Abstract
Macrophages play an important role in the intestinal mucosal immune system. However, they are a poorly defined cell population. We therefore determined their phenotype in normal colonic mucosa. Macrophages were isolated from colonic biopsies and surgical specimens by collagenase digestion. Colonic macrophages were positively sorted by anti-CD33 magnetic beads. Flow cytometric triple fluorescence analysis was applied to study CD14, CD16, CD33, CD44, CD11b, CD11c, CD64, HLA-DR, CD80, CD86 and CD3/CD19 expression. CD33 was evaluated as a positive marker for intestinal macrophages. CD33+ cells isolated from normal colonic mucosa showed co-expression of the established intracellular macrophage marker CD68 in FACS analysis. CD33+ cells were capable of phagocytosis. Isolation of this cell population by magnetic anti-CD33 beads and culture resulted in a 4.2–40-fold increase in IL-1β and 4.5–44-fold increase in tumour necrosis factor-alpha (TNF-α) secretion compared with unsorted lamina propria mononuclear cells (LPMC). Of the CD33+ cells, 90.9 ± 6.9% (mean ± s.d.) were CD44+. However, macrophages from colonic mucosa showed only a low expression of CD14 (10.5 ± 3.8%), CD16 (10.1 ± 3.9%), HLA-DR (27.3 ± 9.2%), CD11b (17.4 ± 6.8%), CD11c (17.8 ± 10.4%). Furthermore, expression of CD80 (9.2 ± 4.2%) and CD86 (15.1 ± 7.3%) was low, suggesting a low ability of normal intestinal macrophages to activate T cells and T cell-mediated immune responses. We conclude that CD33 is useful for the isolation and flow cytometric characterization of colonic macrophages. These cells exhibit a single phenotype in normal mucosa (CD33++, CD44++, CD14−, CD16−, CD11b−, CD11c−, HLA-DRlow, CD80−, CD86−) lacking lipopolysaccharide (LPS) receptor and costimulatory molecules.
Keywords: macrophages, intestine, CD33, CD14, CD80, CD86
INTRODUCTION
Macrophages play a key role in the process of inflammation in many different tissues. They are able to secrete proinflammatory cytokines (such as IL-1, tumour necrosis factor (TNF), IL-6, IL-8, MCP-1), free oxygen radicals, proteases and other tissue-degrading enzymes. Intestinal macrophages represent one of the largest compartments of the mononuclear phagocyte system in the body [1]. They are localized preferentially in the subepithelial region and constitute 10–20% of mononuclear cells in the intestinal lamina propria [2,3].
A number of studies dealing with phenotypic characteristics suggested that intestinal macrophages differ markedly from blood monocytes, from macrophages found in other tissues, or from in vitro differentiated macrophages (for a review see [4]). The classical monocyte-specific surface markers CD14 (lipopolysaccharide (LPS) receptor) [5] and CD16 (FcγIII receptor) [6] were found to be almost absent in normal mucosa. The typical macrophage marker CD11b (complement receptor 3 (CR3), a member of the integrin family) was only found in < 5% of intestinal macrophages [7]. Less than 15% expressed CD11a (LFA-1) and < 40% CD11c (complement receptor 4 (CR4)) [7]. CD54 (intercellular adhesion molecule-1 (ICAM-1)) was found to be present in 7% of the intestinal macrophages of normal mucosa [7]. CD25 (IL-2 receptor) was found to be almost absent in situ [8], whereas in isolated intestinal macrophages there seemed to be a constitutive expression [9]. With the exception of the study of Doe and coworkers [5], these studies were performed using immunohistochemical techniques. Because of the low expression of typical macrophage markers, application of flow cytometric analysis has been difficult due to the problem of recognition of intestinal macrophages. Therefore, the first aim of this study was to find a positive marker for intestinal macrophages in flow cytometric analysis.
We identified CD33 as a useful recognition marker for intestinal macrophages in flow cytometric analysis. CD33, a 67-kD glycoprotein, is a member of the immunoglobulin superfamily, and its expression is restricted to myelomonocytic blood cells. As the amount of neutrophils in our lamina propria mononuclear cell (LPMC) fraction was < 1%, CD33 could be used to distinguish between macrophages and lymphocytes. The functions and binding properties of CD33 are still unknown. Recently it has been demonstrated that CD33 is the fourth member of the sialoadhesin family of sialic acid-dependent cell adhesion molecules [10]. The binding to CD33 can be modulated by endogenous sialoglycoconjugates when CD33 is expressed in plasma membranes.
To characterize further the phenotype of human colonic macrophages we monitored the expression of the ‘typical’ macrophage antigens CD14 (LPS receptor), CD16 (FcγIII receptor), CD11b (CR3) and CD11c (CR4). Furthermore, the expression of MHC class II and of the costimulatory molecules CD80 (B7-1) and CD86 (B7-2) [11,12] was analysed. The B7 molecules expressed on antigen-presenting cells (APC) play an important role in the activation of T cells (for a review see [13]). They interact with the CD28 or CTLA-4 molecule on T lymphocytes. The presence or absence of costimulatory molecules on APC significantly influences the qualitative and quantitative nature of the immune response. For this reason the B7 molecules may have an important role in autoimmunity and immune evasion [14,15]. Furthermore, the differential expression of B7-1 and B7-2 differentially activates Th1 or Th2 responses [16].
MATERIALS AND METHODS
Materials
Dulbecco's modified Eagles' medium (DMEM) was from Biochrom (Berlin, Germany); fetal calf serum (FCS) and RPMI 1640 medium for macrophages from Sigma (Taufkirchen, Germany); mouse anti-human CD14, CD16, HLA-DR, CD44, CD68 and CD11b antibodies were purchased from Immunotech (Hamburg, Germany). Anti-human CD33 antibody was from Coulter (Hamburg, Germany). Anti-human CD3, CD19 and CD11c antibodies were from Medac (Hamburg, Germany). For the study of CD80 three different antibodies were tested: clone BB1, FITC-conjugated (Pharmingen, San Diego, CA), clone MAB 104, FITC-conjugated (Immunotech, Marseille, France), clone BB1, PE-conjugated (Becton Dickinson, Heidelberg, Germany). Anti-CD86 was from clone 2331 (FUN1), FITC-conjugated (Pharmingen), but clone IT2.2, PE-conjugated (Pharmingen), was also tested. The PE-conjugated antibodies showed brighter fluorescence, but the number of immunolabelled cells did not differ between the tested antibodies. Hanks' buffered salt solution (HBSS) was obtained from Biochrom, Ficoll from Pharmacia (Freiburg, Germany). FIX&PERM solution for cell permeabilization was obtained from An Der Grub (Kaumberg, Austria). All other reagents and chemicals were from Sigma.
Source of human colonic macrophages
Human colonic macrophages were isolated from biopsies obtained by endoscopy or from surgical specimens (for magnetic bead isolation). The study was approved by the University of Regensburg Ethics Committee. None of the persons (14 for phenotypic characterization, 10 for CD80/CD86 expression, eight for short time culture experiments, five for CD68/CD33 costaining, five for magnetic cell sorting and culture of purified colonic macrophages) suffered from inflammatory bowel disease or was treated with corticosteroids or other immunosuppressive drugs. None of the patients showed macroscopical inflammation of the mucosa. Colonoscopy was done because of screening for colorectal cancer or for polypectomy. Surgery was done because of colorectal cancer. The mean age of the patients was 56 ± 7 years. Six biopsy specimens were collected from each patient. Biopsies were immediately transferred into sterile RPMI 1640 at 4°C supplemented with PenStrep and 10% FCS. All further steps were performed under sterile conditions. No fixation procedure was performed before the labelling steps.
Isolation of human colonic macrophages
Human colonic macrophages were isolated according to a modification of the method of Bull & Bookman [17]. Six biopsies were washed three times in calcium-magnesium-free HBSS. The specimens were incubated twice in calcium-magnesium-free HBSS with 1 mm EDTA for 10 min at 37°C under gentle shaking (10 ml for six biopsies) to remove intestinal epithelial cells. Specimens were washed with HBSS and incubated for 20–30 min in 2 ml RPMI with 1 mg/ml collagenase type I ( = 336 U/ml), 0.1 mg/ml DNAse and 1 mg/ml hyaluronidase without FCS at 37°C. Cells were washed twice with PBS and finally submitted to Ficoll density gradient centrifugation for 20 min at 2000 rev/min (∼ 690 g, without brake) in a Heraeus centrifuge for isolation of mononuclear cells. The interphase was carefully removed and washed with PBS.
Roller-culture of human intestinal macrophages
To determine if the phenotype of normal colonic macrophages changed after removal from their specific milieu in the gut, we cultured colonic macrophages from eight normal donors for 24 h or 48 h. Immediately after isolation and Ficoll centrifugation cells were washed and resuspended in 3 ml RPMI 1640 medium with 10% FCS (+ Pen-Strep, gentamycin and fungizone). The cell suspension was placed in a 12-ml Falcon tube on roller-culture equipment in the incubator. Permanent rolling prevented adhesion of the cells to the tube wall.
Peripheral blood monocytes
Peripheral blood (10 ml) was collected from 12 healthy donors in lithium–heparin tubes. Mononuclear cells were separated by Ficoll density gradient centrifugation. The purity of isolated mononuclear cells was always > 95%. Peripheral blood mononuclear cells (PBMC) were washed twice and were then incubated in the isolation medium (see above) used for the isolation of colonic macrophages (collagenase, DNAse, hyaluronidase) for 20 min under identical conditions to those described above. This step was followed by the immunostaining procedure (see below).
In vitro differentiated macrophages
PBMC were separated by leukapheresis of healthy donors. Monocytes were isolated by countercurrent centrifugal elutriation in a J6M-E Beckmann centrifuge with a standard chamber and a JE-5 rotor at 2500 rev/min and a flow rate of 20 ml/min, as described [18,19]. To induce monocyte to macrophage differentiation purified monocytes were cultured in RPMI 1640 supplemented with 5 × 10−5 m mercaptoethanol, polyvitamins, antibiotics, pyruvate, non-essential amino acids and 2% human AB group serum (endotoxin < 0.1 EU/ml as measured in the Limulus assay) on Teflon foils at 106 cells/ml (Biofolie 25; Heraeus, Hanau, Germany) for 7 or 14 days [20].
In vitro differentiated monocyte-derived dendritic cells
PBMC were separated by leukapheresis of healthy donors. Monocytes were isolated by countercurrent centrifugal elutriation as described above. To induce differentiation to dendritic cells purified monocytes were cultured in RPMI 1640 supplemented with 5 × 10−5 m mercaptoethanol, polyvitamins, antibiotics, pyruvate, non-essential amino acids with addition of 100 U/ml IL-4, 50 U/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) and 50 U/ml interferon-gamma (IFN-γ) on Teflon foils at 106 cells/ml (Biofolie 25; Heraeus) for 14 days.
Immunostaining procedures
Isolated cells were resuspended in PBS with 1% bovine serum albumin (BSA). For each immunolabelling 100 μl of the cell suspension were placed into 1.5-ml polypropylene tubes. To each cell suspension 10 μl of the three antibody solutions were added (total volume of 130 μl). Each FITC-conjugated antibody (CD14, CD16, HLA-DR, CD44, CD11b, CD11c, CD80, CD86) was combined with the CD33–PE conjugate and a mixture of the PerCP-conjugated CD3 and CD19 antibodies. Incubation was performed in the dark for 15–30 min on ice to ensure specific labelling. Cells were washed three times with cold PBS and resuspended in 200 μl PBS. The cell solution was transformed to FACS tubes just before FACS analysis to avoid attachment of macrophages to the tube walls.
For the double-staining of CD68 (FITC) and CD33 (PE) all isolated mononuclear cells from a surgical specimen were treated for 15 min at room temperature with 100 μl FIX&PERM solution A, washed with PBS + 1% BSA and then incubated for 15 min with 100 μl FIX&PERM solution B for permeabilization. Permeabilized cells were incubated for 30 min with the CD68 and CD33 antibodies.
Magnetic cell sorting
Isolated cells were sorted by immunomagnetic beads coated with MoAbs against CD33 (MACS Beads; Miltenyi Biotec, Bergisch Gladbach, Germany) with the help of a separation column and a magnetic separator from the same company in accordance with the manufacturer's recommendations. Briefly, for magnetic labelling the isolated LPMC were washed in sterile and degassed PBS with 5 mm EDTA and 0.5% BSA (MACS buffer) to avoid cell aggregation, and resuspended in 80 ml MACS buffer per 107 total cells. Twenty millilitres MACS Beads were added per 107 total cells, mixed and incubated for 15 min at 6–12°C. Cells were washed and resuspended in 500 ml MACS buffer per 108 cells.
For magnetic sorting a suitable column according to the estimated amount of positive cells was placed in the magnetic field of the separator and washed with MACS buffer. The column was loaded with the magnetically stained cell suspension and washed again. The effluent was collected as negative fraction. The positive fraction was flushed out with MACS buffer in the absence of a magnetic field.
Flow cytometry
Flow cytometry was performed using a FACScan (Becton Dickinson, San Jose, CA). The fluorescence of 10 000 cells was collected on a 1024-channel four-generation log scale through forward light scatter (FSC) and linear scale through right-angle scatter (SSC). Fluorescence emission for fluorescein was determined at 530 nm (FL1), the PE emission (always CD33) was determined at 585 nm (FL2) and the PerCP emission (always CD3/CD19) was determined at 670 nm (FL3). Analysis gates were set around debris, lymphocytes and macrophages on a forward scatter versus side scatter dot plot. Acquisition was performed on unfixed cells. Data acquisition and analysis were performed automatically using LYSIS TM II Software, Version 1.1 (Becton Dickinson) or WIN-MDI Software.
Phagocytosis assay
For phagocytosis assay 106 cells were resuspended in 200 μl RPMI 1640 supplemented with 10% FCS and 200 μl of a latex bead suspension (5 μl Sigma latex beads in 1 ml RPMI), or for fluorescence microscopy 10 μl of FITC-labelled 0.5- or 1-μm beads. Cells were incubated for 2 h at 37°C and washed four times with NKH buffer pH 7.4 (8.0 g NaCl, 0.4 g KCl, 2000 μl HEPES at 1000 ml)
Statistical analysis
Unless otherwise stated all data are given as mean ± s.d. Statistical analysis was performed using Student's t-test for unpaired samples.
RESULTS
Evaluation of CD33 as specific positive marker for colonic macrophages
Optical characteristics of colonic macrophages in flow cytometry
Flow cytometric forward- to side-scatter characteristics of isolated colonic LPMC were found to be not sufficient to separate lymphocytes from macrophages (Fig. 1a), whereas the distinction between lymphocytes and monocytes from peripheral blood (Fig. 1b) and between lymphocytes and macrophages from Teflon cultures (Fig. 1c) could easily be done by their optical characteristics, leading to two distinct populations. CD68, which is an established intracellular marker for macrophages, could not be used because permeabilization for intracellular labelling was followed by a cell loss, probably due to the previous isolation procedure. In addition, a surface marker would allow sorting of the positive cells. Therefore, we searched for a specific surface marker of colonic macrophages. Screening of a number of antibodies directed against typical macrophage antigens (CD14, CD16, CD11b, CD11c, HLA-DR), or antigens present on macrophages but not on lymphocytes (CD33), showed a high expression of CD33 in 11.8 ± 2.2% of the isolated cells. CD14 was expressed in 0.9 ± 0.3%, CD16 in 0.9 ± 0.4%, CD11b in 1.5 ± 0.5%, CD11c in 1.1 ± 0.7% and HLA-DR in 2.4 ± 0.8% of the isolated cells.
Fig. 1.
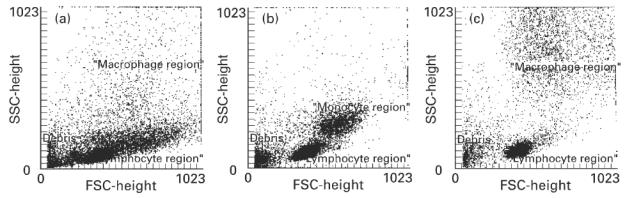
Forward/side scatter characteristics of mononuclear cells from normal colon and from blood, and of in vitro differentiated macrophages. Isolated mononuclear cells from normal colon (a), from peripheral blood (b) or in vitro differentiated macrophages (c) were analysed by FACS (10 000 cells each). It is obvious that the discrimination between lymphocytes and monocytes/macrophages is easy for blood monocytes (b) and in vitro differentiated macrophages (c). In the case of isolated colonic macrophages (a) it is almost impossible to discriminate clearly between the lymphocyte and the macrophage region.
Characterization of the CD33+ cell population
The CD33+ cell population could be easily distinguished from the other cells (Fig. 2a). The side scatter signal was obviously higher than in the remaining cells, indicating higher ‘granularity’. ‘Backgating’ of the CD33+ cells showed forward/side scatter characteristics typical for monocytes/macrophages (Fig. 2b). However, as mentioned before, there were not two distinct populations but a continuous transition between intestinal lymphocytes and macrophages. To ensure that no lymphocytes were analysed for the expression of macrophage surface markers, we performed FACS-triple fluorescence technique which allowed us to have an inclusion (CD33) and an exclusion (CD3/CD19) marker for each measurement. For the calculation of the expression of the analysed macrophage surface markers only the CD33+, CD3/CD19− cell population (lower right quadrant in Fig. 2c) was used. Less than 10% of the cells analysed showed double expression of the macrophage marker CD33 and the lymphocyte markers CD3 and CD19. The double expression of lymphocyte and macrophage surface markers on one particle could be due to lymphocytes attached to macrophages, or not completely sufficient compensation.
Fig. 2.
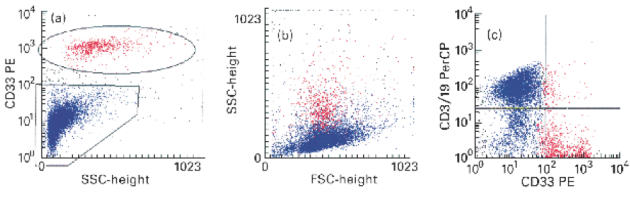
Flow cytometric detection of CD33+ cells from colonic mucosa. (a) Dot plot of side scatter characteristics versus CD33 fluorescence. Ten thousand cells were analysed each. CD33+ cells (red) are easily discriminated from the other cells (blue), which are mainly lymphocytes. (b) ‘Backgating’ of the CD33+ cells (red) shows that these cells have higher side scatter signals compared with the lymphocyte population. The region in the forward/side scatter plot is typical for monocytes/macrophages. (c) Dot plot of CD33 (PE) fluorescence versus CD3/CD19 (PerCP) fluorescence. CD33+ cells (red) are negative for the lymphocyte markers CD3 and CD19 in > 90%. Only very few cells show a positive signal for CD33 and CD3/CD19, probably due to attached or adherent cells.
FACS analysis of the co-expression of CD68 and CD33
To evaluate further the positive recognition of macrophages by anti-CD33, we performed double-staining for intracellular CD68 and CD33. CD68 is a well established intracellular marker for macrophages which has been widely used in immunohistochemical studies. Figure 3a shows that the permeabilization procedure increases the amount of debris. Figure 3b demonstrates the analysis of isotype control antibodies without positive signals. In > 95% of the analysed cells, co-expression of CD68 and CD33 on the cells could be demonstrated (Fig. 3c). Single analysis of the CD68 (Fig. 3d) and CD33 (Fig. 3e) also revealed that the positive cells had the same side scatter characteristics.
Fig. 3.
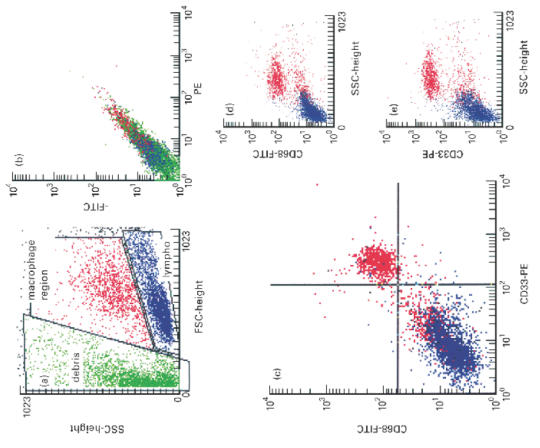
FACS analysis of the co-expression of CD68 and CD33. (a) Forward/side scatter plot of permeabilized colonic macrophages showing an increase in debris (green). Regions were set on the macrophage (red) and lymphocyte population (blue). (b) Isotype control showing no non-specific staining. (c) Co-expression of CD68 and CD33 (upper right quadrant). The cells expressing both antigens have optical characteristics of macrophages. The red cells in the lower left quadrant indicate, again, that optical characteristics are not sufficient to distinguish clearly between colonic lymphocytes and macrophages. There are almost no cells expressing either CD33 (lower right quadrant) or CD68 (upper left quadrant) alone. (d) Side scatter versus CD68 plot showing clearly a positive cell population. (e) Side scatter versus CD33 plot showing a positive population with very similar side scatter characteristics compared with the CD68+ cells.
Phagocytosis assay
Phagocytosis assays showed that about 15% of all isolated LPMC were able to phagocytose latex beads, a typical function of macrophages. Immunocytochemical immunostaining for CD33 (with the same antibody used in flow cytometric determinations) after the phagocytosis assay revealed that 80.5 ± 5.2% of the phagocytosing cells were positive for CD33. On the other hand, 83 ± 5.4% of the CD33+ cells were capable of phagocytosis. To confirm these results we performed a phagocytosis assay with fluorescence (FITC)-labelled latex beads and counterstained the cells with PE-labelled CD68 and CD33 (Fig. 4). Again, we found that > 80% of the CD33+ cells and in addition 90% of the CD68+ cells were capable of phagocytosis.
Fig. 4.
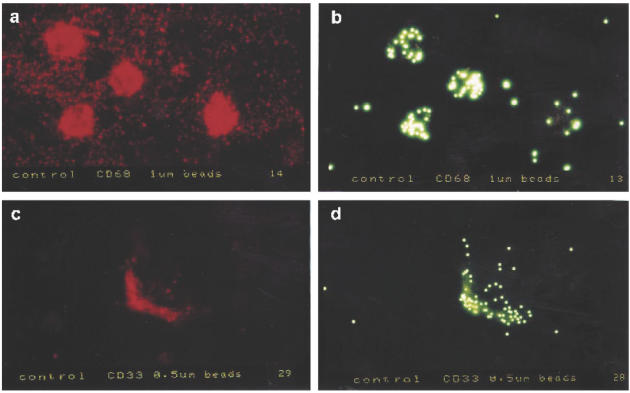
Immunofluorescence demonstration of phagocytosis in CD68+ and CD33+ cells. Human colonic mononuclear cells were isolated and immunostained as described in Materials and Methods. Specimens were obtained from patients with normal colonic histology. The staining for CD68 (a) was brighter compared with CD33 (c) with higher background staining in the cytospins, probably due to spread cytosol during centrifugation. More than 90% of the CD68+ cells (a) and > 80% of the CD33-labelled cells (c) showed phagocytosis of FITC-labelled beads (b,d).
Isolation of CD33+ cells by anti-CD33 magnetic beads
The isolation procedure was performed as described in Materials and Methods. Figure 5a demonstrates the forward/side scatter characteristics before and Fig. 5b after two subsequent separation procedures with MACSbeads. It is clear from the optical characteristics that the large lymphocyte population has almost completely disappeared. After one sorting step CD33+ cells were enriched to 50% of the remaining cells (Fig. 5c). After the second separation step > 90% of the intact cells were CD33+ (Fig. 5d, isotype control shown in Fig. 5e).
Fig. 5.
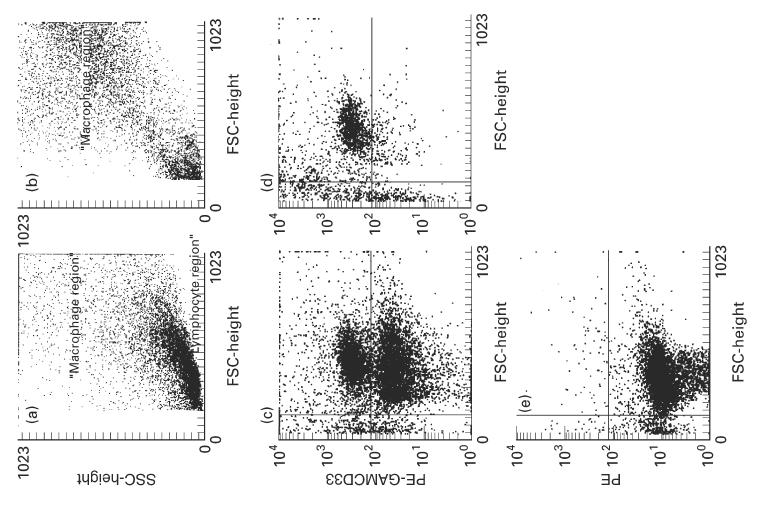
Isolation of CD33+ cells by anti-CD33 magnetic beads. Forward/side scatter characteristics isolated intestinal mononuclear cells before (a) and after (b) two subsequent separation procedures with MACSbeads. The large lymphocyte population has almost completely disappeared in b. (c) Side scatter versus CD33+ cells after one isolation step. CD33+ cells are enriched to about 50% of the cells. (d) After the second separation step > 90% of the intact cells are CD33+. (e) Isotype control showing no non-specific staining.
Culture of isolated CD33+ colonic macrophages
Magnetic bead-isolated CD33+ colonic macrophages were cultured on Primaria six-well or 24-well plates. The cells showed typical macrophage-like morphology after 3 days of culture (Fig. 6a). Incubation with an IgG-isotype control antibody and a secondary peroxidase-conjugated antibody did not show any staining (Fig. 6a). Incubation with the anti-CD68 antibody was followed by a positive staining of > 90% of the cells (Fig. 6b). The same experiments were performed with 7 days cultured in vitro differentiated macrophages. The morphology of these cells was very similar, an isotype control antibody did not stain the cells (Fig. 6c), and the CD68 antibody in parallel to the colonic macrophages stained > 90% of the in vitro differentiated macrophages (Fig. 6d).
Fig. 6.
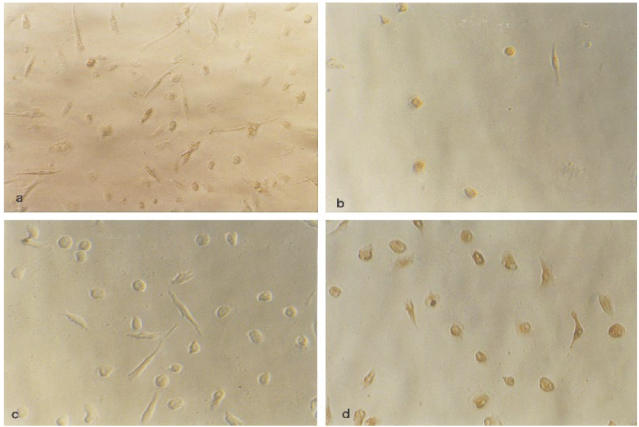
Culture of isolated CD33+ colonic macrophages. CD33+ colonic macrophages isolated by magnetic beads and in vitro differentiated macrophages were cultured on Primaria six-well or 24-well plates. (a) Typical macrophage-like morphology of anti-CD33 isolated cells after 3 days of culture. Cells stained with isotype control and DAB-peroxidase. (b). Incubation with the anti-CD68 antibody. More than 90% of the cells show positive staining. The same experiments were performed with 7 day cultured in vitro differentiated macrophages (c,d). (c) Similar morphology compared with colonic macrophages, staining with isotype control antibody. (d) The CD68 antibody in parallel to the colonic macrophages stained > 90% of the in vitro differentiated macrophages.
IL-1β and TNF-α secretion of cultured CD33+ cells
The secretion of IL-1β and TNF-α after 24 h of culture was measured in medium from LPMC and the corresponding CD33-MACSbead-isolated colonic macrophages. CD33+ cells from five different isolations secreted 4.2–40-fold more IL-1β (Fig. 7a) and 5.5–44.4-fold more TNF-α (Fig. 7b). Isolated and cultured intestinal epithelial cells did not express mRNA for or secrete any IL-1β and TNF-α (data not shown). In addition, intestinal fibroblasts isolated from the lamina propria of surgical specimens did not produce and secrete IL-1β, indicating that the determined increase in cytokine secretion by the CD33+ population was due to the fact that these cells are macrophages.
Fig. 7.
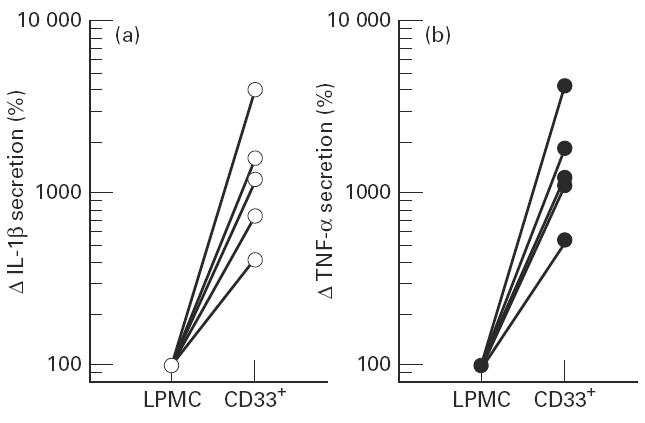
IL-1β and tumour necrosis factor-alpha (TNF-α) secretion of cultured CD33+ cells. (a) IL-1β secretion of lamina propria mononuclear cells (LPMC) and sorted CD33+ cells from five different isolations. CD33+ cells secrete 4.2–40-fold more IL-1 than the LPMC. (b) TNF-α secretion after 24 h of culture in the medium of LPMC and the corresponding CD33-MACSbead-isolated colonic macrophages. Isolated CD33+ cells secreted between 4.5- and 44-fold more TNF-α.
Phenotypic characterization of colonic macrophages
Expression of typical macrophage markers on colonic macrophages from normal mucosa
Figure 8a–f shows the expression of FITC-labelled anti-CD14 (a), anti-CD16 (b), anti-HLA-DR (c), anti-CD44 (d), anti-CD11b (e) and anti-CD11c (f) versus CD33. As summarized in Table 1, macrophage surface expression of CD14, CD16, CD11b, CD11c and HLA-DR in colonic mucosa was low.
Fig. 8.
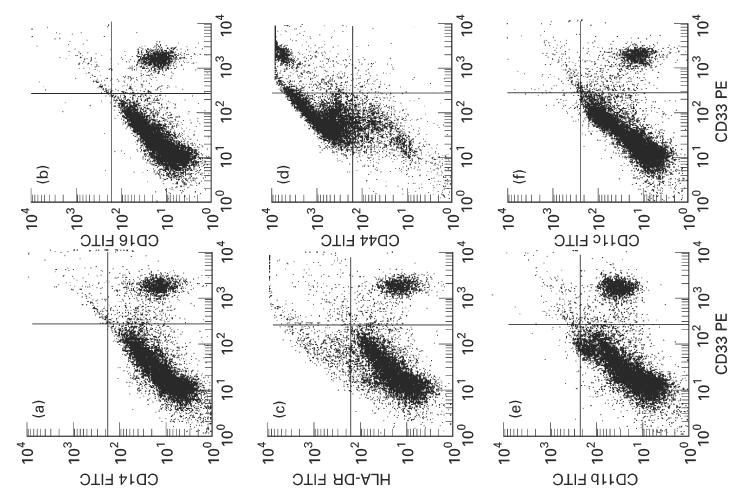
Phenotype of mononuclear cells isolated from normal colonic mucosa. (a) Dot plot of CD33 (PE) fluorescence versus CD14 (FITC) fluorescence. CD33+/CD14− and CD33+/CD14+ cells are easily discriminated from the other cells. (b) Dot plot of CD33 (PE) fluorescence versus CD16 (FITC) fluorescence. (c) Dot plot of CD33 (PE) fluorescence versus HLA-DR (FITC) fluorescence. (d) Dot plot of CD33 (PE) fluorescence versus CD44 (FITC) fluorescence. (e) Dot plot of CD33 (PE) fluorescence versus CD11b (FITC) fluorescence. (f) Dot plot of CD33 (PE) fluorescence versus CD11c (FITC) fluorescence. Ten thousand cells were analysed each. There was only a low number of CD14+, CD16+, HLA-DR+, CD11b+ and CD11c+ macrophages (CD33+).
Table 1.
Phenotypic characterization of macrophages
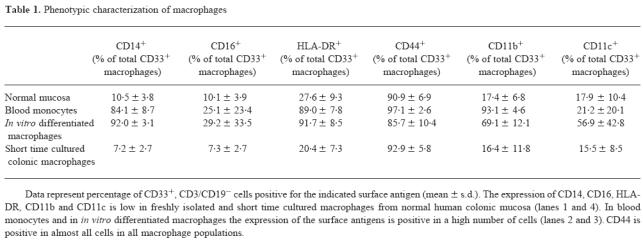
To exclude that the finding of a very low number of colonic macrophages expressing typical macrophage markers was due to digestion of the antigens during the isolation procedure, we analysed peripheral blood monocytes after applying the same isolation conditions as used for colonic mononuclear cells. The expression of antigens on PBMC was not different from published data (see Table 1).
Colonic macrophages were also substantially different from in vitro differentiated and cultivated macrophages. Data are given in Table 1.
Characterization of CD33+ colonic macrophages after short time culture
To elucidate if the low expression of typical macrophage surface markers in colonic macrophages compared with blood monocytes or macrophages differentiated in Teflon bags was due to a rapidly reversible down-regulation of the markers in the intestinal milieu or due to a specific differentiation process, we cultured freshly isolated colonic macrophages for 24 h or 48 h in roller bottles to avoid adherence under sterile conditions without the presence of LPS. Analysis of short time cultured colonic macrophages revealed that there was no difference compared with freshly isolated colonic macrophages (Table 1). The data show that the phenotype of colonic macrophages cannot be changed within 48 h, and may therefore be due rather to a specific differentiation process, and not only to a transient regulation by a specific milieu.
Expression of CD80 and CD86 on colonic macrophages from normal mucosa
CD33 was used as colonic macrophage marker. In vitro differentiated dendritic cells were used as a positive control for the expression of CD80 and CD86, which were positive on almost all of the dendritic cells positive for CD33 (Fig. 9). The expression of CD80 (B7-1) was low on colonic macrophages (9.2 ± 4.2%) (Fig. 9a). The expression of CD86 (B7-2) was only slightly higher (15.2 ± 7.3%, range 6–20%) (Fig. 9b).
Fig. 9.
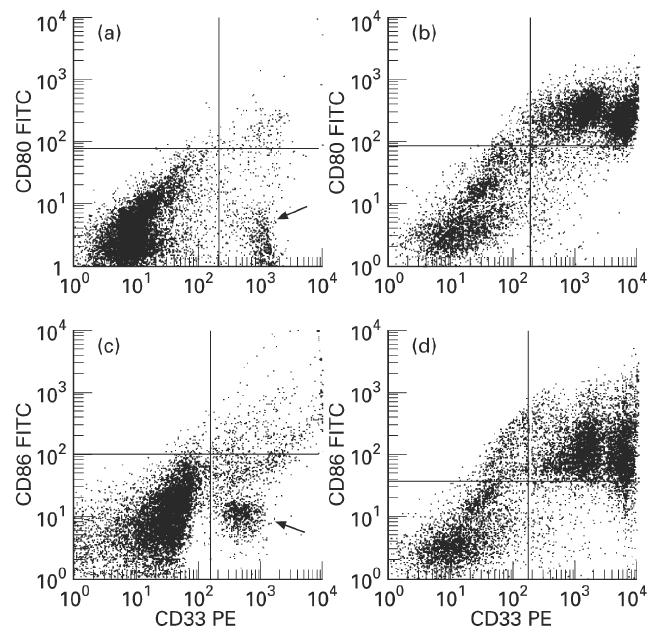
Expression of CD80 (B7-1) and CD86 (B7-2) on colonic macrophages and in vitro differentiated dendritic cells. (a,c) Dot plot of CD33 (PE) fluorescence versus CD80 (FITC) fluorescence (a) or versus CD86 (FITC) fluorescence (c) of colonic macrophages. Most of the population of colonic macrophages lacks CD80 and CD86 expression (arrows). Only a few cells are positive for CD80 or CD86 (upper right quadrant). (b,d) Dot plot of CD33 (PE) fluorescence versus CD80 (FITC) fluorescence (b) or versus CD86 (FITC) fluorescence (d) of in vitro differentiated dendritic cells. Almost all dendritic cells (CD33+) express CD80 and CD86 (upper right quadrants).
DISCUSSION
In this study CD33 was evaluated as a positive marker for colonic macrophages and was used for the isolation of pure macrophage preparations from normal colon. In addition, a detailed analysis of the phenotype of colonic macrophages in normal mucosa is presented. For the first time flow cytometric triple fluorescence technique was applied. A panel of antibodies against typical macrophage surface antigens and molecules involved in antigen presentation and costimulation of T cells was used. Because it became obvious that optical properties (forward/side scatter characteristics) were not sufficient for the clear identification of the macrophage population, we evaluated CD33 as a positive marker for intestinal macrophages. It could be shown that CD68 and CD33 are co-expressed on the same cell population with optical characteristics in FACS analysis typical for macrophages. CD33 could be used for the isolation of a cell population showing typical macrophage morphology in culture. The isolated cells secreted up to 45-fold more IL-1β and TNF-α compared with LPMC. The CD33+ cells were capable of phagocytosis.
The role of CD33 in macrophage function is not clear, and factors influencing its expression on macrophages have not been determined. However, the constant expression, in contrast to most other typical surface antigens, on colonic macrophages suggests a role in intestinal macrophage function. CD68 could not be routinely used in flow cytometric characterization of intestinal macrophages. Permeabilization of the cells for intracellular labelling of CD68 was followed by a loss of cells during the permeabilization procedure and a large increase in debris.
Only 83% of the phagocytic cells were CD33+. There may be a small population of phagocytic cells without CD33 expression, and there may be a small population of CD33+ cells without phagocytic activity, but hitherto it has not been possible to characterize these cells further.
In general there is a change in the understanding of the route of differentiation of macrophages and dendritic cells from their precursor cells. In recent years it has been shown that cells with dendritic features can be differentiated from monocytes by incubation with certain cytokines (IL-4, GM-CSF, IFN-γ). These in vitro differentiated dendritic cell populations are not homogeneous. There are monocyte-derived dendritic cells (MODC) that still show expression of CD14 and are capable of phagocytosis. These data suggest that the classical definition of dendritic cells and macrophages characterizes only the two poles of a line of possible intermediate differentiation forms. Goerdt and coworkers therefore support a unifying concept of different types of ‘custocytes’ [22].
Using CD33 as a positive marker for colonic macrophages, we showed that only few macrophages in colonic mucosa express CD14, CD16, HLA-DR, CD11b or CD11c. CD44 was detected on almost all cells. In vitro differentiated macrophages show a very different phenotype. However, the phenotype of alveolar macrophages is similar to that of colonic macrophages. Normal alveolar macrophages show a low level of expression of CD14 and CD11b [23]. Only during inflammatory lung diseases is the number of CD14+ and CD11b+ macrophages increased [24–28]. For the analysis of these antigens with low expression in normal alveolar macrophages, flow cytometry was found to be useful, giving more reliable results than the alkaline immunophosphatase technique [25,26]. Ziegler-Heitbrock and coworkers described a correlation between the expression of CD14 on alveolar macrophages and the impairment of lung function in pulmonary sarcoidosis [27].
In addition, similar results were obtained for liver macrophages. In an immunohistochemical characterization using intracellular CD68 as a recognition marker, most liver macrophages were negative for CD14 [29,30]. In rats, CD14 and CD11b were not expressed in normal liver macrophages, whereas an increase in expression of both antigens was observed in cholestatic animals [31].
Compared with intestinal macrophages, in vitro differentiated macrophages show a different phenotype, with high expression of CD14, CD16, HLA-DR and CD11b. No in vitro incubation conditions are known to lower the expression of CD14 to the level observed in colonic macrophages. After culture of the monocyte-like cell line U937 with LPS, CD14 expression was even induced [32], as was seen with blood monocytes [19]. Under conditions found in the intestine with high levels of LPS a high expression of CD14 on macrophages would have been expected. On the other hand, there may be only a low contact with LPS when the mucosal barrier is intact.
The low expression of MHC class II molecules on intestinal macrophages in our study is surprising. To control these results we checked HLA-DR expression on monocytes and on in vitro differentiated macrophages subjected to the same incubation protocol with collagenase, hyaluronidase and DNase. The HLA-DR expression on these cells was high, and correlated with data from the literature.
One reason for the low number of cells positive for HLA-DR expression in our study might be that we regarded cells positive for the investigated antigens when the mean fluorescence was above 102 arbitrary units to avoid interference with the autofluorescence of the cells. Therefore cells with low expression of HLA-DR might have been regarded as negative for HLA-DR expression in our study. The disruption of the tissue during the isolation of intestinal macrophages may lead to loss of dendrites and pseudopodia. This could lead to a loss of surface molecules that are not randomly distributed over the cell. However, there are no reports on clustering of MHC II molecules on dendrites of macrophages.
An interesting finding is the low expression of the costimulatory molecules B7-1 (CD80) and B7-2 (CD86) on colonic macrophages. Recent reports suggested that blocking of B7-1 or B7-2 in vivo affects the type of immune response by altering the differentiation of T-helper (Th) cells [13,14,33]. Th0 cells can differentiate into Th1 cells, which regulate cell-mediated immunity and secrete mainly IL-2, IFN-γ and TNF-β, or into Th2 cells, which regulate humoral immune responses and secrete IL-4, IL-5 and IL-10 (for a review see [34]). In several models of autoimmune diseases a Th2 immune response was associated with disease resistance, while induction of a Th1 response led to a progressive disease. This has been shown, for example, in experimental allergic encephalitis (EAE) [14]. In Crohn's disease an increase of Th1-like cytokines has also been observed [35]. In contrast, immune reactions against intracellular pathogens require a Th1 response, while a Th2 response results in a progressive disease. Treatment of animals with EAE with an anti-B7-1 antibody resulted in the generation of effector T cells with a Th2 phenotype, treatment with an anti-B7-2 antibody resulted in a Th1 response [14]. These findings show that the costimulatory molecules B7-1 (CD80) and B7-2 (CD86) play an important role for the regulation of the type of immune response. A lack of these molecules could lead to antigen desensitization and anergy [13], which could be of importance in the normal intestinal mucosa. In the case of CD80 and CD86, alveolar macrophages resemble intestinal macrophages as well. They do not costimulate T cells via the CD28 pathway and do not express CD80 or CD86 even after stimulation with IFN-γ [36]. Since immune reactions within the lung are associated with inflammatory injury of pulmonary tissue, it has been suggested that the reduced expression of B7-1 and B7-2 might be advantageous [35]. A similar situation might exist in the intestinal mucosa. The lack of expression of costimulatory molecules could be an important mechanism involved in the induction of peripheral tolerance to abundant antigens to which the mucosa is continuously exposed. Thus, intestinal macrophages are the second APC population hitherto known lacking costimulatory molecules. This is important for understanding their role in the normal intestinal mucosa.
In conclusion, our study shows that intestinal macrophages from normal mucosa exhibit a single phenotype (CD33++, CD44++, CD14−, CD16−, CD11b−, CD11c−, HLA-DRlow, CD80−, CD86−), which is different from that of blood monocytes or of in vitro differentiated macrophages and dendritic cells, but resembles that of alveolar macrophages. It is intriguing to study which factors induce the typical differentiation of intestinal macrophages. Our data support the concept that intestinal macrophages might have different roles in the mucosal immune system from antigen presentation and T cell stimulation. Further studies on functional characteristics of these cells have to address this question. The new opportunity to isolate these cells via CD33 may be important for these investigations.
Acknowledgments
We grateful acknowledge the cooperation of E. Treher, W. Vogt and S. Gruene, who supplied the biopsy samples. This study was supported by Deutsche Forschungsgemeinschaft (DFG): An 111/6-1.
References
- 1.Lee SH, Starkey PM, Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J Exp Med. 1985;161:475–89. doi: 10.1084/jem.161.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavli P, Doe WF. Intestinal macrophages. In: MacDermott RP, Stenson WF, editors. Inflammatory bowel disease. New York: Elsevier; 1992. pp. 177–88. [Google Scholar]
- 3.Donnellan WL. The structure of the colonic mucosa. The epithelium and subepithelial reticulohistiocytic complex. Gastroenterol. 1965;49:496–514. [PubMed] [Google Scholar]
- 4.Andus T, Rogler G, Daig R, Falk W, Schölmerich J, Gross V. Inflammatory bowel disease. In: Tygat GNJ, Bartelsman JFWM, van Deventer SJH, editors. The role of macrophages. Dordrecht: Kluwer: 1995. pp. 281–97. [Google Scholar]
- 5.Grimm MC, van Pavli P, de Pol E, Doe WF. Evidence for a CD14+ population of monocytes in inflammatory bowel disease mucosa—implications for pathogenesis. Clin Exp Immunol. 1995;100:291–7. doi: 10.1111/j.1365-2249.1995.tb03667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahida YR, Patel S, Gionchetti P, Vaux D, Jewell DP. Macrophage subpopulations in lamina propria of normal colon and inflamed colon and terminal ileum. Gut. 1989;30:826–34. doi: 10.1136/gut.30.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malizia G, Calabrese A, Cottone M, Raimondo M, Trejdosiewicz LK, Smart CJ, Oliva L, Pagliano L. Expression on leukocyte adhesion molecules by mucosal mononuclear phagocytes in inflammatory bowel disease. Gastroenterol. 1991;100:150–9. doi: 10.1016/0016-5085(91)90595-c. [DOI] [PubMed] [Google Scholar]
- 8.Mahida YR, Patel S, Wu K, Jewell DP. Interleukin 2 receptor expression by macrophages in inflammatory bowel disease. Clin Exp Immunol. 1988;74:382–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Smith DP, Brandes ME, Wahl SM, Mai UEH. The accumulation of mononuclear phagocytes in inflamed mucosa is likely due to the recruitment of circulating monocytes and not lamina propria macrophages. FASEB J. 1991;5:981. (Abstr.). [Google Scholar]
- 10.Freeman SD, Kelm S, Barber EK, Crocker PR. Characterization of CD33 as a member of the sialoadhesin family of cellular interaction molecules. Blood. 1995;85:2005–12. [PubMed] [Google Scholar]
- 11.Caux C, Vanbervliet B, Massacrier C, Azuma M, Okumura K, Lanier LL, Banchereau J. B70/B7-2 is identical to CD86 and is the major functional ligand for CD28 expressed on dendritic cells. J Exp Med. 1994;180:1841–7. doi: 10.1084/jem.180.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engel P, Gribben JG, Freemen GJ, et al. The B7-2 (B70) costimulatory molecule expressed by monocytes and activated B lymphocytes is the CD86 differentiation antigen. Blood. 1994;84:1402–7. [PubMed] [Google Scholar]
- 13.Thompson CB. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T-helper cell differentiation. Cell. 1995;81:979–82. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- 14.Harlan DM, Abe R, Le KP, June CH. Potential roles of the B7 and CD28 receptor families in autoimmunity and immune evasion. Clin Immunol Immunpathol. 1995;75:99–111. doi: 10.1006/clin.1995.1058. [DOI] [PubMed] [Google Scholar]
- 15.Guerder S, Meyerhoff J, Flavell R. The role of the T cell costimulator B7-1 in autoimmunity and the induction and maintenance of tolerance to peripheral antigen. Immunity. 1994;1:155–66. doi: 10.1016/1074-7613(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 16.Kuchroo VK, Das MP, Brown JA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 17.Bull DM, Bookman MA. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977;59:966–74. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreesen R, Brugger W, Scheibenbogen C, Kreutz M, Leser HG, Rehm A, Löhr G. Surface phenotype analysis of human monocyte to macrophage maturation. J Leuk Biol. 1990;47:490. doi: 10.1002/jlb.47.6.490. [DOI] [PubMed] [Google Scholar]
- 19.Brugger W, Reinhardt D, Galanos C, Andreesen R. Inhibition of in vitro differentiation of human monocytes to macrophages by lipopolysaccharides (LPS): phenotypic and functional analysis. Int Immunol. 1991;3:221–7. doi: 10.1093/intimm/3.3.221. [DOI] [PubMed] [Google Scholar]
- 20.Andreesen R, Picht J, Löhr G. Primary cultures of human blood-borne macrophages grown on hydrophobic Teflon membranes. J Immunol Methods. 1983;56:295. doi: 10.1016/s0022-1759(83)80019-2. [DOI] [PubMed] [Google Scholar]
- 21.Schreiber S, MacDermott RP, Raedler A, Pinnan R, Bertovich MJ, Nash GS. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterol. 1991;101:1020–30. doi: 10.1016/0016-5085(91)90729-5. [DOI] [PubMed] [Google Scholar]
- 22.Goerdt S, Kodelja V, Schmuth M, Orfanos CE, Sorg C. The mononuclear phagocyte–dendritic cell dichotomy: myths, facts, and a revised concept. Clin Exp Immunol. 1996;105:1–9. doi: 10.1046/j.1365-2249.1996.d01-740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbosa IL, Gant VA, Hamblin AS. Alveolar macrophages from patients with bronchogenic carcinoma and sarcoidosis similarly express monocyte antigens. Clin Exp Immunol. 1991;86:173–8. doi: 10.1111/j.1365-2249.1991.tb05791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Striz I, Wang YM, Teschler H, Sorg C, Costabel U. Phenotypic markers of alveolar macrophage maturation in pulmonary sarcoidosis. Lung. 1993;171:293–303. doi: 10.1007/BF03215872. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Arellano JL, Losa-Garcia JE, Orfao-Matos A, Gonzalez M, de-la-Cruz JL, Jiminez A, de-Castro S. Comparison of two techniques (flow cytometry and alkaline immunophosphatase) in the evaluation of alveolar macrophage immunophenotype. Diagn Cytopathol. 1993;9:259–65. doi: 10.1002/dc.2840090304. [DOI] [PubMed] [Google Scholar]
- 26.Wasserman K, Subklewe M, Pothoff G, Banik N, Frederick-Schell E. Expression of surface markers on alveolar macrophages from symptomatic patients with HIV-infection as detected by flow cytometry. Chest. 1994;105:1324–34. doi: 10.1378/chest.105.5.1324. [DOI] [PubMed] [Google Scholar]
- 27.Pforte A, Schiessler A, Gais P, Beer B, Ehlers M, Schutt C, Ziegler-Heitbrock HW. Expression of CD14 correlates with lung function impairment in pulmonary sarcoidosis. Chest. 1994;105:349–54. doi: 10.1378/chest.105.2.349. [DOI] [PubMed] [Google Scholar]
- 28.Pforte A, Schiessler A, Gais P, Beer B, Strobel M, Ehlers M, Schutt C, Ziegler-Heitbrock HW. Increased expression of the monocyte differentiation antigen CD14 in extrinsic allergic alveolitis. Monaldi Arch Chest Dis. 1993;48:607–12. [PubMed] [Google Scholar]
- 29.Tomita M, Yamamoto K, Kobashi H, Ohmoto M, Tsuji T. Immunohistochemical phenotyping of liver macrophages in normal and diseased liver. Hepatol. 1994;20:317–25. [PubMed] [Google Scholar]
- 30.Matsuura K, Ishida T, Setoguchi M, Higuchi Y, Akizuki S, Yamamoto S. Upregulation of mouse CD14 expression in Kupffer cells by lipopolysaccharide. J Exp Med. 1994;179:1671–6. doi: 10.1084/jem.179.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tracy TF, Fox ES. CD14-lipopolysaccharide receptor activity in hepatic monocytes after cholestatic liver injury. Surg. 1995;118:371–7. doi: 10.1016/s0039-6060(05)80347-2. [DOI] [PubMed] [Google Scholar]
- 32.Ikewaki N, Tamauchi H, Inoko H. Modulation of cell surface antigens and regulation of phagocytic activity mediated by CD11b in the monocyte-like cell line U937 in response to lipopolysaccharide. Tissue Antigens. 1993;42:125–32. doi: 10.1111/j.1399-0039.1993.tb02178.x. [DOI] [PubMed] [Google Scholar]
- 33.Lenschow DJ, Ho SC, Sattar H, Rhee L, Gray G, Nabavi N, Herold KC, Bluestone JA. Different effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes on the nonobese diabetic mouse. J Exp Med. 1995;181:1145–55. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–51. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 35.Niessner M, Volk BA. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR) Clin Exp Immunol. 1995;101:428–35. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chelen CJ, Fang Y, Freeman GJ, et al. Human alveolar macrophages present antigen ineffectively due to defective expression of B7 costimulatory cell surface molecules. J Clin Invest. 1995;95:1415–21. doi: 10.1172/JCI117796. [DOI] [PMC free article] [PubMed] [Google Scholar]


