidation by murine and human pulmonary alveolar macrophages. J Protozool. 1991;38:234S–6S. [PubMed] [Google Scholar]
- 43.Kawakami K, Zhang T, Qureshi MH, Saito A. Cryptococcus neoformans inhibits nitric oxide production by murine peritoneal macrophages stimulated with interferon-γ and lipopolysaccharide. Cell Immunol. 1997;180:47–54. doi: 10.1006/cimm.1997.1166. [DOI] [PubMed] [Google Scholar]
- 44.Espinel-Ingroff A, Kish CW, Jr, Kerkering TM, et al. Collaborative comparison of broth macrodilution and microdilution antifungal susceptibility tests. J Clin Microb. 1992;30:3138–45. doi: 10.1128/jcm.30.12.3138-3145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Little JR, Wolf JE. Immunologic effects of amphotericin B. Clin Immunol News. 1987;8:183–5. [Google Scholar]
- 46.Gelfand JA, Kimball K, Burke JF, Dinarello CA. Amphotericin B treatment of human mononuclear cells in vitro results in secretion of tumor necrosis factor and interleukin-1. Clin Res. 1988;36:456. [Google Scholar]
- 47.Wolf JE, Massof SE. In vivo activation of macrophage oxidative burst activity by cytokine and amphotericin B. Infect Immun. 1990;58:1296–300. doi: 10.1128/iai.58.5.1296-1300.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tohyama M, Kawakami K, Saito A. Anticryptococcal effect of amphotericin B is mediated through macrophage production of nitric oxide. Antimicrob Agents Chemother. 1996;40:1919–23. doi: 10.1128/aac.40.8.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]



