Abstract
To assess the eosinophil response to Plasmodium falciparum infection a cohort of initially parasite-free Ghanaian children was followed for 3 months. Seven of nine children who acquired an asymptomatic P. falciparum infection showed increase in eosinophil counts, while a decrease was found in seven of nine children with symptomatic malaria, and no change was observed in 14 children who remained parasite-free. In a hospital-based study, paediatric patients with cerebral malaria (CM), severe anaemia (SA), or uncomplicated malaria (UM) had uniformly low eosinophil counts during the acute illness followed by eosinophilia 30 days after cure. Plasma levels of eosinophil cationic protein (ECP) and eosinophil protein X (EPX) were measured as indicators of eosinophil activation. In spite of the low eosinophil counts, ECP levels were increased on day 0 and significantly higher in patients with CM (geometric mean (95% confidence interval) 8.5 ng/ml (6.8–10.7 ng/ml)) than in SA (4.7 ng/ml (3.0–7.5 ng/ml)) and UM patients (4.3 ng/ml (3.6–5.3 ng/ml), P < 0.001). A similar pattern was found for EPX. It thus appears that the low eosinophil counts may be due to tissue sequestration and destruction rather than decreased production. The plasma levels of the granule proteins correlated with levels of tumour necrosis factor and soluble IL-2 receptor, implicating inflammatory responses and T cell activation as causes of the eosinophil activation. By contrast, the eosinophil induction did not appear to be part of a Th2-like response. Eosinophil granule proteins may be important in both control of malaria infection and the pathogenesis of severe malaria.
Keywords: malaria, Plasmodium falciparum, eosinophils, eosinophil cationic protein, eosinophil protein X, cerebral malaria
INTRODUCTION
Acute malaria in adults with no or limited previous exposure to Plasmodium infection is usually associated with low eosinophil counts [1,2], followed by persistent eosinophilia in a proportion of patients after cure [3]. In children from endemic areas of Africa, malaria is similarly associated with decreased numbers of eosinophils in peripheral blood, although at the same time the bone marrow is rich in eosinophil precursors [4]. It thus appears that although acute malaria induces eosinophil production, eosinophil release from the bone marrow is blocked, or the cells are consumed in the inflamed tissues at a higher rate than they are produced. Eosinophils might be stimulated either directly by the parasites or by cytokines or other mediators produced during the malaria attack. Eosinophils may play a role in protection against malaria by induction of parasite killing [5], but they may also contribute to pathology by release of granule proteins such as eosinophil cationic protein (ECP) and eosinophil protein X/eosinophil-derived neurotoxin (EPX) [6]. EPX, and in particular ECP, are potent neurotoxins [7], and they may consequently contribute to the symptoms of cerebral malaria (CM). The present study aimed at investigating the eosinophil-inducing capacity of malaria parasites in healthy children and malaria patients from an area in which P. falciparum is endemic.
PATIENTS AND METHODS
Study population and patients
Field study
A longitudinal study of a cohort of 300 children took place in Dodowa, 50 km north-east of Accra between April 1994 and August 1995. The town has hyperendemic malaria transmission, with peak incidence of clinical attacks during the rainy season between May and November [8]. Approximately 18% of healthy children living in the town excrete eggs of intestinal helminths (unpublished data), whereas schistosomiasis and filariasis have not been recorded.
The cohort was followed by weekly clinical examinations and monthly blood films for the detection of malaria parasites. If a child had fever defined as axillary temperature > 37.5°C at the weekly examination, a blood film was investigated for parasites as well. Furthermore, the field workers stayed in the town and were approached by the parents whenever a child had fever. Blood films were investigated on the same day and treatment with chloroquine was instituted without delay, i.e. in most cases on the first day of fever. Children with asymptomatic parasitaemia were not treated. For the present study, children were included in June–August 1995 if they met the following criteria: no parasitaemia in May and June 1995, no parasitaemia in August, no malaria parasites other than P. falciparum in July. The group was divided into three groups, i.e. those without parasitaemia in July, those with asymptomatic parasitaemia in July, and those with a clinical malaria attack between the investigations in June and August.
Hospital-based study
Patients were enrolled at the Department of Child Health (Korle-Bu Teaching Hospital, Accra) during the same 3-month period. Only patients with strictly defined uncomplicated malaria (UM), CM, or severe malarial anaemia (SA) were included. The exact inclusion and exclusion criteria have been described elsewhere [9]. All patients were investigated before treatment with chloroquine and 30 days after cure, in some cases children were also investigated on days 3 and 7 after initiation of treatment. From each patient blood films were prepared and 20 ml venous blood collected in heparinized vacutainer tubes for separation of plasma and peripheral blood mononuclear cells (PBMC) by density centrifugation. In most cases the processing was initiated within 30 min of blood collection, but in some cases samples were stored at 20°C for up to 2 h. Plasma samples were frozen immediately after centrifugation and stored at −40°C until use.
Parents of all enrolled children gave signed informed consent for the participation, and the studies were approved by the Ministry of Health, Ghana and the Ethical and Protocol Review Committee at the University of Ghana Medical School.
Examination of blood films
Malaria parasites were investigated in thick and thin Giemsa-stained blood films. A slide was declared negative after examination of 1000 leucocytes in the thick film. Eosinophil relative frequencies were determined by differential counting and, for the hospital study, the absolute eosinophil count was obtained by multiplying the frequency with total leucocytes.
Measurement of ECP and EPX
Plasma levels of ECP and EPX were determined by ‘sandwich’ ELISA using the biotin-avidin peroxidase method as described [10,11]. The assays measure ECP and EPX in the range 15–1000 pg/ml and 60–2000 pg/ml, respectively. Before measurement the samples were diluted in PBS containing 0.1% Tween 20, 0.1% n-cetyl-NNN-trimethyl-ammonium bromide (CTAB), 20 mm EDTA, and 0.2% human serum albumin pH 7.4.
Plasma levels of cytokines and cytokine receptors were measured by the following commercial ELISA kits: IL-5, soluble IL-4 receptor (sIL-4R) (both Quantikine, R&D Systems, Minneapolis, MN), tumour necrosis factor (TNF; BioSource Europe, Fleurus, Belgium), and sIL-2R (Genzyme, Cambridge, MA).
Plasma total IgE was measured by a standard ‘sandwich’ ELISA using polyclonal goat anti-human IgE for coating, alkaline phosphatase-conjugated polyclonal goat anti-human IgE as secondary antibody, and p-nitrophenyl phosphate as substrate (all reagents from Sigma, St Louis, MO). IgE concentrations were calculated from standard curves using IgE standard serum (Behring, Marburg, Germany). Samples were tested at 1:1000 dilution and, if values were outside of the standard curve, tested again at appropriate dilution to allow determination of values between 1 and 10 000 kU/l.
Statistical analysis
Data were compared by Student's t-test, one-way anova, one-way anova for repeated measurements, and two-way anova for repeated measurements as well as by linear regression analysis. Data were normalized by log (x) or log (x + 1) transformation when necessary. All calculations were done using SigmaStat software (Jandell Scientific, San Rafael, CA). P < 0.05 was considered significant.
RESULTS
Field study
Almost 90% of the children in the study cohort were excluded because they had parasitaemia at the screenings in May, June and/or August, or did not fulfil the criteria for various other reasons. Thus, blood films from 32 children were investigated: 14 children without parasitaemia at all three observations, nine children with asymptomatic parasitaemia, and nine children with clinical malaria in between two aparasitaemic months. Eosinophil frequency varied substantially at the individual level, but did not differ significantly between the groups at the beginning of the observation period (Fig. 1, P = 0.6). In the group that remained aparasitaemic the frequency remained stable throughout the study period (Fig. 1, P = 0.9). A significant drop in eosinophils was observed during acute illness, returning to initial values after cure (Fig. 1, P = 0.005). In contrast, a significant increase in eosinophil frequency was observed in the children with asymptomatic parasitaemia, and this increase persisted after parasitaemia had cleared (Fig. 1, P = 0.003). At the time of infection, eosinophil frequency was significantly higher in asymptomatic children than in clinical malaria (Fig. 1, P = 0.02).
Fig. 1.

Frequency of eosinophils in blood films from children observed for three consecutive months. All children were uninfected at the beginning (June) and at the end (August) of the observation period. Uninfected, children without parasitaemia for three consecutive months; malaria, children with symptomatic Plasmodium falciparum malaria in July; asymptomatic, children with asymptomatic P. falciparum infection in July. Bars indicate geometric mean, error bars indicate s.e.m. **Significantly different from previous month by repeated measurement anova, P < 0.01.
Hospital study
Seventy-three patients were enrolled in the study, 25 with UM, 10 with SA, and 38 with CM. The geometric mean eosinophil count in all patient groups before treatment was 0.01 × 109 cells/l. Forty-six patients returned for 30 day follow up, when the geometric mean (95% confidence interval) eosinophil count was 0.30 × 109 cells/l (0.11–0.52 cells/l), 0.05 × 109 cells/l (0.0–0.11 cells/l), and 0.20 × 109 cells/l (0.11–0.28 cells/l) for UM, SA, and CM, respectively. This rise was significant in all patient groups (P < 0.01), but there was no difference between the groups (P = 0.2 by one-way anova). Twenty-two patients were examined on days 3, 7 and 30 after beginning of treatment. As shown in Fig. 2, eosinophil counts increased by day 3 in all groups and showed a further, significant rise by day 30 (P < 0.001). The frequencies did not differ between the groups (P = 0.5 by two-way repeated measurement anova).
Fig. 2.

Eosinophil counts before (day 0) and after (days 3, 7, and 30) treatment of cerebral malaria (n = 10), severe malarial anaemia (n = 5), and uncomplicated malaria (n = 7). Values are geometric mean and s.e.m.
The field study led us to hypothesize that P. falciparum infection induces eosinophil production, but that the excess production in clinical malaria is out-balanced by increased sequestration or destruction due to inflammatory processes in the tissues. In order to test this hypothesis, we measured plasma concentrations of two proteins, ECP and EPX, which show increased values in conditions with eosinophil activity [12–14]. As shown in Fig. 3, plasma levels of ECP were significantly higher in all patient groups on day 0 compared with day 30 after cure (P < 0.05). In contrast, EPX levels were similar at the two observation points (Fig. 4, P = 0.7). Both ECP and EPX levels were significantly higher in CM patients than in the other groups before treatment, but not after cure (Figs 3 and 4; day 0, P < 0.001; day 30, P > 0.6). There was a significant positive correlation between ECP and EPX levels (data not shown, r = 0.8, P < 0.001). On day 30 there was significant correlation between eosinophil counts and both ECP and EPX (data not shown, r = 0.3, P = 0.04 and r = 0.4, P = 0.004, respectively).
Fig. 3.
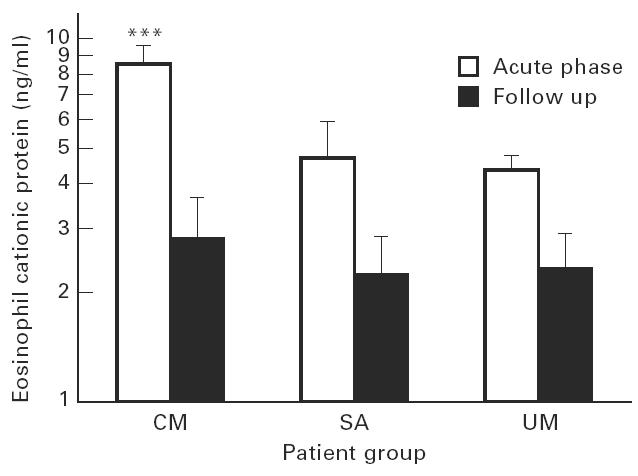
Plasma concentrations of eosinophil cationic protein (ECP) in acute malaria and at 30-day follow up. Cerebral malaria (CM), n = 38; severe malarial anaemia (SA), n = 10; uncomplicated malaria (UM), n = 25. Bars are geometric means with s.e.m. ***Significantly higher than other patient groups, P < 0.001.
Fig. 4.

Plasma concentration of eosinophil protein X (EPX; eosinophil-derived neurotoxin) before and after treatment. Presentation as in Fig. 3.
IL-5 is an important eosinophil-stimulating cytokine associated with Th2-like immune responses [15,16]. IL-5 was, however, not detectable in any of the plasma samples at a sensitivity of 7.8 pg/ml. As alternative markers of Th2-like responses the association of ECP with plasma levels of soluble IL-4 receptor (sIL-4R) and total IgE was evaluated. Neither IL-4R nor IgE showed any correlation with ECP (r = 0.1, P = 0.3 and r = − 0.1, P = 0.3, respectively; data not shown). Conversely, there was a significant positive correlation between the proinflammatory cytokine, TNF, and ECP in all patient groups (Fig. 5a, r = 0.6, P < 0.001), and a similar correlation was found with EPX (data not shown, r = 0.5, P < 0.001). Furthermore, there was a positive correlation between sIL-2R, a marker of T cell activation [17] and both ECP (Fig. 5b, r = 0.5, P < 0.001) and EPX (data not shown, r = 0.4, P < 0.001).
Fig. 5.

Linear correlation analysis between eosinophil cationic protein (ECP) and (a) tumour necrosis factor and (b) soluble IL-2 receptor in malaria patients before treatment. Symbols as in Fig. 2.
DISCUSSION
Our data strongly indicate that P. falciparum infections induce eosinophilia. First, our longitudinal field study showed a marked rise in eosinophil frequency in children with asymptomatic infection, whereas no change was observed in uninfected controls. Second, even though eosinophil counts were markedly depressed, markers of eosinophil function were elevated in acute malaria. A number of previous investigations have shown that cure of acute P. falciparum malaria is followed by increasing eosinophil counts in both non-immune American adults, semi-immune Thai adults, and semi-immune African children [1–4]. In these studies, the induction of eosinophils was variously attributed to a direct response to the parasites, an indirect response to anti-malarial drugs, or reflecting release of eosinophils following temporary bone marrow suppression. In the present study we found prolonged increases in eosinophil counts in untreated asymptomatic infections, arguing against a drug-induced response. Malaria causes extensive changes in bone marrow structure and function, but eosinophil precursors are usually abundant [4,18]. Another stimulus of eosinophils could be immunological responses to the infections. There was a positive association between levels of TNF and sIL-2R and both ECP and EPX in the malaria patients, indicating that inflammatory reactions or T cell activation may play a role in eosinophil induction during acute illness. Asymptomatic infections are associated with only slight elevation of TNF and sIL-2R ([19] and Kurtzhals et al., unpublished data), and it is possible that different mechanisms are responsible for the induction of eosinophilia in asymptomatic children and eosinophil activation in patients with acute malaria. On the other hand, with a frequency of chloroquine-resistant P. falciparum in Southern Ghana of around 8% [20], recurrent asymptomatic parasitaemia could have contributed to the high eosinophil counts in some of the clinical day 30 samples.
The importance of eosinophils in malaria remains speculative. In a single study, secretory products from eosinophils were shown to inhibit in vitro growth of P. falciparum in a dose-dependent manner, with most pronounced effects on the late asexual stages of the parasite [5]. This inhibition was partly due to ECP at concentrations comparable with the plasma concentrations observed in the present study. Local ECP concentrations may be far higher in inflamed tissues where parasites and eosinophils get in close contact, and may thus play a significant role in parasite killing. Conversely, the fact that ECP and EPX were significantly higher in cerebral than in uncomplicated malaria suggests that eosinophils may also induce pathological responses in malaria, possibly through their function as neurotoxins [7]. Although we could not demonstrate a correlation between IgE levels and eosinophil granule proteins, it should be noted that high levels of IgE have been demonstrated in CM [21]. In this case it was suggested that IgE complexes could contribute to cerebral symptoms via TNF induction [22]. Further studies are needed to elucidate the interplay between eosinophils, IgE and TNF in the pathogenesis of CM.
The eosinophilia of helminth infections is usually much more pronounced than that induced by malaria, and levels of ECP and EPX are higher in filariasis and schistosomiasis than in malaria [12]. Helminth infections are associated with a predominant Th2-like immune response, resulting in production of the cytokines IL-4 and IL-5 and immunoglobulins of the IgE class [23]. IL-5 is a potent activator of eosinophils [16] and may play a crucial role in helminth-induced eosinophilia. In the present study plasma IL-5 was below detection level in all patients and there was no correlation between IL-4 receptor or total IgE and markers of eosinophil function. It thus appears that the eosinophil induction observed in malaria was not associated with Th2-like responses. However, both epidemiological data and experimental studies in mice suggest that helminth infections can decrease the severity of malaria [24,25]. If eosinophils play a role in protection against malaria it is possible that concomitant infection with helminths could mediate protection against malaria by inducing eosinophilia.
EPX was not elevated to the same extent as ECP, although the concentration of the two molecules showed a highly significant correlation and both were associated with CM. Measurement of ECP and EPX may give falsely high values due to degranulation of eosinophils in the samples if they are not processed immediately [26]. Although this problem is more pronounced in serum than in EDTA plasma, it does occur in heparin plasma, especially in the case of EPX [26]. This would not affect the day 0 samples, which contained no or very few eosinophils, but may have caused falsely high values of EPX on day 30. However, recent data suggest that the release of ECP and EPX may vary independently [13,27], and it is thus possible that the different degree of ECP and EPX elevation during acute illness was a true phenomenon.
In conclusion, an eosinophilia is induced in malaria and concentrations of eosinophil secretory proteins are higher in CM than uncomplicated cases or cases of severe malarial anaemia. The possible protective or pathological role of eosinophils in malaria deserves further investigation.
Acknowledgments
John Tsakpo of the Centre for Tropical Clinical Pharmacology and Therapeutics, Korle-Bu Teaching Hospital is thanked for invaluable technical assistance. Ben Abuaku, Charles Atiogbe, and the health workers in Dodowa are thanked for carrying out of the fieldwork. The project was supported by the ENRECA project of the Danish International Development Agency (DANIDA) and the Danish Research Council for Development Research (RUF). L.H. is a Weimann Senior Research Fellow.
References
- 1.Davis TME, Ho M, Supanaranond W, Looareesuwan S, Pukrittayakamee S, White NJ. Changes in the peripheral blood eosinophil count in falciparum malaria. Acta Trop. 1991;48:243–5. doi: 10.1016/0001-706x(91)90052-l. [DOI] [PubMed] [Google Scholar]
- 2.Reiley CG, Barrett O. Leukocyte response in acute malaria. Am J Med Sci. 1971;262:153–8. doi: 10.1097/00000441-197109000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Shanks GD, Wilairatanaporn C. Eosinophilic response to falciparum malaria infections. SE Asian J Trop Med Public Health. 1992;23:795–7. [PubMed] [Google Scholar]
- 4.Abdalla SH. Peripheral blood and bone marrow leucocytes in Gambian children with malaria: numerical changes and evaluation of phagocytosis. Ann Trop Paed. 1988;8:250–8. doi: 10.1080/02724936.1988.11748582. [DOI] [PubMed] [Google Scholar]
- 5.Waters LS, Taverne J, Tai P-C, Spry CJF, Targett GAT, Playfair JHL. Killing of Plasmodium falciparum by eosinophil secretory products. Infect Immun. 1987;55:877–81. doi: 10.1128/iai.55.4.877-881.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durack DT, Ackerman SJ, Loegering DA, Gleich GJ. Purification of human eosinophil-derived neurotoxin. Proc Natl Acad Sci USA. 1981;78:5165–9. doi: 10.1073/pnas.78.8.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredens K, Dahl R, Venge P. The Gordon phenomenon induced by the eosinophil cationic protein and eosinophil protein X. J Allergy Clin Immunol. 1982;70:361–6. doi: 10.1016/0091-6749(82)90025-2. [DOI] [PubMed] [Google Scholar]
- 8.Afari EA, Appawu M, Dunyo S, Baffoe-Wilmot A, Nkrumah FK. Malaria infection, morbidity and transmission in two ecological zones in Southern Ghana. Afr J Health Sci. 1995;2:312–5. [PubMed] [Google Scholar]
- 9.Kurtzhals JAL, Rodrigues O, Addae M, Commey JOO, Nkrumah FK, Hviid L. Reversible suppression of bone marrow response to erythropoietin in Plasmodium falciparum malaria. Br J Haematol. 1997;97:169–74. doi: 10.1046/j.1365-2141.1997.82654.x. [DOI] [PubMed] [Google Scholar]
- 10.Reimert CM, Venge P, Kharazmi A, Bendtzen K. Detection of eosinophil cationic protein (ECP) by an enzyme-linked immunosorbent assay. J Immunol Methods. 1991;138:285–90. doi: 10.1016/0022-1759(91)90177-h. [DOI] [PubMed] [Google Scholar]
- 11.Reimert CM, Minuva U, Kharazmi A, Bendtzen K. Eosinophil protein X/eosinophil derived neurotoxin (EPX/EDN): detection by enzyme-linked immunosorbent assay and purification from normal human urine. J Immunol Methods. 1991;141:97–104. doi: 10.1016/0022-1759(91)90214-z. [DOI] [PubMed] [Google Scholar]
- 12.Tischendorf FW, Brattig NW, Büttner DW, Pieper A, Lintzel M. Serum levels of eosinophil cationic protein, eosinophil-derived neurotoxin and myeloperoxidase in infections with filariae and schistosomes. Acta Trop. 1996;62:171–82. doi: 10.1016/s0001-706x(96)00038-1. [DOI] [PubMed] [Google Scholar]
- 13.Carlson M, Håkansson L, Kämpe M, Stålenheim G, Peterson C, Venge P. Degranulation of eosinophils from pollen-atopic patients with asthma is increased during pollen season. J Allergy Clin Immunol. 1992;89:131–9. doi: 10.1016/s0091-6749(05)80050-8. [DOI] [PubMed] [Google Scholar]
- 14.Hällgren R, Bjelle A, Venge P. Eosinophil cationic protein in inflammatory synovial effusions as evidence of eosinophil involvement. Ann Rheum Dis. 1997. ; in press. [DOI] [PMC free article] [PubMed]
- 15.Salgame P, Abrams JS, Clayberger C, et al. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279–82. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 16.Sanderson CJ. Interleukin-5: an eosinophil growth and activation factor. Dev Biol Stand. 1997;69:23–29. [PubMed] [Google Scholar]
- 17.Rubin LA, Kurman CC, Fritz ME, et al. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985;135:3172–7. [PubMed] [Google Scholar]
- 18.Wickramasinghe S, Phillips RE, Looareesuwan S, Warrel DA, Hughes M. The bone marrow in human cerebral malaria: parasite sequestration within sinusoids. Br J Haematol. 1987;66:295–306. doi: 10.1111/j.1365-2141.1987.tb06913.x. [DOI] [PubMed] [Google Scholar]
- 19.Riley EM, Rowe P, Allen SJ, Greenwood BM. Soluble plasma IL-2 receptors and malaria. Clin Exp Immunol. 1993;91:495–9. doi: 10.1111/j.1365-2249.1993.tb05930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afari EA, Dunyo S, Appawu M, Nkrumah FK. In vivo seasonal assessment of Plasmodium falciparum sensitivity to chloroquine in two different malaria endemic communities in Southern Ghana. Afr J Health Sci. 1994;1:112–5. [PubMed] [Google Scholar]
- 21.Perlmann H, Helmby H, Hagstedt M, et al. IgE elevation and IgE anti-malarial antibodies in Plasmodium falciparum malaria: association of high IgE levels with cerebral malaria. Clin Exp Immunol. 1994;97:284–92. doi: 10.1111/j.1365-2249.1994.tb06082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perlmann P, Perlmann H, Flyg BW, et al. Immunoglobulin E, a pathogenic factor in Plasmodium falciparum malaria. Infect Immun. 1997;65:116–21. doi: 10.1128/iai.65.1.116-121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zwingenberger K, Hohmann A, Cardoso de Brito M, Ritter M. Impaired balance of interleukin-4 and interferon-gamma production in infections with Schistosoma mansoni and intestinal nematodes. Scand J Immunol. 1991;34:243–51. doi: 10.1111/j.1365-3083.1991.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 24.Murray J. The biological suppression of malaria: an ecological and nutritional interrelationship of a host and two parasites. Am J Clin Nutr. 1978;31:1363–6. doi: 10.1093/ajcn/31.8.1363. [DOI] [PubMed] [Google Scholar]
- 25.Christensen N, Furu P, Kurtzhals JAL, Odaibo A. Heterologous synergistic interactions in concurrent experimental infection in the mouse with Schistosoma mansoni,Echinostoma revolutum,Plasmodium yoelii,Babesia microti, and Trypanosoma brucei. Parasitol Res. 1988;74:544–51. doi: 10.1007/BF00531632. [DOI] [PubMed] [Google Scholar]
- 26.Reimert CM, Poulsen LK, Bindslev-Jensen C, Kharazmi A, Bendtzen K. Measurement of eosinophil cationic protein (ECP) and eosinophil protein X/eosinophil derived neurotoxin (EPX/EDN) J Immunol Methods. 1993;166:183–90. doi: 10.1016/0022-1759(93)90359-f. [DOI] [PubMed] [Google Scholar]
- 27.Reimert CM, Skov PS, Poulsen LK. A microtiter assay for activation of eosinophils. Allergy. 1998. ; in press. [DOI] [PubMed]


