Abstract
Fas (APO-1/CD95)-mediated apoptosis plays an important role in liver cell destruction in viral hepatitis. Using sandwich ELISA, we measured serum levels of soluble Fas (sFas) in patients with hepatocellular carcinoma (HCC) who were positive for hepatitis B surface antigen (HBsAg) or anti-hepatitis C virus (HCV) antibody. sFas levels were significantly higher in HCC patients (median 4.07 ng/ml; range 0.14–29.18 ng/ml) than levels in age-matched healthy donors (0.29 ng/ml; 0–4.90 ng/ml) (P < 0.0001) and HBsAg or anti-HCV antibody-positive patients with liver cirrhosis (LC) (2.16 ng/ml; 0.24–8.39 ng/ml) (P = 0.0015). An arbitrary cut-off level of 3.03 ng/ml (mean + 3 s.d. of controls) revealed the positive frequency of sFas in each group: 1.7% in healthy subjects, 25.9% in LC, and 59.0% in HCC (sensitivity 59.0% and specificity 74.1%). All HCC sera tested contained transmembrane-deleted sFas and some contained another sFas lacking the Fas C-terminal. The positive frequency of either sFas (59.0%) or α-fetoprotein (AFP) (57.4%) in HCC patients reached 77.0%. HCC patients with multiple tumour foci (7.53 ng/ml; 1.40–29.18 ng/ml) had significantly higher sFas levels than did patients with a solitary tumour (2.70 ng/ml; 0.14–19.0 ng/ml) (P = 0.003). In all of the sFas-positive patients with a solitary tumour, surgical removal of the tumour reduced sFas levels to the negative in the first post-op week. These findings suggest that sFas may be closely linked with HCC and may be a candidate for a clinical parameter for HCC.
Keywords: soluble Fas, hepatitis B virus, hepatitis C virus, liver cirrhosis, hepatocellular carcinoma
INTRODUCTION
As apoptotic cell death plays an important role in development and homeostasis of multicellular organisms, the failure of cells to undergo apoptosis might be involved in the pathogenesis of a variety of human diseases, including autoimmune diseases, viral infection, and malignancies [1,2]. Fas/APO-1 (CD95) is a type 1 transmembrane glycoprotein that signals sensitive cells to die by apoptosis upon ligation with anti-Fas/APO-1 MoAb [3,4] or the natural ligand for Fas, Fas ligand (FasL) [5]. Fas is constitutively expressed in a wide range of tissues [5–7]. Although use of Fas-defective autoimmune lpr mice [8–10] contributed much to clarify the function of Fas in lymphoid tissues [11], little is known of the role of Fas in non-lymphoid organs. Much attention has recently been directed to the Fas/FasL system in the liver. When apoptosis-inducing anti-Fas antibody was intraperitoneally injected into mice, normal but not lpr mice rapidly died of extensive liver damage resembling fulminant hepatitis [12]. In immunohistochemical studies in patients with chronic hepatitis [13–15], Fas protein was over-expressed by hepatocytes, especially in actively inflammatory areas. In contrast, FasL messenger RNA (mRNA) was highly expressed by liver-infiltrating mononuclear cells in the presence of chronic hepatitis [14,16], and by hepatocytes in alcoholic liver disease [14]. Intriguingly, Fas-null mice showed substantial liver hyperplasia as well as an accelerated lymphadenopathy [17]. Hepatocellular carcinomas (HCC) lose the expression of Fas and express FasL, which may kill attacking lymphocytes [18]. Thus, the Fas/FasL system may be closely linked to the cell injury induced by hepatitis virus infection and the regulation of tumourigenesis in the liver.
Several isoforms of soluble Fas (sFas) are generated by a mode of alternative splicing, exon skipping [19–22]. Serum levels of sFas were elevated in patients with systemic lupus erythematosus (SLE) [19–23], leukaemias [24] and solid tumours [25]. As normal hepatocytes, human hepatoma cell lines tested expressed detectable amounts of Fas on their surface. In addition, 5–10% of Fas cDNA prepared from each hepatoma line showed the in-frame deletion of exon 6, thus encoding for a transmembrane (TM)-deleted sFas [26]. We report here, for the first time, that serum sFas is elevated in patients with HCC compared with patients with liver cirrhosis (LC) and healthy controls, and that most of the sFas molecules in HCC sera retain the cytoplasmic region. Our results suggest that sFas may contribute to the escape of HCC from immune surveillance and its positivity needs an extensive examination for the complication of HCC in patients with LC.
PATIENTS AND METHODS
Patients and sera
This study included 88 Japanese patients, 27 with LC and 61 with HCC, who were followed at Hokkaido University Hospital and affiliated hospitals. Characteristics of these patients are summarized in Table 1. All patients were positive for hepatitis B surface antigen (HBsAg) or anti-hepatitis C virus (HCV) antibody. Patients with liver diseases of other aetiology were excluded. Diagnosis of LC was made histologically (n = 5) and/or by clinical characteristics, laboratory tests, abdominal ultrasound and computed tomography (CT). Diagnosis of HCC was made histologically (n = 39) and/or by laboratory tests, abdominal ultrasound, CT, magnetic resonance imaging, and selective hepatic arteriography. None of the patients had active varicose bleeding or encephalopathy at the time of investigation. Patients with severe complications and other diseases, such as overt infectious diseases, autoimmune diseases, haematological disorders, renal insufficiency and metastatic tumours, were excluded from this analysis. None of these patients had received chemotherapy, transarterial embolization, percutaneous ethanol injection therapy, or surgical treatments. Physical examination and laboratory tests were done at the time blood samples were taken. Blood samples used as normal controls were obtained from 59 age-matched healthy individuals who were negative for both HBsAg and anti-HCV antibody and had normal liver function. Informed consent was obtained from all patients and healthy donors. Individual serum samples were divided into aliquots and stored at −70°C until analysed.
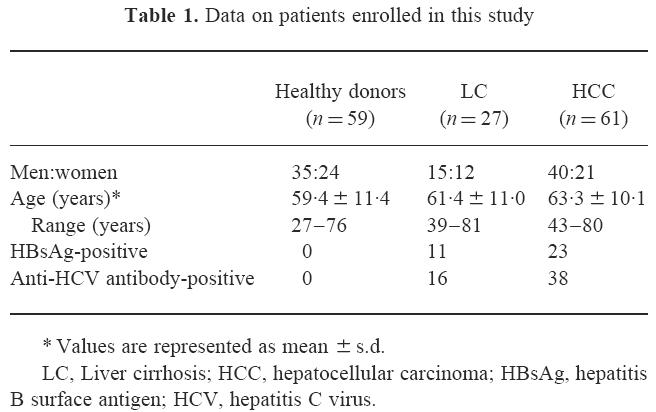
Table 1.
Data on patients enrolled in this study
Measurement of sFas
Serum concentrations of sFas were measured by a sandwich ELISA, as previously described [23]. Briefly, each well of microtitre plates (Sumilon, MS-8496F; Sumitomo Bakelite Co., Tokyo, Japan) was coated with 50 μl of anti-Fas MoAb (10 μg/ml) (DX3; Oncogene Research Products, Cambridge, MA) for 3 h at 37°C, followed by blocking of free sites with 100 μl of Block Ace (Dainihon Pharmaceutical Co., Osaka, Japan) for 2 h at 37°C. Test sera (50 μl) were added neat in duplicates and incubated for 3 h at 37°C, followed by 50 μl of biotinylated anti-Fas MoAb (10 μg/ml) (DX2; PharMingen, San Diego, CA) for 1 h at 37°C, and by 50 μl of streptavidin-conjugated alkaline phosphatase (Zymed Labs, Inc., South San Francisco, CA) for 1 h at 37°C. The wells were finally incubated with 100 μl of diethanolamine buffer (pH 9.8) containing p-nitrophenyl phosphate (1 mg/ml) for 1 h at room temperature. Five washes with 0.05% Tween 20–PBS pH 7.4 were performed between steps. In some experiments, rabbit anti-Fas C-terminal peptide antibody (anti-FasC) [23] was used as detector antibody. Predetermined culture supernatant from COS-7 cells transfected with sFas complementary DNA-inserted expression vector served as standards in all assays.
Statistical analysis
For statistical analyses, a Macintosh computer was used with a software package (StatView; Abacus Concepts, Inc., Berkeley, CA) with χ2 test, Mann–Whitney U-test, Wilcoxon's singed rank test and Spearman's rank correlation test. P < 0.05 was considered statistically significant.
RESULTS
Serum sFas levels in patients with LC and HCC
Serum concentrations of sFas in 88 patients and 59 healthy donors were determined, using the sandwich ELISA described above. Individual levels of serum sFas in three groups are shown in Fig. 1. In healthy controls, serum sFas levels (median, mean ± s.d., range) were 0.29 ng/ml, 0.60 ± 0.81 ng/ml, 0–4.90 ng/ml and were independent of gender. In patient groups, sera exhibited higher levels of sFas (median, range), as follows: 2.16 ng/ml, 0.24–8.39 ng/ml in patients with LC; 4.07 ng/ml, 0.14–29.18 ng/ml in patients with HCC. These levels of sFas were significantly higher than those in healthy controls (Mann–Whitney U-test, P < 0.0001 for LC and HCC). Furthermore, sFas levels were significantly higher in patients with HCC than in those with LC (Mann–Whitney U-test, P = 0.0015). When the cut-off level was set at 3.03 ng/ml (mean + 3 s.d. of controls), the positive frequency of serum sFas was 1.7%, 25.9% and 59.0% in healthy controls, LC and HCC patents, respectively. Thus, serum sFas levels had a sensitivity of 59.0% and a specificity of 74.1% for the detection of HCC from LC. The positivity of sFas in HCC patients was significantly greater than that in healthy controls and LC patients (χ2 test, P < 0.0001 and P = 0.0085, respectively).
Fig. 1.
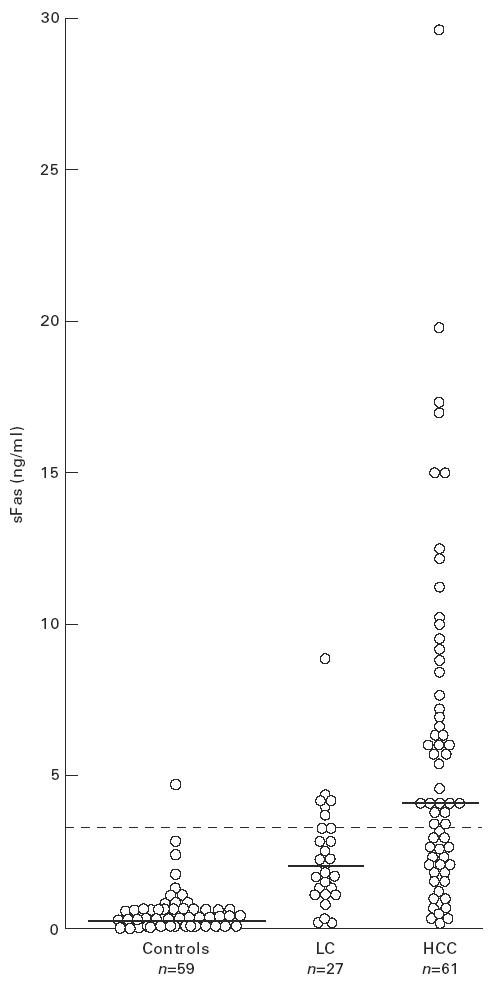
Levels of serum sFas in patients with liver cirrhosis (LC) and hepatocellular carcinoma (HCC). Sera randomly obtained from patients and healthy donors were assayed for sFas levels by sandwich ELISA using DX3 and DX2 anti-Fas MoAb. Horizontal bars and dotted line represent median values and the cut-off value of sFas (3.03 ng/ml), respectively.
Characterization of sFas in HCC sera
To characterize the molecular structure of sFas, HCC sera were analysed for sFas derived from Fas mRNA transcript lacking TM-encoding exon 6. Since this spliced form of sFas mRNA retains the cytoplasmic portion, biotinylated rabbit anti-FasC antibody was used as detector antibody. There was a significant correlation between sFas levels obtained by two different detector antibodies (Spearman's rank correlation test, P = 0.001) (data not shown). However, sFas levels in one third of HCC serum samples tested appeared higher in the standard DX3/DX2 system than in the DX3/anti-FasC system.
Serum sFas levels in HCC patients with a solitary tumour and those with multiple tumour foci
We next examined the correlation between the levels of serum sFas and multiplicity in 51 patients with nodular HCC who were subgrouped into solitary (n = 32) and multiple type (n = 19) at the time of blood sampling. Figure 2 shows the distribution of individual levels of serum sFas in these subgroups. Serum sFas levels (median, range) were significantly higher in HCC patients with multiple tumour foci (7.53 ng/ml, 1.40–29.18 ng/ml) than in those with a solitary tumour (2.70 ng/ml, 0.14–19.0 ng/ml) (Mann–Whitney U-test, P = 0.003). The difference in the positive frequency was statistically significant between HCC patients with multiple tumour foci (78.9%) and a solitary tumour (40.6%) (χ2 test, P = 0.0179). There was, however, no significant correlation between sFas levels and the size of a solitary tumour.
Fig. 2.
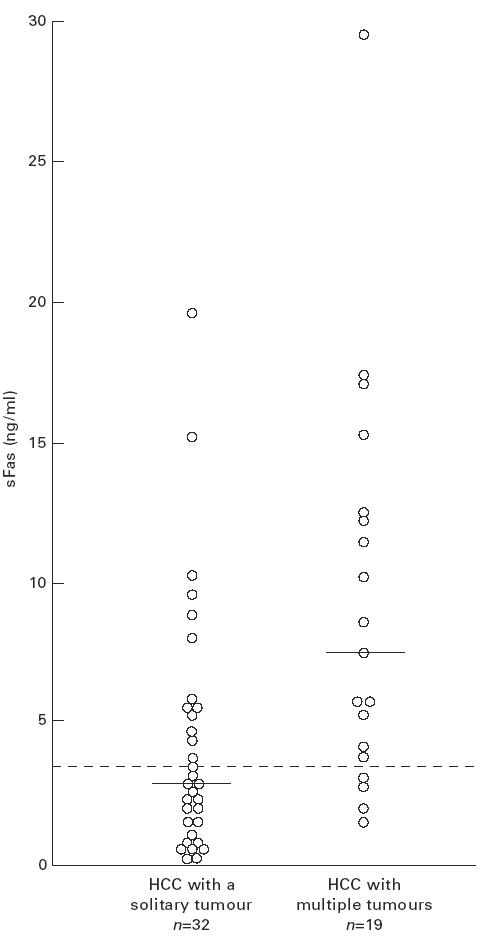
Levels of serum sFas in hepatocellular carcinoma (HCC) patients with a solitary tumour and in those with multiple tumours. Horizontal bars and dotted line represent median values and the cut-off value of sFas (3.03 ng/ml), respectively.
Changes of serum sFas levels in HCC patients with a solitary tumour after surgical removal of the tumour
The solitary tumour in 21 HCC patients was excised and levels of serum sFas (median, range) before and after surgery were compared for each patient. Surprisingly, pre-op sFas levels (3.74 ng/ml, 0.4–19.0 ng/ml) were significantly reduced to 1.15 ng/ml, 0.07–2.63 ng/ml in the first post-op week (Wilcoxon's signed rank test, P < 0.0001). All of 13 sFas-positive HCC patients (6.0 ng/ml, 3.19–19.0 ng/ml) became sFas-negative (1.17 ng/ml, 0.60–2.63 ng/ml) by 1 week post-op, and all but one patient maintained the same levels for another 1 week (Fig 3).
Fig. 3.
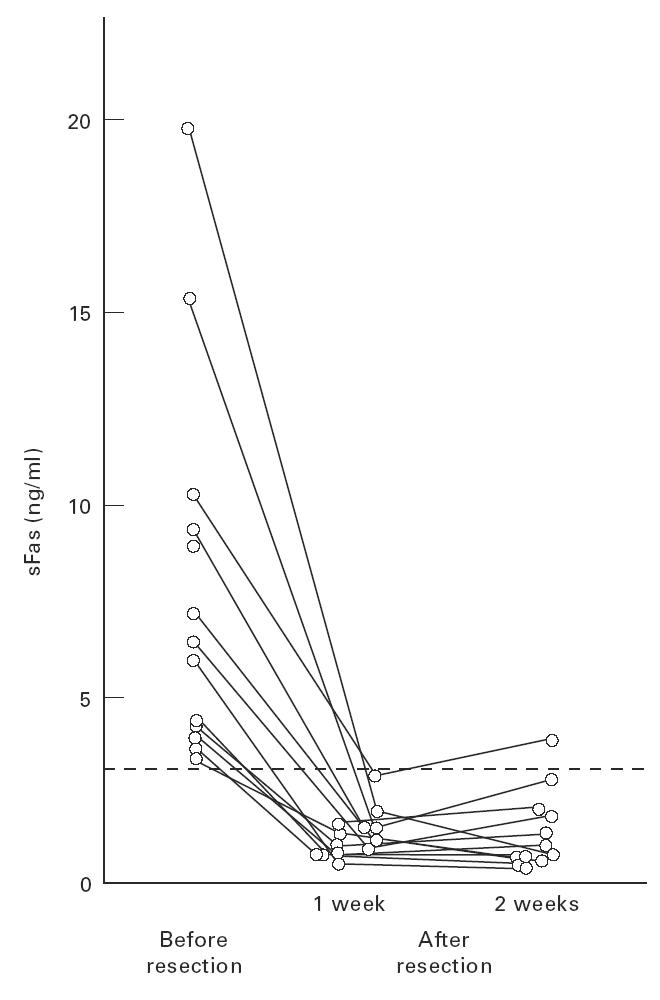
Comparisons between the levels of serum sFas in sFas-positive patients with a solitary tumour of hepatocellular carcinoma (HCC) before and after surgical resection of the tumour. For 21 HCC patients with a solitary tumour who underwent surgical removal of the tumour, the levels of serum sFas in 13 sFas-positive patients were plotted. Horizontal dotted line indicates the cut-off value of sFas (3.03 ng/ml).
DISCUSSION
Although the biological role of the Fas/FasL system in malignant tumours has not been firmly established, this system may function as one of the tumour suppressor systems via FasL-bearing cytotoxic T lymphocytes (CTL) or natural killer (NK) cells. Therefore, failure of Fas triggering due to loss of Fas/FasL expression or function may prolong the survival of tumour cells and result in the development and progression of malignancy.
sFas is potentially able to inhibit anti-Fas MoAb- or FasL-mediated apoptosis. In fact, chimeric sFas fusion molecule, composed of the extracellular domain of Fas and the Fc portion of human IgG, abrogates Fas-dependent CTL activity, in a dose-dependent manner [27]. Recombinant TM-deleted sFas also suppresses the apoptotic activity of anti-Fas MoAb [20,22], soluble FasL (sFasL) [22], and FasL-positive transfectants (our unpublished observations). In addition to hepatoma cell lines [26], human cell lines such as osteosarcoma [28] and malignant glioma [29] do express TM-deleted sFas mRNA, and this sFas molecule was secreted into the culture supernatants. Thus, sFas may function as an inhibitor of the Fas/FasL system and escape of tumour cells from immune surveillance may occur. An in vivo experiment for the effect of sFas on the growth of HCC tumour transfected with sFas cDNA is now in progress.
Our findings clearly indicate the significant elevation of serum levels of sFas in patients with HCC, compared with LC and healthy donors. In HCC, the levels of sFas correlated with the multiplicity of tumour focus rather than the size of a solitary tumour. It is not clear whether sFas levels are dependent on the number of tumour foci or the total mass volume. Further analysis for the accurate measurement of these parameters from all the imaging films available will be necessary. Most interestingly, in all sFas-positive HCC patients who had undergone surgical removal of each solitary tumour, the levels of sFas rapidly decreased by 78.8% (mean of percentage reduction; range 50.6–93.8%) at 1 week post-op, and all these patients became sFas-negative. These findings strongly suggest that sFas may be produced by or closely linked with the tumour cells. The lack of correlation between sFas levels and tumour size, however, may not support the production of sFas by the tumour itself. One possibility to explain this conflicting result is that HCC cells, especially those inside the tumour, may lose the capacity to produce sFas due to an insufficient blood supply as the tumour grows. Another possibility is that sFas may derive from infiltrating lymphocytes or unidentified cells which are highly activated by HCC. In either possibility, sFas levels may be tightly related to tumour multicentricity rather than to the size of a unifocal tumour. Recently, we obtained evidence that sFas in synovial fluids from patients with rheumatoid arthritis might be derived from neutrophils [30]. We have not yet identified the cellular source of sFas in HCC patients, although we have found that liver has the highest expression of sFas mRNA in human organs tested (our unpublished observations). Unfortunately, we cannot discriminate sFas from membranous-type Fas protein in tissue sections because there is no epitope specific for sFas molecule which lacks the TM region.
Since our sandwich ELISA detects any form of sFas which contains DX3 and DX2 epitopes in the extracellular domain of Fas, sFas-positive HCC sera may contain sFas of the shedding type [31] as well as TM-deleted sFas. Another ELISA using DX3 and anti-FasC revealed that all HCC serum samples tested contained TM-deleted sFas, and one third of them contained another sFas molecule(s) lacking the Fas C-terminal. This is in sharp contrast to sFas in SLE sera, some of which showed negligible levels of TM-deleted sFas [23]. In any form of sFas, the N-terminal domain seems most important to function as an inhibitor of the Fas/FasL system [22].
About 40% of our patients with HCC showed no significant elevation of serum sFas levels. There is recent evidence that some tumour cells, including HCC, do express functional FasL which can counterattack Fas-positive lymphocytes [18,32,33]. Since human FasL is rapidly cleaved off by matrix metalloproteinase [34], increased levels of sFasL may interfere with sFas detection in our assay system, but such is not the case. Our DX3/DX2 system excluded the influence on sFas detection by sFasL [23].
Because of the expression of Fas in a wide range of tissues, sFas itself is non-specific. We compared sFas levels in patients with HCC and LC, both entities caused by hepatitis virus infection. Since most HCC develop at the later stage of LC, early diagnosis of HCC in LC patients is clinically of considerable importance. α-fetoprotein (AFP) is most available as a tumour marker for HCC. The positivity and serum levels of sFas did not correlate with those of AFP (χ2 test, P = 0.1343 and Spearman's rank correlation test, P = 0.1019, respectively). HCC patients with high levels of sFas did not always exhibit high levels of AFP, and vice versa. It is noteworthy that the positive frequency of either sFas (59.0%) or AFP (57.4%) in HCC patients reached up to 77.0%. We propose that sFas is closely linked with HCC and, in combination with AFP, increases sensitivity for the detection of HCC in patients with LC. Prospective studies on LC patients and a further follow up of post-operative HCC patients will be needed to determine whether serum sFas levels could be a clinically predictable marker for HCC.
Acknowledgments
We thank Drs S. Isogawa, M. Watanabe, T. Tsushima and H. Yashima for providing data and sera, and M. Ohara for helpful comments. This work was supported in part by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Science, Sports and Culture of Japan, and grants from The Cell Science Research Foundation, Osaka, and the Akiyama Foundation, Sapporo, Japan.
References
- 1.Bursch W, Oberhammer F, Schulte-Hermann R. Cell death by apoptosis and its protective role against disease. Trends Pharmacol Sci. 1992;113:245–51. doi: 10.1016/0165-6147(92)90077-j. [DOI] [PubMed] [Google Scholar]
- 2.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 3.Yonehara S, Ishli A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–56. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oehm A, Behrmann I, Falk W, et al. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily: sequence identity with the Fas antigen. J Biol Chem. 1992;267:10709–15. [PubMed] [Google Scholar]
- 5.Suda T, Takahashi T, Golstein P, et al. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–78. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe-Fukunaga R, Brannan CI, Itoh N, et al. The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J Immunol. 1993;148:1274–9. [PubMed] [Google Scholar]
- 7.Leithauser F, Dhein J, Mechtersheimer G, et al. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993;69:415–29. [PubMed] [Google Scholar]
- 8.Watanabe-Fukunaga R, Brannan CI, Copeland NG, et al. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–7. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 9.Adachi M, Watanabe-Fukunaga R, Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc Natl Acad Sci USA. 1993;90:1756–60. doi: 10.1073/pnas.90.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi S, Hirano T, Kakinuma M, et al. Transcriptional repression and differential splicing of Fas mRNA by early transposon (ETn) insertion in autoimmune lpr mice. Biochem Biophys Res Commun. 1993;191:617–24. doi: 10.1006/bbrc.1993.1262. [DOI] [PubMed] [Google Scholar]
- 11.Singer GG, Abbas AK. The Fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–71. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 12.Ogasawara J, Watanabe-Fukunaga R, Adachi M, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–9. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 13.Hiramatsu N, Hayashi N, Katayama K, et al. Immunohistochemical detection of Fas antigen in liver tissue of patients with chronic hepatitis C. Hepatology. 1994;19:1354–9. [PubMed] [Google Scholar]
- 14.Galle PR, Hofmann WJ, Walczak H, et al. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–30. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mochizuki K, Hayashi N, Hiramatsu N, et al. Fas antigen expression in liver tissues of patients with chronic hepatitis B. J Hepatol. 1996;24:1–7. doi: 10.1016/s0168-8278(96)80178-4. [DOI] [PubMed] [Google Scholar]
- 16.Mita E, Hayashi N, Iio S, et al. Role of Fas ligand in apoptosis induced by hepatitis C virus infection. Biochem Biophys Res Commun. 1994;28:468–74. doi: 10.1006/bbrc.1994.2483. [DOI] [PubMed] [Google Scholar]
- 17.Adachi M, Suematsu S, Kondo T, et al. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nature Genet. 1995;11:294–300. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- 18.Strand S, Hofmann WJ, Hug H, et al. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells. A mechanism of immune evasion? Nature Med. 1996;2:1361–6. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 19.Cheng J, Zhou T, Liu C, et al. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994;263:1759–62. doi: 10.1126/science.7510905. [DOI] [PubMed] [Google Scholar]
- 20.Cascino I, Fucci G, Papoff G, et al. Three functional soluble forms of the human apoptosis-inducing Fas molecule are produced by alternative splicing. J Immunol. 1995;154:2706–13. [PubMed] [Google Scholar]
- 21.Liu C, Cheng J, Mountz JD. Differential expression of human Fas mRNA species upon peripheral blood mononuclear cell activation. Biochem J. 1995;310:957–63. doi: 10.1042/bj3100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papoff G, Cascino I, Eramo A, et al. An N-terminal domain shared by Fas/APO-1 (CD95) soluble variants prevents cell death in vitro. J Immunol. 1996;156:4622–30. [PubMed] [Google Scholar]
- 23.Jodo S, Kobayashi S, Kayagaki N, et al. Serum levels of soluble Fas/APO-1 (CD95) and its molecular structure in patients with systemic lupus erythematosus (SLE) and other autoimmune diseases. Clin Exp Immunol. 1997;107:89–95. doi: 10.1046/j.1365-2249.1997.d01-901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knipping E, Debatin KM, Stricker K, et al. Identification of soluble APO-1 in supernatants of human B- and T-cell lines and increased serum levels in B and T-cell leukemias. Blood. 1995;85:1562–9. [PubMed] [Google Scholar]
- 25.Midis GP, Shen Y, Owen-Schaub LB. Elevated soluble Fas (sFas) levels in nonhematopoietic human malignancy. Cancer Res. 1996;56:3870–4. [PubMed] [Google Scholar]
- 26.Natoli G, Ianni A, Costanzo A, et al. Resistance to Fas-mediated apoptosis in human hepatoma cells. Oncogene. 1995;11:1157–64. [PubMed] [Google Scholar]
- 27.Hanabuchi S, Koyanagi M, Kawasaki A, et al. Fas and its ligand in a general mechanism of T-cell-mediated cytotoxicity. Proc Natl Acad Sci USA. 1994;91:4930–4. doi: 10.1073/pnas.91.11.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owen-Schaub LB, Angelo LS, Radinsky R, et al. Soluble Fas/APO-1 in cells: a potential regulator of apoptosis. Cancer Lett. 1995;94:1–8. doi: 10.1016/0304-3835(95)03834-j. [DOI] [PubMed] [Google Scholar]
- 29.Weller M, Malipiero U, Rensing-Ehl A, et al. Fas/APO-1 gene transfer for human malignant glioma. Cancer Res. 1995;55:2936–44. [PubMed] [Google Scholar]
- 30.Mukai M, Kobayashi S, Jodo S, et al. Characterization and source of soluble Fas in synovial fluids from patients with RA. Arthritis Rheum. 1997;40:S293. [Google Scholar]
- 31.Black PH. Shedding from the cell surface of normal and cancer cells. Adv Cancer Res. 1980;32:75–199. doi: 10.1016/s0065-230x(08)60361-9. [DOI] [PubMed] [Google Scholar]
- 32.O'Connell J, O'Sullivan GC, Collins JK. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–82. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hahne M, Rimoldi D, Schröter M, et al. Melanoma cell expression of Fas (APO-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–6. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 34.Kayagaki N, Kawasaki A, Ebata T, et al. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182:1777–83. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]


