Abstract
Colonic epithelial cells of patients with UC express DAF in relation to the severity of mucosal inflammation. The aim of this study was to determine whether this factor in stool could be used as a marker of disease activity in UC patients. Stool DAF was measured by use of an immunoassay in 181 stool specimens obtained from 55 patients with UC of various levels of disease activity. Stool DAF concentrations in patients whose UC was active (0.0–785.6 ng/g stool; median 47.1 ng/g; n = 115) were significantly higher than concentrations in patients whose disease was inactive (0.0–48.6 ng/g; median 0.0 ng/g; n = 66) (P < 0.0001). Values in active UC patients also were higher than those in control patients with diarrhoea (0.0–30.0 ng/g; median 0.0 ng/g; n = 26) (P < 0.0001) and in control subjects without apparent colorectal disease (0–20.4 ng/g; median 0.0 ng/g; n = 44) (P < 0.0001). The elevated levels of stool DAF obtained from UC patients in relapse declined markedly in specimens collected after the disease went into remission following medical therapy. Stool DAF levels correlated with the severity of endoscopic and histological findings and the degree of DAF expression on the colonic epithelia. Our results suggest that the measurement of stool DAF is useful as a non-invasive means of monitoring intestinal disease activity in patients with UC.
Keywords: ulcerative colitis, decay-accelerating factor, CD55, inflammatory bowel disease
INTRODUCTION
In the management of UC, assessment of disease activity is essential. At present, disease activity is assessed by consideration of subjective symptoms, clinical features, and findings of radiography and colonoscopy. Simple, non-invasive methods for measuring the degree of mucosal inflammation have been needed.
Recently, we showed that DAF (CD55), a glycoprotein involved in regulation of the complement cascade [1], is prominently expressed on colorectal cancer cells [2] and is detectable in stools of patients with colorectal cancer [3]. We have shown also that colonic epithelial cells in UC express DAF on their apical cell surface in relation to the severity of mucosal inflammation [4]. It seems likely that DAF is shed into the colonic lumen of UC patients with active inflammation. Consequently, it seems reasonable that determination of DAF in stools could be a non-invasive method for measuring the degree of disease activity in UC. In the present study, we measured stool DAF concentrations in patients with UC to determine the validity of this possibility.
PATIENTS AND METHODS
Patients
Fifty-five patients with UC (23 women and 32 men, age range 16–68 years, mean age 37 years) were studied. The diagnosis of UC was based on history, clinical symptoms, and endoscopic and histological findings. Twenty-six patients had total colitis, 13 had left-sided colitis, and 16 had proctitis. Several spontaneous stool samples (1–5 g) were obtained from each of the patients during times of various degrees of disease activity. Disease activity was categorized on the basis of clinical features and laboratory data according to the criteria of Truelove & Witts [5]. A total of 181 stool specimens was collected and examined. One hundred and fifteen of the specimens were taken when the patients' disease was in a clinically active stage; the remaining 66 specimens were taken when the disease was in remission.
Thirty-nine stool samples were obtained as normal controls from 23 patients (18 women and five men, age range 31–60 years, mean age 52 years) who underwent colonoscopy for abdominal symptoms but showed no apparent colorectal disease, and from 21 healthy volunteers (five women and 16 men, age range 26–42 years, mean age 32 years). Stool samples were obtained also from 26 patients with diarrhoea (12 women and 14 men, age range 15–72 years, mean age 37 years) who had these conditions: irritable bowel syndrome, n = 12; infectious colitis, n = 11; antibiotics-induced colitis, n = 2; colonic tuberculosis, n = 1. Informed consent was obtained from each patient.
Determination of DAF in stool specimens
The stools were weighed, suspended in an equal volume of PBS containing 1% bovine serum albumin, 0.05% Tween 20 and 1 mm PMSF, then centrifuged at 20 000 g for 15 min. Supernatants were collected and kept frozen at −80°C until use. Samples were coded, and the person doing the DAF assay had no knowledge of their origin.
Stool DAF was measured by an ELISA, using 1C6 mouse monoclonal and rabbit polyclonal antibodies to DAF as described [3,6]. A calibration curve was obtained from several dilutions of known quantities of purified DAF, and the concentrations of DAF in samples were calculated. Because our preliminary study showed that stool DAF concentrations obtained from patients with active UC were 10–100 times higher than those from colorectal cancer patients, we diluted the stool samples so that their optical density (OD) values fitted in the calibration curve. Samples were analysed in duplicate, and the results are presented as ng/g stool. In some of the stool samples analysed for DAF, haemoglobin concentrations were also determined, by use of an immunological test for faecal haemoglobin (Lumipule HemSp; Fujirebio Inc., Tokyo, Japan).
Endoscopic and histologic examination
In 45 patients with UC, a total of 65 colonoscopic examinations was performed within 1 week before or after stool specimens were obtained (43 examinations were done when patients were in the clinically active stage and 22 in remission). The stool DAF concentrations and endoscopic or histological findings were compared. Biopsy specimens were taken from the lesion with the most severe endoscopic findings when the disease was active and from the rectum when the disease was in remission. The severity of mucosal damage based on endoscopic findings and degrees of mucosal inflammation based on histological features were graded according to the method described by Matts [7]. Colonoscopic and histological findings were graded by two gastroenterologists (M.M. and H.O.) who had no knowledge of the stool DAF values of patients under examination.
Immunohistochemistry
Two biopsy specimens were obtained during each colonoscopic examination. One was fixed in formalin and processed for routine histological examination. The other was fixed in a periodate-lysine-paraformaldehyde fixative [8] for immunohistochemical staining of DAF, by use of an indirect peroxidase-labelled antibody method with 1C6 mouse MoAb to DAF as described [2,4]. The expression of DAF on the epithelial cells in the colonic mucosa was semiquantified blindly according to an arbitrarily defined score [4].
Statistical analysis
For statistical analysis, Mann–Whitney U-test, Wilcoxon's signed rank test, and Kruskal–Wallis test were used. Correlation was assessed using Spearman's rank correlation coefficient.
RESULTS
Stability of DAF in stools from UC patients
Because proteolytic enzymes such as elastase are present in stools of UC patients [9], we first evaluated the stability of DAF in stools from patients with active UC. As shown in Table 1, DAF concentrations in stool specimens from two patients with active UC were found similar within 24 h after defecation, whether the specimens were kept at 4°C or at room temperature. This series of measurements also established the reproducibility of the assay.
Table 1.
Stability of DAF in stools from two patients with active UC
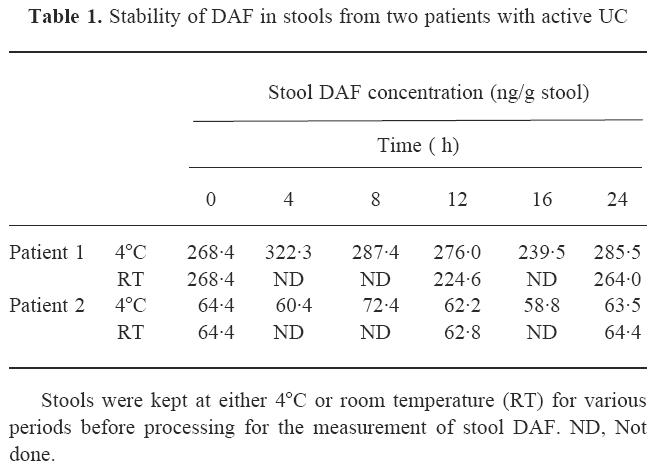
Stool DAF concentrations in UC patients and controls
The distribution of stool DAF concentrations in each patient group is shown in Fig. 1. Stool DAF concentrations in UC patients whose disease was active (0.0–785.6 ng/g; median 47.1 ng/g stool) were significantly higher than those in patients whose disease was inactive (0.0–48.6 ng/g; median 0.0 ng/g) (P < 0.0001). Values in active UC patients were also higher than those in control patients with diarrhoea (0.0–30.0 ng/g; median 0.0 ng/g) (P < 0.0001) and in control subjects without apparent colorectal disease (0–20.4 ng/g; median 0.0 ng/g) (P < 0.0001) (Mann–Whitney U-test).
Fig. 1.
Stool DAF concentrations in control subjects without apparent colorectal disease (normal control), control patients with diarrhoea due to causes other than UC (diarrhoea control), and UC patients when the disease was active or inactive. Active UC versus inactive UC, diarrhoea control, and normal control, P < 0.0001 (Mann–Whitney U-test).
Effects of medical treatment on stool DAF concentrations
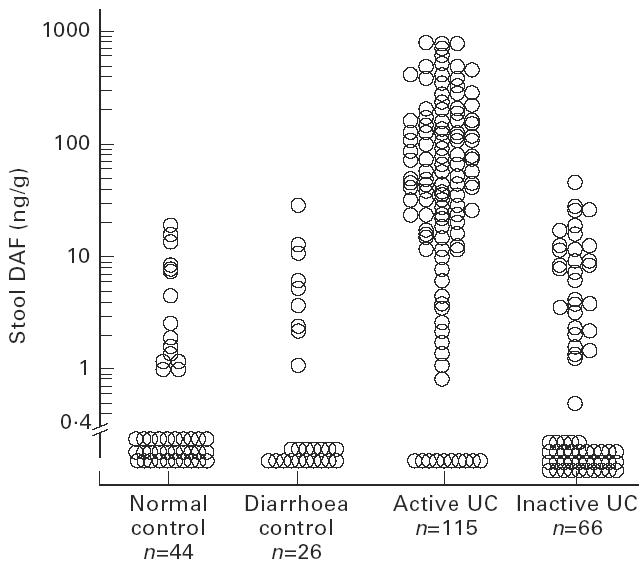
We evaluated the effects of medical treatment on stool DAF concentrations. A pair of stool samples was obtained from each of 19 UC patients (11 patients with total colitis, two with left-sided colitis, six with proctitis). The first specimens were taken when the patients' disease was active, and the second when the disease was in remission after medical therapy. The elevated levels of stool DAF (16.4–729.2 ng/g; median 218.0 ng/g) in patients with active disease fell to much lower levels in specimens collected when the disease had gone into remission (0.0–48.6 ng/g; median 1.2 ng/g) (P = 0.0001; Wilcoxon signed rank test) (Fig. 2).
Fig. 2.
Effects of medical treatment on stool DAF concentrations in patients with UC. Stool DAF concentrations in specimens obtained from patients with active disease versus concentrations in those obtained after the disease had gone into remission following medical therapy. P = 0.0001 (Wilcoxon signed rank test).
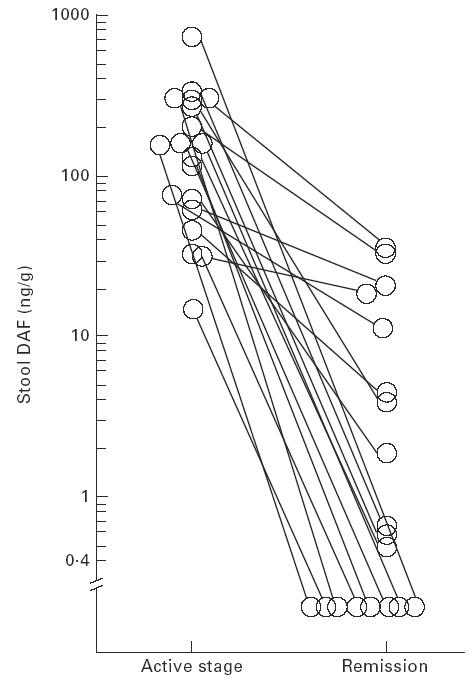
Serial determination of stool DAF concentrations in one case of UC with relapse and remission is presented (Fig. 3). Stool DAF concentrations correlated well with disease activity according to the frequency of bowel movements, clinical symptoms and the severity of endoscopic and histological findings.
Fig. 3.
Serial levels of stool DAF concentrations in one case of UC with relapse and remission. The patient, a 53-year-old woman with left-sided colitis, had been hospitalized three times within 2 years due to clinical relapse. Endoscopic findings were graded according to Matts [7]: grade 1, normal; grade 2, mild granularity of the mucosa, with mild contact bleeding; grade 3, marked granularity and oedema of the mucosa, contact bleeding, and spontaneous bleeding; grade 4, severe ulceration of mucosa with haemorrhage. Histological findings were graded according to Matts [7]: grade 1, normal appearance; grade 2, some infiltration of the mucosa or lamina propria with either round cells or polymorphs; grade 3, much cellular infiltration of the mucosa, lamina propria, and submucosa; grade 4, presence of crypt abscesses, with much infiltration of all layers of the mucosa; grade 5, ulceration, erosion, or necrosis of the mucosa, with cellular infiltration of some or all of its layers.
Haemoglobin and DAF concentrations in stool
Because DAF is present on erythrocytes [1], and stools from patients with active UC often contain blood, elevated levels of DAF concentrations in UC patients' stools might be caused by increased amounts of faecal blood. To examine this possibility, concentrations of haemoglobin and DAF were determined in 51 stool specimens from patients with active UC. The DAF and haemoglobin concentrations were not correlated (Spearman's rank correlation coefficient, r = 0.16, n = 51, P = 0.263).
Relationships between stool DAF concentrations and colonoscopic and histological gradings in patients with UC
In patients with UC, stool DAF concentrations and the severity of mucosal injury evaluated by endoscopic findings were significantly correlated (Spearman's rank correlation coefficient, r = 0.58, n = 65, P < 0.0001) (Fig. 4). However, there was no significant relationship of stool DAF concentrations to the extent of area involved as determined endoscopically (P = 0.229) (Kruskal–Wallis test). Stool DAF concentrations correlated significantly with the severity of mucosal inflammation as determined by histological findings (Spearman's rank correlation coefficient, r = 0.52, n = 65, P < 0.0001) (Fig. 5).
Fig. 4.
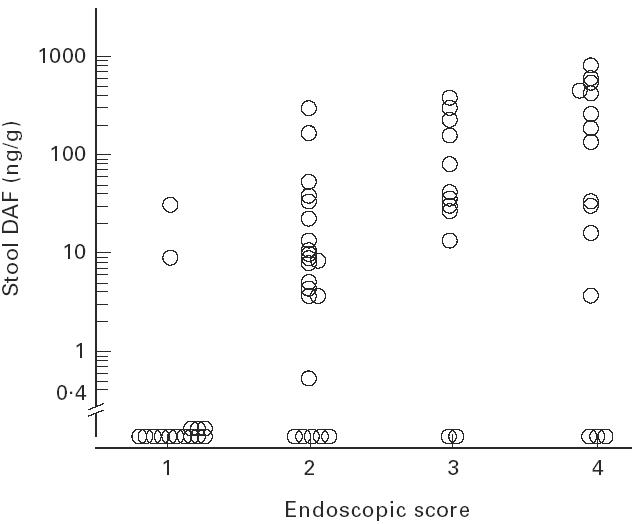
Stool DAF concentration by endoscopic grade (see legend to Fig. 3) in UC. Spearman's rank correlation coefficient, r = 0.58 (P < 0.0001).
Fig. 5.
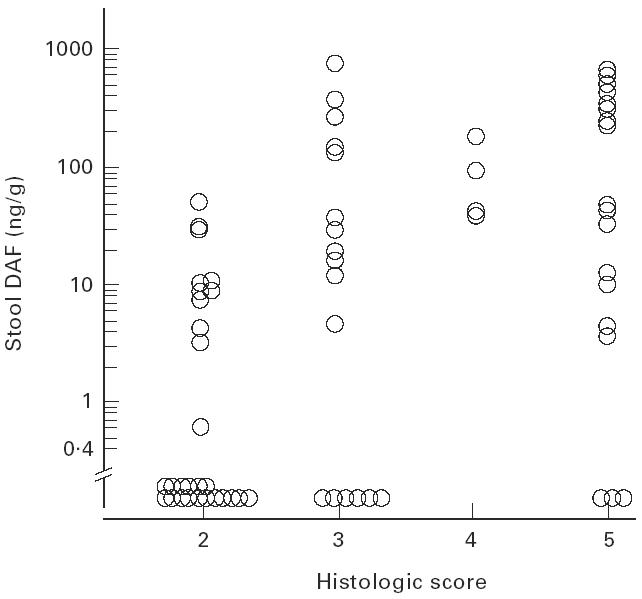
Stool DAF concentration by histological grade (see legend to Fig. 3) in UC. Spearman's rank correlation coefficient, r = 0.52 (P < 0.0001).
Correlation between stool DAF concentrations and tissue DAF expression
In accordance with our previous observation [4], DAF staining of the colonic mucosa of UC patients was increased in relation to the severity of mucosal inflammation. Also, there was a significant correlation between stool DAF concentrations and the grade of histological DAF expression (Spearman's rank correlation coefficient, r = 0.71, n = 65, P < 0.0001) (Fig. 6).
Fig. 6.
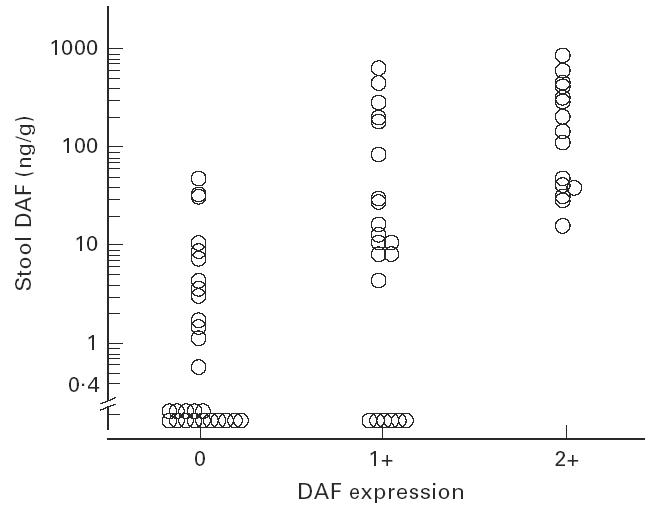
Correlation between stool DAF concentrations and tissue DAF expression in UC. Spearman's rank correlation coefficient, r = 0.71 (P < 0.0001). The expression of DAF on the colonic epithelial cells was semiquantified and graded: lack of staining, 0; specific staining on less than half of the epithelial cells, 1+; specific staining on more than half of the epithelial cells, 2+.
DISCUSSION
In this study, we determined the amount of DAF in stools from patients with UC and found that stool DAF concentrations were significantly elevated in patients with active disease compared with concentrations in patients with inactive disease, patients with diarrhoea due to causes other than UC, and subjects without apparent colorectal disease. The elevated levels of stool DAF in patients with active UC dropped markedly in specimens collected after the diseases went into remission following medical therapy. In addition, stool DAF concentrations correlated with endoscopic and histological gradings. As expected from the fact that DAF is resistant to proteolytic enzymes such as trypsin [10] and is stable in heat treatment [11], measurement of DAF in stool specimens yielded consistent results during 24 h after defecation even when kept at room temperature. Thus, the measurement of stool DAF appears to be a practical and reliable non-invasive means of assessing intestinal disease activity in patients with UC.
DAF in stool of UC patients possibly comes from several sources. The first is from colonic epithelial cells in the mucosal lesion. DAF is only sporadically expressed on colonic epithelial cells in normal conditions [2,4], but the colonic epithelia of active UC over-express DAF on the luminal surface [4]. Release of DAF has been observed from the surface of several kinds of cells [12–14]. Thus, DAF is probably released into the colonic lumen from the epithelial cells which over-express DAF in UC. Indeed, stool DAF concentrations correlated well with the grade of DAF expression assessed by immunohistochemical staining of the colonic mucosa. DAF is a glycoprotein that is attached to the cell membrane through a glycosyl phosphatidylinositol (GPI) anchor [15]. One proposed role of the GPI anchor is to enable the efficient release by hydrolytic enzymes which sever the anchor. In support of this possibility, a soluble form of DAF exists in various body fluids [12] and a cell-associated GPI-specific phospholipase D is shown to release DAF from HeLa cells [16]. Thus, DAF in stools of UC patients could be released by enzymatic cleavage at the GPI anchor. Another possible mechanisms is that DAF is released in the form of intact vesicles budding from the cell surface, as some tumour cells are capable of vesiculating under certain conditions [17,18], or that DAF is derived by secretion from the cells. We are examining the forms of DAF in stools of UC patients to help understand the mechanism of DAF release.
DAF in stool also may come from inflammatory cells passing into the colonic lumen. In UC, inflammatory cells, which synthesize and release DAF from their membrane [13], enter the colonic lumen through the diseased mucosa. Indeed, several proteins derived from polymorphonuclear cells, such as elastase [9] and lactoferrin [19], reportedly are increased in stools of patients with active UC. In addition, DAF in stool may be derived from erythrocytes, whose plasma membranes are rich in DAF [1]. However, we found no correlation between the amounts of faecal DAF and haemoglobin.
Radiographic, colonoscopic and histological examination of the colon are useful and informative for the assessment of disease activity of UC, but these invasive examinations may be a burden to patients when the disease is active. Urinary albumin [20] and several proteins in stool such as tumour necrosis factor-alpha [21] have been suggested as markers of intestinal inflammation. Here we demonstrate that stool DAF concentrations can reflect the endoscopic and histological findings of mucosal inflammation in UC. Stool DAF cannot serve as a specific marker of UC, however, because it is detected also in patients who have colorectal cancer [3]. Nevertheless, our observations indicate that measurement of stool DAF concentrations may be an additional non-invasive test of intestinal disease activity in patients with UC.
Acknowledgments
The authors thank Dr William R. Brown (University of Colorado School of Medicine) for assistance in preparation of the manuscript and Drs Kimihiro Shimo, Toshirou Maga, Seiyunu Suzuki, Nobumasa Ikeda, Masao Yoshioka, Shigeatsu Fujiki and Jun Tomoda (Okayama University Medical School) for support of this work. This work was supported by Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports and Culture, Tokyo, Japan.
References
- 1.Nicholson-Weller A, Burge J, Fearon DT, et al. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982;129:184–9. [PubMed] [Google Scholar]
- 2.Inoue H, Mizuno M, Uesu T, et al. Distribution of complement regulatory proteins, decay-accelerating factor, CD59/homologous restriction factor 20 and membrane cofactor protein in human colorectal adenoma and cancer. Acta Med Okayama. 1994;48:271–7. doi: 10.18926/AMO/31112. [DOI] [PubMed] [Google Scholar]
- 3.Mizuno M, Nakagawa M, Uesu T, et al. Detection of decay accelerating factor in stool specimens of patients with colorectal cancer. Gastroenterol. 1995;109:826–31. doi: 10.1016/0016-5085(95)90390-9. [DOI] [PubMed] [Google Scholar]
- 4.Uesu T, Mizuno M, Inoue H, et al. Enhanced expression of decay accelerating factor and CD59/homologous restriction factor 20 on the colonic epithelium of ulcerative colitis. Lab Invest. 1995;72:587–91. [PubMed] [Google Scholar]
- 5.Truelove SC, Witts LJ. Cortisone in ulcerative colitis. Final report on a therapeutic trial. BMJ. 1955;2:1041–8. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujita T, Inoue T, Ogawa K, et al. The mechanism of action of decay-accelerating factor (DAF). DAF inhibits the assembly of C3 convertases by dissociating C2a and Bb. J Exp Med. 1987;166:1221–8. doi: 10.1084/jem.166.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matts SGF. The value of rectal biopsy in the diagnosis of ulcerative colitis. Qua J Med. 1961;30:393–407. [PubMed] [Google Scholar]
- 8.McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–83. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- 9.Adeyemi EO, Hodgson HJF. Faecal elastase reflects disease activity in active ulcerative colitis. Scand J Gastroenterol. 1992;27:139–42. doi: 10.3109/00365529209165434. [DOI] [PubMed] [Google Scholar]
- 10.Sugita Y, Negoro T, Matsuda T, et al. Improved method for the isolation and preliminary characterization of human DAF (decay accelerating factor) J Biochem. 1986;100:143–50. doi: 10.1093/oxfordjournals.jbchem.a121686. [DOI] [PubMed] [Google Scholar]
- 11.Nakano Y, Sugita Y, Ishikawa Y, et al. Isolation of two forms of decay-accelerating factor (DAF) from human urine. Biochim Biophys Acta. 1991;1074:326–30. doi: 10.1016/0304-4165(91)90171-c. [DOI] [PubMed] [Google Scholar]
- 12.Medof ME, Walter EI, Rutgers JL, et al. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987;165:848–64. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tausk F, Fey M, Gigli I. Endocytosis and shedding of the decay accelerating factor on human polymorphonuclear cells. J Immunol. 1989;143:3295–302. [PubMed] [Google Scholar]
- 14.Tsuji S, Kaji K, Nagasawa S. Decay-accelerating factor on human umbilical vein endothelial cells. Its histamine-induced expression and spontaneous rapid shedding from the cell surface. J Immunol. 1994;152:1404–10. [PubMed] [Google Scholar]
- 15.Medof ME, Walter EI, Roberts WL, et al. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochem. 1986;25:6740–7. doi: 10.1021/bi00370a003. [DOI] [PubMed] [Google Scholar]
- 16.Metz CN, Brunner G, Choi Muira NH, et al. Release of GPI anchored membrane proteins by a cell-associated GPI-specific phospholipase D. EMBO J. 1994;13:1741–51. doi: 10.1002/j.1460-2075.1994.tb06438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Black PH. Shedding from normal and cancer-cell surfaces. N Engl J Med. 1980;303:1415–6. doi: 10.1056/NEJM198012113032411. [DOI] [PubMed] [Google Scholar]
- 18.Masella R, Cantafora A, Guidoni L, et al. Characterization of vesicles, containing an acylated oligopeptide, released by human colon adenocarcinoma cells. NMR and biochemical studies. FEBS Letters. 1989;246:25–29. doi: 10.1016/0014-5793(89)80246-7. [DOI] [PubMed] [Google Scholar]
- 19.Sugi K, Saitoh O, Hirata I, et al. Fecal lactoferrin as a marker for disease activity in inflammatory bowel disease: comparison with other neutrophil-derived proteins. Am J Gastroenterol. 1996;91:927–34. [PubMed] [Google Scholar]
- 20.Mahmud N, McDonald GSA, Kelleher D, et al. Microalbuminuria correlates with intestinal hisopathological grading in patients with inflammatory bowel disease. Gut. 1996;38:99–103. doi: 10.1136/gut.38.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braegger CP, Nicholls S, Murch SH, et al. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89–91. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]


