Abstract
Effects of oxidative stress on stimulation-dependent signal transduction, leading to IL-2 expression, were studied. Purified quiescent human blood T lymphocytes were subjected to: (i) acute exposure to hydrogen peroxide; (ii) chronic exposure to hydrogen peroxide; and (iii) acute exposure to ionizing radiation. The cells were then stimulated for 6 h. DNA-binding activities (determined by the electrophoretic mobility shift assay) of three transcription factors: NFκB, AP-1 and NFAT, were abolished in the lymphocytes by all three modes of oxidative stress. The lymphocytes exhibited lipid peroxidation only upon exposure to the lowest level of hydrogen peroxide used (20 μm). All three modes of oxidative stress induced catalase activity in the lymphocytes. The only exception was hydrogen peroxide at 20 μm, which did not induce catalase activity. We conclude that: (i) suppression of specific transcription factor functions can potentially serve as a marker of exposure to oxidative stress and its effects on human lymphocytes; (ii) lipid peroxidation is only detectable in human lymphocytes upon exposure to weak oxidative stress which does not induce catalase activity; (iii) therefore, transcription factor DNA-binding activities are more sensitive to oxidative stress than lipid peroxidation.
Keywords: oxidative stress, immunosuppression, NFκB, NFAT, AP-1
INTRODUCTION
T lymphocytes are activated following the binding of a ligand to the antigen receptor complex. One of the early manifestations of this interaction is the transcriptional activation of the IL-2 gene [1]. IL-2 is a pivotal lymphokine involved in B and T lymphocyte, as well as natural killer (NK) cell regulation [1]. The modulation of IL-2 transcription by nuclear proteins can serve as a general readout that would be affected by any abnormality occurring earlier in the activation pathway. A transcriptional enhancer in the promoter region of the IL-2 gene responds to signals generated after activation through the T cell antigen receptor [2]. A number of positive regulatory elements have been identified in this region, including: NFAT, AP-1, NFκB, EGR-1, AP-3, Oct-1, and Sp1 [3–5]. Transcription factors binding to the first three, which are expressed in primary human T cells only upon stimulation, are plausible targets for suppression of T cell activation.
A variety of distinct biochemical changes in lymphocytes and in various other target cells is induced by the oxidants hydrogen peroxide and hydroxyl radical. These changes include alterations in enzymatic activities, lipid peroxidation and damage to DNA. Ionizing radiation can be used as a means of introducing oxygenating radicals into lymphocytes in a geometrically and temporally precise way. The absorption of radiation involves splitting H2O molecules (the most common constituent of cells) into hydroxyl radicals and H• radicals which are initially distributed in proportion to the radiation dose distribution [6]. In addition, irradiation of dissolved O2 will produce the superoxide radical, HO2•, also following the radiation dose distribution. The superoxide radical has intermediate reactivity between that of strongly reactive hydroxyl radicals and relatively weakly reactive hydrogen peroxide.
We have previously described a new mechanism of IL-2 down-regulation [7]. Endogenous hydrogen peroxide produced by monocytes and endogenously produced or exogenously added polyamines provide down-regulatory signals for IL-2 production by human peripheral blood T cells. The interaction between polyamine oxidase (PAO; EC 1.4.3.4, monoamine oxidase) and the polyamine spermidine generates products (including hydrogen peroxide) over 2 days that provide chronic low-level oxidative stress, suppressing IL-2 production. Furthermore, we found that PAO activity suppressed protein tyrosine phosphorylation, calcium mobilization and nuclear DNA-binding activities [8].
The objective of this study was to compare the effects of three modes of inducing oxidative stress in human lymphocytes on stimulation-induced transcription factors, in order to facilitate the development of functional markers for the exposure to, and effect of, oxidative stress in man. Oxidative stress was exerted on unstimulated cells, since the vast majority of peripheral blood lymphocytes (PBL) are in a quiescent state. The modes studied were: (i) high levels of reagent hydrogen peroxide generating short but acute stress; (ii) PAO activity generating extracellularly low levels of hydrogen peroxide for 2 days; and (iii) electron irradiation generating both extra- and intracellularly mainly hydroxyl radicals.
MATERIALS AND METHODS
Cells
T cells from the peripheral blood of healthy donors were studied. Cells were incubated for all assays in a serum-free medium, because fetal calf serum (FCS) contains PAO activity [7]. Therefore, RPMI 1640 with Nutridoma-HU supplement (Boehringer Mannheim Biochemicals, Indianapolis, IN) was used.
Lymphocyte preparation
Cells were purified by Ficoll–Hypaque (Pharmacia Fine Chemicals, Uppsala, Sweden) density gradient centrifugation. The resultant mononuclear cell preparation was allowed to adhere to plastic dishes to remove macrophages and other adherent cells.
Non-adherent mononuclear cells were mixed with a suspension of neuroaminidase-treated sheep erythrocytes and incubated at 37°C for 15 min, followed by centrifugation and further incubation at 4°C for 45 min. Thereafter, the rosetted cells were obtained by centrifugation through Ficoll–Hypaque. The erythrocytes in the cell pellet were lysed by exposure to 0.83% NH4Cl. The rosetted cells contained > 98% CD3+ T cells, and 0.4–1% M3+ monocytes as determined by flow cytometry.
Oxidative stress
These modes were used: (i) a short high-level extracellular stress—reagent hydrogen peroxide was added directly at 20, 50, 100 and 200 μm for 2 h. We have found that these levels suppress IL-2 production in human blood lymphocytes without affecting cell viability [9]; (ii) a longitudinal low level extracellular stress—lymphocytes were preincubated for 2 days with a commercial preparation of PAO (a monoamine oxidase which oxidizes polyamines at a 2.5-fold higher rate than benzylamine [7]; Sigma, St Louis, MO) at 5 × 10−4 U/ml and spermidine at 5 μm. This exposure generates gradually 5 μm hydrogen peroxide over 2 days and suppresses IL-2 production in response to mitogenic stimulation [7]; (iii) electron radiation generating both extra- and intracellularly mainly hydroxyl radicals—lymphocytes were exposed to a radiation dose of 6 Gy for 5 min. This dose produces non-lethal cellular responses [10] and generates oxidants per time unit at about 20-fold higher levels than mode (ii), but for a much shorter period of time. We used a 2.5-MeV Van de Graaff accelerator that generated electrons to a maximum energy of 1.8 MeV. The cells were exposed in suspension to high energy x-rays generated by stopping the electron beam in a tantalum plate. Doses were continuously monitored by means of parallel plate ionization chambers coupled with a stable, vibrating reed electrometer.
Oxidative stress was exerted and the cells were washed and rested for 2 h before stimulation in order to exclude any possible effects on the assay used.
Measurements of transcription factor activities
For T cell stimulation we used phytohaemagglutinin (PHA; 1 μg/ml) + tetradecanoyl phorbol acetate (TPA; 5 ng/ml), for 6 h at 37°C, 5% CO2, before collecting the cells for nuclear extraction.
DNA-binding determination by the electrophoretic mobility shift assay
Preparation of nuclear extracts
Cells were washed and nuclear extracts were prepared according to a modification of the method of Schreiber et al. [11]. This method is suitable for small numbers of cells and therefore appropriate (based on our experience; [8,12]) for studies of PBL. Cells were washed and resuspended in Tris-buffered saline, transferred to an Eppendorf tube and repelleted. The cell pellet was resuspended in a buffer containing 10 mm HEPES, 10 mm KCl, 0.1 mm EDTA, 0.1 mm EGTA, 1 mm DTT, 1 mm PMSF, 5 μg/ml aprotinin, 5 μg/ml antipain, 100 μm benzamidine, 5 μg/ml leupeptin, 5 μg/ml pepstatin, 5 μg/ml soybean trypsin chymotrypsin inhibitor, pH 7.9. The cells were allowed to swell on ice for 15 min and NP-40 at 0.625% was added. The tube was vortexed for 10 s and centrifuged for 30 s in a microfuge. The nuclear pellet was resuspended in a buffer containing 20 mm HEPES, 0.4 m NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm DTT, and the seven aforementioned protease inhibitors, pH 7.9. The tube was vigorously rocked on wet ice for 15 min on a shaking platform, and the nuclear extract was centrifuged for 5 min to remove insoluble nuclear matrix. The protein concentration of the supernatant was determined (Bradford method; BioRad Protein Assay Kit (Hercules, CA)). Aliquots were stored at −70°C.
DNA–protein interactions
DNA probes containing the binding sites from the IL-2 promoter region [3] were purchased from Genosys (The Woodlands, TX). The probe for NFAT-1 spans between nucleotides −255 and −285: 5′-GGAGGAAAAACTGTTTCATACAGAAGGCGTT-3′. The probe for AP-1 spans between nucleotides −140 and −156: 5′-TTCAAAGAGTCATCAG-3′. The probe for NFκB spans between nucleotides −190 and −214: 5′-TAACAAACAGGGATTTCACCTACAT-3′.
The probes were labelled with 32P-ATP using T4 polynucleotide kinase (Promega, Madison, WI). For the binding assay, 10 000 ct/min DNA probe (≈ 0.2 ng) were combined with 2 μg poly (dI-dC) (a non-specific competitor DNA), 3 μg bovine serum albumin (BSA; a protein carrier) and 10 μg nuclear extract in a final reaction volume of 20 μl. The binding reaction mixture was incubated for 15 min in a 30°C water bath. The protein–DNA complexes were detected on a 4% low ionic strength native polyacrylamide gel. The gel was dried under vacuum and autoradiographed.
Measurements of lipid peroxidation
Quantitative peroxide assay
A lipid-compatible formulation of the PeroXOquant Quantitative Peroxide Assay (Pierce Chemical Co., Rockford, IL) was used. This assay is adapted to measure cellular hydroperoxides. To differentiate between hydrogen peroxide and peroxides of cellular molecules (such as lipid peroxides) we followed the recommendations of the manufacturer and regarded any catalase (7000 U/ml)-inhibitable measurement as representing hydrogen peroxide. In the assay, peroxides convert Fe2+ to Fe3+ in a sulphuric acid solution. The Fe3+ complexes with the xylenol orange dye to yield a purple product with absorbance at 540–600 nm. The molar extinction coefficient of the xylenol orange–Fe3+ complex is 1.5 × 104 m−1 cm−1 in 25 mm H2SO4 at room temperature. Five million cells were lysed by sonication (two 10-s pulses with a 10-s interval) and incubated for 15–20 min at room temperature in the following working solution (10 times the volume of the sonicate): 0.25 mm ammonium ferrous (II) sulphate, 25 mm H2SO4, 4 mm butylated hydroxytoluene (BHT), 125 μm xylenol orange in methanol. Results were read at 595 nm in a microtitre plate reader. For calibration and validation, a series of hydrogen peroxide solutions at concentrations between 1 μm and 1 mm were prepared and assayed. Results were calculated per protein concentration as determined by the Bradford method. Since the peroxide assay allows measurement of peroxides without lipid extraction, a blank without ammonium ferrous (II) sulphate and H2SO4 was used to subtract endogenous iron (and other transition metal) readings [13].
Measurements of catalase activity
Ten million cells were lysed by sonication (two 10-s pulses with a 10-s interval) in 0.5 ml PBS. The resultant sonicate was centrifuged at 14 000 g for 10 min at 4°C. Catalase activity was measured in the supernatant. Supernatant (50 μl) was mixed with 600 μl of 15 mm H2O2 in a cuvette. The kinetics of the decrease in light absorbance at 240 mm (H2O2 decomposition) were determined for 3 min in a DU 640 spectrophotometer (Beckman, Fullerton, CA). A cuvette containing only PBS served as blank. A cuvette without a sample was used to ensure that H2O2 did not decompose spontaneously under our experimental conditions. Enzymatic activity was expressed as the rate constant of a first-order reaction (k) divided by the protein concentration. A1 and A2 refer to the absorbance before and after a given time interval of measurement (t), respectively. k = (2.3/t) (log A1/A2) (s−1. mg protein−1) [14,15].
Reagents
All reagents were purchased from Sigma, unless otherwise stated.
Statistical analysis
Data were analysed, where appropriate, using Student's t-test.
RESULTS
Transcription factor DNA-binding studies
Since this study aimed to develop markers of oxidative stress-induced suppression of cellular function, we studied the ability of DNA sequences from the IL-2 promoter to bind to proteins present in nuclei of lymphocytes that are stimulated by mitogens and are commencing proliferation. IL-2 is central to the cellular immune response, and inability to express this gene would result in cellular dysfunction of T lymphocytes. Three DNA-binding activities present in activated lymphocytes were studied: NFκB, AP-1 and NFAT. The cells were subjected to oxidative stress: an enzymatic activity (PAO) generating hydrogen peroxide, irradiation and four concentrations of hydrogen peroxide administered directly to the cells. None of these stresses affected lymphocyte viability, which remained at 95%, as determined by trypan blue exclusion. Figure 1 demonstrates the effect of oxidative stress on NFκB DNA-binding in T lymphocytes. While unstimulated cells (Unst.) did not express NFκB DNA binding, stimulated cells (Stim.) did express this activity, and the interaction was specific, as shown by its prevention in the presence of a specific competitor (an excess of unlabeled NFκB DNA, Stim. + Comp.). All the types and levels of oxidative stress we employed completely abolished the induction of NFκB DNA-binding in stimulated T lymphocytes.
Fig. 1.
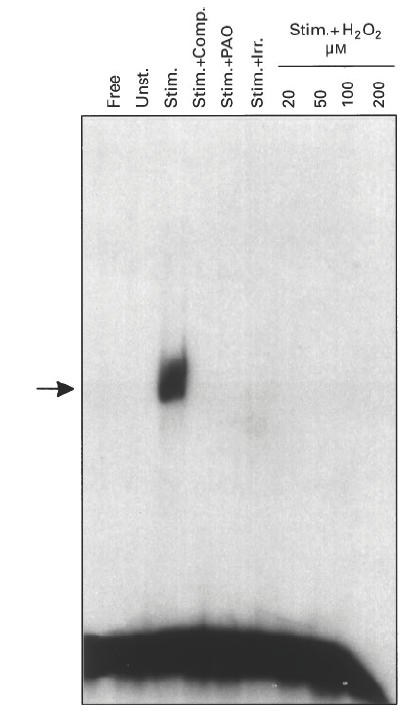
Suppression of NFκB DNA-binding by oxidative stress. T cells were pretreated with polyamine oxidase (Stim. + PAO, for 2 days at 5 × 10−4 U/ml + spermidine at 5 μm, and then washed and incubated for 2 h in fresh medium), or irradiation (Stim. + Irr., for 5 min at 6 Gy and then incubated for 2 h in fresh medium), or hydrogen peroxide (Stim. + H2O2, for 2 h at the indicated concentration and then washed and incubated for 2 h in fresh medium). Cells were then stimulated with phytohaemagglutinin (PHA; 1 μg/ml) + tetradecanoyl phorbol acetate (TPA; 5 ng/ml) for 6 h. In addition, control cultures of untreated cells were either not stimulated (Unst.) or stimulated with PHA + TPA for 6 h (Stim.). Nuclear extracts were prepared and 10 μg of protein were incubated with 32P-labelled NFκB sequence and electrophoresed. The lanes were loaded with DNA without nuclear extract (Free), DNA with extract from untreated and unstimulated cells (Unst.), DNA with extract from untreated and stimulated cells (Stim.), same as Stim. + 50× excess of unlabelled probe (Stim. + Comp.), and DNA with extracts from pretreated cells that were also stimulated (Stim. + PAO, Stim. + Irr., Stim. + H2O2). The gel was dried and autoradiographed. The arrow marks the specific DNA–protein complex.
As can be seen in Figs 2 and 3, protein binding activities to two other DNA sequences (AP-1 and NFAT) were only expressed in stimulated T cells and were abolished by exposing the cells to oxidative stress, similar to the results obtained with the NFκB sequence.
Fig. 2.
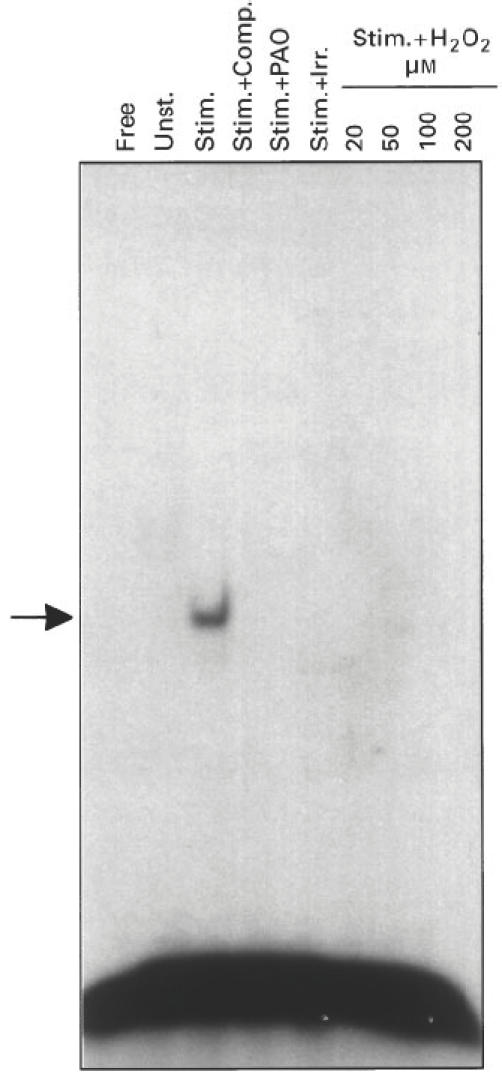
Suppression of AP-1 DNA-binding by oxidative stress. Same as Fig. 1, except that 32P-labelled AP-1 sequence was used.
Fig. 3.
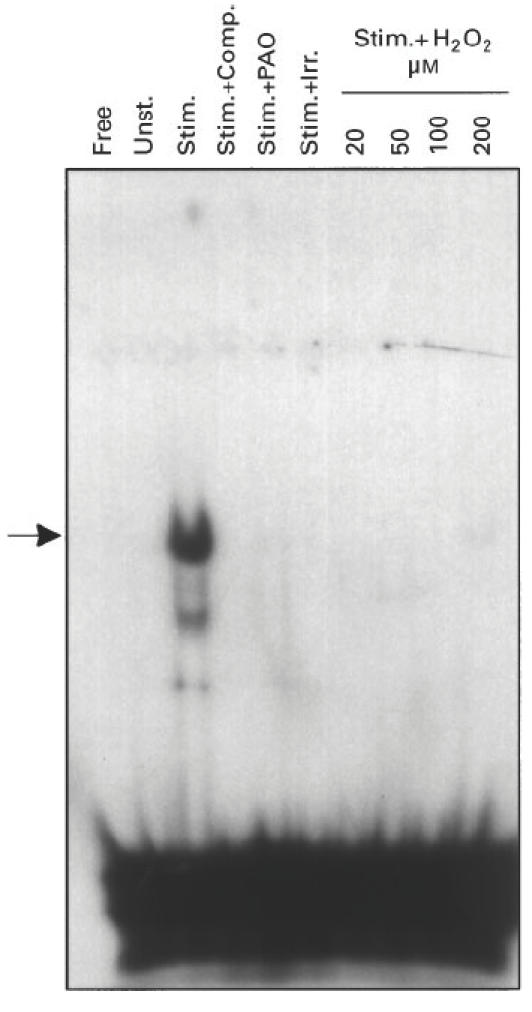
Suppression of NFAT DNA-binding by oxidative stress. Same as Fig. 1, except that 32 P-labelled NFAT sequence was used.
Lipid peroxide determination in lymphocytes exposed to oxidative stress
Since the goal of these studies was to develop markers of oxidative stress in lymphocytes, we measured lipid peroxidation as a biochemical parameter of exposure to oxidants. The basal level of lipid peroxides in T lymphocytes was 2.4 ± 0.7 nmol lipid hydroperoxides/mg protein. Only treatment with hydrogen peroxide at 20 μm for 2 h induced a rise in cellular peroxides to 11.9 ± 1.8 nmol lipid hydroperoxide/mg protein (P < 0.0005), while the other treatments (hydrogen peroxide at 50–200 μm, polyamine oxidase and irradiation) did not induce any rise in the levels of lipid peroxides above basal levels. A possible explanation of these findings is that the direct biochemical damage was repaired within 2 h after the exposures. The lowest concentration of hydrogen peroxide may not have been sufficient to induce appropriate levels of antioxidant defences, allowing the lipid peroxidation to be detected. To investigate this possibility, levels of the major antioxidative enzyme, catalase, were measured in T lymphocytes exposed to oxidative stress.
Catalase determination in lymphocytes exposed to oxidative stress
Every oxidative stress exposure, except for hydrogen peroxide at 20 μm, induced a significant rise in cellular catalase activity above the basal level (Fig. 4).
Fig. 4.
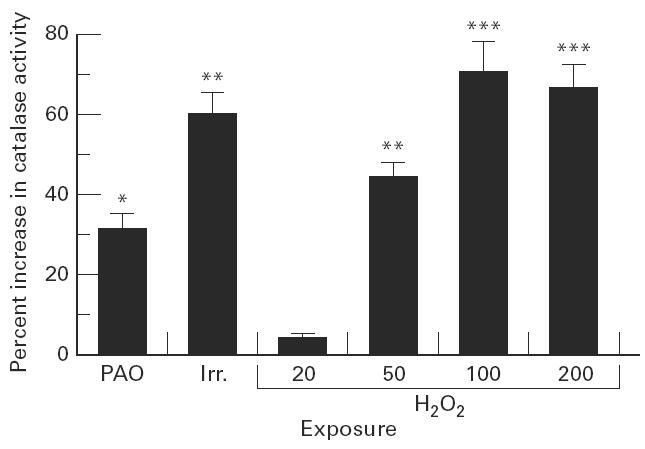
Enhancement of catalase activity by oxidative stress. T cells were exposed to polyamine oxidase (PAO, for 2 days at 5 × 10−4 U/ml + spermidine at 5 μm, and then washed and incubated for 2 h in fresh medium), or irradiation (Irr., for 5 min at 6 Gy and then incubated for 2 h in fresh medium), or hydrogen peroxide (H2O2 at the indicated concentration, μm, for 2 h and then washed and incubated for 2 h in fresh medium). Cells were lysed and cytosolic catalase activity was measured by following kinetically the decomposition of hydrogen peroxide in a spectrophotometer, and calculated per protein concentration. Catalase activity in untreated cells was 1.2 ± 0.09 (sec−1. mg protein−1). Results shown are the increase in catalase activity in stressed cells over untreated cells ± s.d. The increase in catalase activity was significant at *P < 0.01; **P < 0.005; ***P < 0.0005.
DISCUSSION
Three modes of oxidative stress—hydrogen peroxide, polyamine oxidase activity generating hydrogen peroxide gradually, and irradiation—suppressed the activation-dependent DNA-binding activities of three transcription factors, NFκB, AP-1 and NFAT, in human peripheral blood T lymphocytes. Only exposure to hydrogen peroxide at 20 μm generated measurable lipid peroxidation products in T lymphocytes, while this was the only exposure that did not induce an increase in cellular catalase activity.
We have previously reported [8] that exposure to polyamine oxidase results in suppression of transmembrane signal transduction in human peripheral blood T lymphocytes. This leads to suppression of the activation-dependent expression of transcription factors in the nucleus, and finally to inhibition of the transcription of the IL-2 gene. In the current study, we compared the effects of different types and levels of oxidative stress on nuclear signal transduction in exposed human lymphocytes. Three transcription factor DNA-binding activities were suppressed in T lymphocytes by every condition of oxidative stress employed. The fact that we did not detect a dose–response dependence in the suppression of transcription factor activity by hydrogen peroxide suggests that suppressing early signalling events by oxidative stress [8] results in an all-or-none effect on distal signalling steps in the nucleus.
The DNA-binding activities of NFκB and AP-1 are induced upon exposure to oxidants [16–18]. The apparent contradiction with our results may be resolved by recognizing that in our system (but not in the other studies mentioned) cells were incubated for 2 h in fresh medium after the exposures, followed by stimulation for 6 h, and only then were DNA-binding activities determined. Therefore, we are assessing the effects of oxidative stress on T cell mitogenic activation rather than the direct effect on transcription factor activities. We have previously found that oxidative stress suppresses early signal transduction steps, protein tyrosine phosphorylation and calcium mobilization [8]. Therefore, the eventual suppression of transcription factor activities may actually reflect early effects of oxidative stress on lymphocyte transmembrane signal transduction. In this context, the decline in IL-2 production by human T lymphocytes from aged persons in response to in vitro stimulation is associated with impaired activation of AP-1 and NFAT [19]. In view of the oxygen radical-related theory of ageing, this is potentially an example of T cell suppression at the signal transduction level by oxidative stress in vivo.
We found that radiation suppressed the expression of DNA-binding activities in activated T lymphocytes. Radiation of human lymphocytes in vitro was previously found to suppress constitutive surface marker expression [20] and enhance micronuclei occurrence following stimulation [21].
Since NFκB and AP-1 were recently found to be the most important IL-2 cis-regulatory elements in normal T cells [22], and we have shown that the acute and chronic modes of oxidative stress used in this study suppress IL-2 production by human lymphocytes [7,9], our results strongly suggest that suppression of transcription factor function caused by inducing oxidative stress in human lymphocytes contributes to down-regulation of IL-2 production and cellular activation. IL-2 is a major growth factor regulating T lymphocyte proliferation [23]. Therefore, suppression of nuclear signalling events that control IL-2 expression reflects not only exposure but also the detrimental effect of exposure to oxidative stress inducers.
Direct measurements of lipid hydroperoxides did not detect increased levels following oxidative stress, except for exposure of T lymphocytes to the lowest concentration of hydrogen peroxide. The same hydrogen peroxide concentration (20 μm) was the only one from among the oxidative stress exposures employed in the current study that did not induce a rise in catalase activity. Therefore, we suggest that when the oxidative stress induces an antioxidative response, i.e. catalase activity, lipid peroxidation is not detectable. PAO did not induce lipid peroxidation, although the enzymatic activity generates only 5 μm H2O2, because the intensity of the cellular stress is determined not only by the concentration of the oxidant but also by the duration of the exposure (in the case of PAO, 2 days). Accordingly, PAO exposure induced an increase in cellular catalase activity.
On the other hand, all three modes of oxidative stress resulted in suppression of cellular function that was clearly evident even 8–26 h after the exposures, as judged by transcription factor activities. Our results suggest that a functional parameter (nuclear signal transduction) is much more sensitive than a structural parameter (lipid peroxidation) as a marker of oxidative damage to human blood lymphocytes.
These results suggest that transcription factor functions can potentially be used as markers of blood lymphocyte exposure to oxidants (including hydrogen peroxide and hydroxyl radicals) which generate either acute or chronic stresses. Our studies were conducted with human PBL, which are readily available, and should therefore be amenable to development into population-based markers of environmental exposure to oxidants. Ex vivo peripheral blood cells can also be used for follow up of exposed individuals.
Acknowledgments
These studies have been supported by the Department of the Army Grant no. DAMD17-95-1-5058. The content of the information does not necessarily reflect the position or the policy of the government, and no official endorsement should be inferred.
References
- 1.Imboden JB, Weiss A. The initiation of human T lymphocyte activation. Prog in Allerg. 1988;42:246–9. [PubMed] [Google Scholar]
- 2.Crabtree G. Contingent genetic regulatory events in T lymphocyte activation. Sci. 1989;243:355–61. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- 3.Granelli-Piperno A, Nolan PI. Nuclear transcription factors that bind to elements of the IL-2 promoter: induction requirements in primary human T cells. J Immunol. 1991;147:2734–9. [PubMed] [Google Scholar]
- 4.Hoyos B, Ballard DW, Bohnlein E, et al. kB-specific DNA binding proteins: role in the regulation of human interleukin-2 gene expression. Sci. 1989;244:457–60. doi: 10.1126/science.2497518. [DOI] [PubMed] [Google Scholar]
- 5.Skerka C, Decker EL, Zipfel PF. A regulatory element in the human interleukin 2 gene promoter is a binding site for the zinc finger proteins Sp1 and EGR-1. J Biol Chem. 1995;270:22500–6. doi: 10.1074/jbc.270.38.22500. [DOI] [PubMed] [Google Scholar]
- 6.Buxton GV. Basic radiation chemistry in liquid water. In: Baxendale JM, Buse F, editors. Dordrecht: D. Reidel; 1982. pp. 241–60. The study of fast processes and transient species by electron pulse radiolysis. [Google Scholar]
- 7.Flescher E, Bowlin TL, Talal N. Polyamine oxidation down-regulates IL-2 production by human peripheral blood mononuclear cells. J Immunol. 1989;142:907–12. [PubMed] [Google Scholar]
- 8.Flescher E, Ledbetter JA, Schieven GL, et al. Longitudinal exposure of human T lymphocytes to weak oxidative stress suppresses transmembrane and nuclear signal transduction. J Immunol. 1994;153:4880–9. [PubMed] [Google Scholar]
- 9.Flescher E, Bowlin TL, Ballester A, et al. Increased polyamines may downregulate interleukin 2 production in rheumatoid arthritis. J Clin Invest. 1989;83:1356–62. doi: 10.1172/JCI114023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns FJ, Sargent E. The induction and repair of DNA breaks in rat epidermis irradiated with electrons. Radiat Res. 1981;87:137–44. [PubMed] [Google Scholar]
- 11.Schreiber E, Matthia P, Muller MM, Shaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucl Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flescher E, Vela-Roch N, Ogawa N, et al. Abnormality of Oct-1 DNA binding in T cells from Sjögren's syndrome patients. Eur J Immunol. 1996;26:2006–11. doi: 10.1002/eji.1830260906. [DOI] [PubMed] [Google Scholar]
- 13.Nourooz-Zadeh J, Tajaddini-Sarmadi J, Wolff SP. Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-Xylenol Orange assay. Anal Biochem. 1994;220:403–9. doi: 10.1006/abio.1994.1357. [DOI] [PubMed] [Google Scholar]
- 14.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 15.Zelikoff JT, Wang W, Islam N, et al. Assays of reactive oxygen intermediates and antioxidant enzymes: potential biomarkers for predicting the effects of environmental pollution. In: Ostrander GK, editor. Techniques in aquatic toxicology. New York: Lewis Publishers; 1996. pp. 287–306. [Google Scholar]
- 16.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 1991;10:2247–58. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schieven GL, Kirihara J, Myers DE, et al. Reactive oxygen intermediates stimulate tyrosine phosphorylation in human lymphoid cells, triggering calcium mobilization and induction of NF-κB DNA binding activity. Blood. 1993;84:1212–20. [Google Scholar]
- 18.Devary Y, Gottlieb R, Lau L, Karin M. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol Cell Biol. 1991;11:2804–11. doi: 10.1128/mcb.11.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whisler RL, Beiqing L, Chen M. Age-related decreases in IL-2 production by human T cells are associated with impaired activation of nuclear transcriptional factors AP-1 and NF-AT. Cell Immunol. 1996;169:185–95. doi: 10.1006/cimm.1996.0109. [DOI] [PubMed] [Google Scholar]
- 20.Rowley DA, Kelley WA, Manders JH. Flow cytometric analysis of lymphocyte surface markers following a 1-Gy dose of gamma radiation. Aviat Space Environ Med. 1993;64:528–33. [PubMed] [Google Scholar]
- 21.Holmen A, Karlsson A, Bratt I, Hogstedt B. Micronuclei and mitotic index in B-, T4- and T8-cells treated with mitomycin C and γ-irradiation. Mutat Res. 1994;309:93–99. doi: 10.1016/0027-5107(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 22.Hughes CCW, Pober JS. Transcriptional regulation of the interleukin-2 gene in normal human peripheral blood T cells. J Biol Chem. 1996;271:5369–77. doi: 10.1074/jbc.271.10.5369. [DOI] [PubMed] [Google Scholar]
- 23.McCrady CW, Ely CM, Westin E, Carchman RA. Coordination and reversibility of signals for proliferative activation and interleukin-2 mRNA production in resting human T lymphocytes by phorbol ester and calcium ionophore. J Biol Chem. 1988;263:18537–44. [PubMed] [Google Scholar]


