Abstract
Experimental autoimmune prostatitis (EAP) is a disease that could be considered an experimental model of human non-bacterial prostatitis. In this experimental model, male rats are intradermally immunized with a saline extract of male sex accessory glands (RAG) in an adequate adjuvant. The prostatitis observed in the immunized animals develops as a consequence of the immune response against RAG antigens, and the histological lesion is strikingly similar to the pattern of prostatic inflammation observed in the human disease. In this study, we purified one of the prostatic autoantigens recognized by the autoantibodies in our model. Amino acid sequence analysis identified the purified protein as prostatein or rat prostatic steroid binding protein, a member of the uteroglobin superfamily. Prostatein was recognized not only by the humoral autoimmune response, but also by the cellular autoimmune response. Certainly, the DTH response and lymph node cell proliferative assays against prostatein in immunized animals yielded positive results. Prostatein is not only the target of the autoimmune response in animals immunized with the whole extract, but also an inducing antigen of the disease. Purified prostatein, when incorporated to an adequate adjuvant, elicited cellular and humoral autoimmune response and lesion in the prostate gland. The identification of one of the target antigens in autoimmune prostatitis has provided a further refinement and characterization of our model, which could serve for a better understanding of the aetiology, pathogenesis and pathophysiology of non-bacterial prostatitis.
Keywords: prostatein, rat prostatic steroid binding protein, autoimmunity, non-bacterial prostatitis, estramustine binding protein
INTRODUCTION
Chronic inflammatory prostatic disease(s) of non-infectious origin is often difficult to diagnose and even more difficult to treat. Patients with this disease have symptoms and signs of prostatitis, but infecting organisms cannot be demonstrated [1]. It is estimated that its incidence exceeds that of bacterial prostatitis by as much as eight-fold [2]. Unfortunately, non-bacterial prostatitis is the form of prostatitis about which least is known. Further investigation is clearly needed to delineate the aetiology, pathogenesis and pathophysiology of the pathology.
One reason for the lack of significant progress towards understanding the aetiology and pathophysiology of non-bacterial prostatitis is the absence of an adequate animal model in which to study this disease process. Keetch et al. [3] reported that injection of syngeneic prostate antigens in mice induces prostatic inflammation similar to the human disease, and suggested that it may be an autoimmune process. In our laboratory we have developed an autoimmune prostatitis in Wistar rats that could be considered a valid model for clinical non-bacterial prostatitis. In this experimental model, male rats are intradermally immunized with a saline extract of male sex accessory glands (RAG) in an adequate adjuvant [4–6]. The male sex accessory glands in the rat consist of the prostate, seminal vesicle and coagulating gland. The lesion observed in the immunized animals develops as a consequence of the immune response against RAG antigens. Previous studies have provided evidence for the existence of autoreactive T cells [7], autoantibodies [8,9], and cytotoxic T cells [10] as well as tisular alterations characterized by mononuclear infiltration into the prostate and, to a minor degree, into the seminal vesicle [11]. A higher proportion of degranulated mast cells has also been described in immunized animals [6,12]. These histological findings are strikingly similar to the pattern of prostatic inflammation defined by Meares & Stamey [13].
The identification of the target antigens in autoimmune diseases is always an important step towards understanding the aetiology of this group of pathologies. In this study, we purified one of the prostatic autoantigens recognized by the antibodies. Amino acid sequence analysis identified the purified protein as prostatein or rat prostatic steroid binding protein, a member of the uteroglobin superfamily. We also examined its ability to induce autoimmune prostatitis in male Wistar rats, and concluded that when incorporated into an adequate adjuvant, prostatein elicits cellular and humoral autoimmune responses and lesion in the prostate gland.
MATERIALS AND METHODS
Animals and antigens
Normal and immunized male Wistar rats, ≈ 3 months old, were used in this study.
RAG excised from Wistar rats were homogenized in 0.01 m PBS at pH 7.2 with protease inhibitors with an Ultraturrax homogenizer. The homogenate was centrifuged at 10 000 g for 30 min and the supernatant was used as RAG homogenate. For the immunization procedure, the RAG homogenate was chemically modified (MRAG) as described previously by coupling the RAG saline extract to diazonium derivatives of sulphanilic and arsanilic acid [14]. Chemical modification does not change the specificity of the immune response, but plays an adjuvant role [8,14].
To obtain the cytosolic proteins of rat prostate (RP) the respective 10 000 g supernatant was centrifuged at 100 000 g for 1 h at 4°C. Homogenates from various tissues were similarly prepared.
Immunization
Rats (n = 6) were intradermally injected on days 0 and 30 with 5 mg of MRAG emulsified with 0.5 ml of Freund's complete adjuvant (FCA). Sera were obtained on days 30 and 45, after one or two injections of MRAG, respectively. In other experiments, rats (n = 3) were intradermally injected on days 0 and 30 with 150 μg of the purified protein chemically modified as previously described [14] and emulsified with 0.5 ml of FCA. In this case, sera were obtained on days 30 and 37, after one or two injections of prostatein, respectively.
Gel electrophoresis and immunoblotting
Cytosolic proteins of RP were separated electrophoretically in 15% SDS–polyacrylamide minigels (BioRad Labs, Richmond, CA) under non-reducing conditions according to the Laemmli gel method [15]. Gels were electrotransferred to nitrocellulose membrane as previously described [16]. The blotted membranes were blocked for 1 h at 37°C with 5% skim milk powder in PBS and were then probed for 3 h at room temperature with normal and autoimmune sera diluted 1:50. A goat IgG anti-rat IgG labelled with peroxidase (Sigma Chemical Co., St Louis, MO) was used as second antibody. The reaction was visualized with 4-chloro-1-naphtol-H2O2.
Isolation of the RP autoantigen of 20 kD
Purification of the antigen of 20 kD was achieved by an electroelution procedure using an electroelution cell and following the manufacturer's instructions (Model 422 Electro-Eluter; BioRad) [17]. Briefly, a preparative SDS–polyacrilamide gel containing ≈ 8 mg electrophoresed RP was run as described above and the gel band excised according to its electrophoresis mobility, and its recognition by a representative autoimmune serum in immunoblotting analysis performed in a section of the same gel. Then the band was electrophoretically eluted from the minced gel in 25 mm Tris, 192 mm glycine, 0.1% SDS.
N-terminal amino acid sequence of the RP autoantigen of 20 kD
To analyse the N-terminal amino acid sequence, 20 μg purified protein was resolved by 15% SDS–PAGE under non-reducing conditions and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Watford, UK) as described by Matsudaira [18]. The protein band was stained with 0.1% coomassie blue R-250 after electroblotting. Sequence analysis was performed in the Laboratorio Nacional de Péptidos y Proteínas (LANAIS-PRO), using an Applied Biosystems Model 477A pulsed-liquid sequencer.
The amino acid sequence was screened for homologies with known sequences in the data bank of the National Biochemical Research Foundation-Protein Identification Research (NBRF-PIR).
DTH test
Immunized and normal rats were challenged in their left footpads with 1 μg of the electroeluted protein or 10 μg of RP dissolved in 0.1 ml of PBS and with PBS alone in their right footpads. The animals were then observed 24 h later for the development of swelling, induration and redness at the site. The footpad swelling was measured with a micrometer. Net footpad swelling was determined by calculating the differences in swelling at a given time, minus the footpad thickness at the time zero, minus the swelling due to the saline alone.
Proliferative assay
Single lymph node cell suspensions were prepared by mincing and gently homogenizing the aseptically dissected lymph nodes in Hanks' balanced salt solution (HBSS). Live lymphocytes were counted by trypan blue exclusion and then diluted with RPMI medium to the desired final concentration. Irradiated (15 Gy) spleen mononuclear cells from normal rats were used as antigen-presenting cells (APC). The APC (4 × 105 cells/well) were incubated 2 h with RP (130 μg/ml) or prostatein (20 μg/ml) at 37°C in a humidified atmosphere of 5% CO2 and then washed twice. Lymph nodes from normal and immunized rats (100 000/well) were added to the pulsed APC and cultured in triplicate in 96-well flat-bottomed microculture. After 72 h of culture, each well was pulsed with medium containing 1 μCi of methyl-3H-thymidine (3H-TdR) and 18 h later harvested onto fibreglass filters and counted using standard liquid scintillation techniques. The proliferative response was expressed as the average ct/min of replicates with antigen minus the average ct/min of replicates without antigen (Δct/min) ± s.e.m. In each experiment, lymph nodes from one to three rats were assayed separately.
ELISA
Multiwell plates were coated with 50 μl/well of 40 μg/ml prostatein solution in 0.05 m carbonate, pH 9.6 at 4°C overnight. The plates were then blocked by filling each well with skim milk powder 1% in PBS. After washing the plates with PBS–Tween 20 (0.05%), 50 μl of diluted serum samples were added and incubated for 1 h at 37°C. Appropriate dilutions of normal sera were also included in each plate. The plates were then incubated with peroxidase-conjugated anti-rat IgG. The reaction was developed with o-phenylenediamine–H2O2. Absorbance was measured at 490 nm in a Titertek Multiskan. Estimation of antibody activity was expressed as a binding index calculated as the relation of optical density (OD) of the test sera versus OD of the control sera. A binding index < 2.0 was deemed negative.
Histology
Prostates from immunized and control rats were studied by light microscopy. Prostates were removed 7 days after the last immunization with prostatein–FCA. The specimens were immediately placed in 10% formalin for at least 24 h, dehydrated in alcohol, cleared in xilol, and embedded in paraffin. The glands were sectioned at 10 μm. After cutting, the sections were stained with haematoxylin–eosin. For identification of mast cells, sections were stained in toluidine blue at pH 3.5. The degree of inflammation in glands was assessed using a scale of 0–4+: 0, no inflammation; 1+, mild, but definite perivascular cuffing with mononuclear or mast cells; 2+, moderate perivascular cuffing; 3+, marked perivascular cuffing with some parenchymal inflammatory cells; and 4+, marked perivascular cuffing and numerous mononuclear and mast cells in the parenchyma.
Quantification of mast cell degranulation
Mast cells were considered degranulated if (at ×400 magnification) extracellular granulae, indistinct boundaries, uneven staining of various parts of the cells, or distinct intracellular vacuolization within the cells was observed.
RESULTS
Detection and organ specificity of a 20-kD cytosolic prostate autoantigen
Cytosolic rat prostate antigens (RP) were analysed by SDS–PAGE under non-reducing conditions and immunoblotting. Sera obtained from different immunized animals (n = 6) on day 45 (after two immunizations) but not on day 30 (after one immunization) recognized at least one specific antigen of low molecular weight (≈ 20 kD). Normal control sera were also tested at the same dilutions and did not react with this polypeptide band (Fig. 1).
Fig. 1.
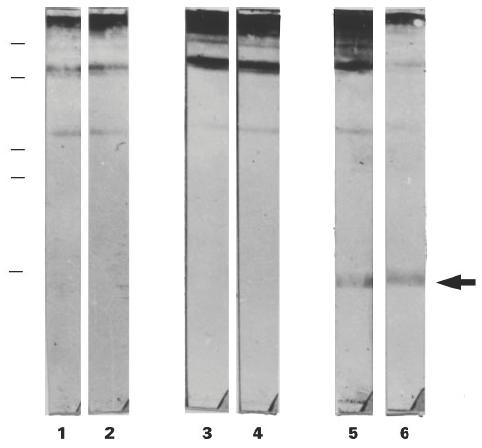
Immunoblotting of antigens in the cytosolic fraction of rat prostate separated in 15% SDS–PAGE under non-reducing conditions. Lanes 1 and 2 were incubated with normal sera diluted 1:50; lanes 3 and 4 were incubated with sera obtained after one immunization with modified rat accessory gland saline extract (MRAG)–Freund's complete adjuvant (FCA) diluted 1:50; lanes 5 and 6 were incubated with sera obtained after two immunizations with MRAG–FCA diluted 1:50. In each case two representative sera out of six are shown. The molecular weights of the markers from top to bottom are: 84, 63, 35, 32 and 21.5 kD.
Treatment of RP with 2-mercaptoethanol (2-ME) resulted in a great loss of antigenicity, showing that disulphide bonds are involved in the binding site of the autoantibodies (Fig. 2, lane A).
Fig. 2.
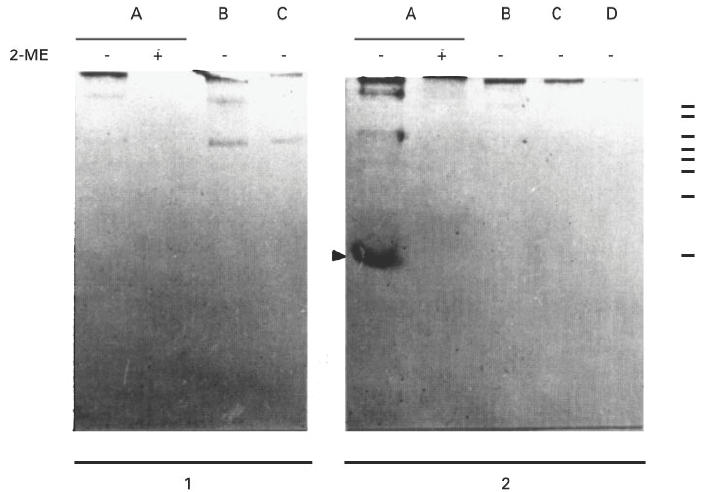
Immunoblotting of antigens in the cytosolic fraction of rat prostate (lane A), heart (lane B), liver (lane C) and lung (lane D) with or without treatment of the samples with 2-mercaptoethanol (2-ME). Normal serum (1) or autoimmune serum obtained after two immunizations (2) were used at a dilution of 1:50. The molecular weights of the markers from top to bottom are: 195, 112, 84, 63, 52, 35, 32 and 21.5 kD.
Organ specificity of the 20-kD autoantigen
To determine if the polypeptide band recognized by the autoantibodies was specifically located in the prostate gland, or if it was an ubiquitous antigen, 100 000 g supernatants from various organs such as heart, liver and lung were separated by SDS–PAGE under non-reducing conditions and immunoblotting analysis was performed. Although the autoimmune sera obtained on day 45 definitely reacted with the polypeptide band of 20 kD found in RP, they did not react with any antigen from another organ. These results suggest that the RP protein of 20 kD is an important organ-specific autoantigen recognized by the humoral response in this experimental model (Fig. 2).
Purification and N-terminal amino acid sequence of the 20-kD autoantigen
Purification of the 20-kD antigen was achieved by an electroelution procedure. Briefly, RP was separated in SDS-polyacrilamide preparative gels as described above, the gel band of interest excised was then electrophoretically eluted from the minced gel. The purified protein was resolved on 15% SDS–PAGE, wherein it migrated at 20 kD under non-reducing conditions. It was also recognized by a representative autoimmune serum in immunoblotting experiments, which also reacted with a polypeptide band of ≈ 40 kD (Fig. 3). Probably, this later antigen represents a dimeric form of the electroeluted protein. Direct sequencing of the intact protein was successful: N-terminal amino acid sequence gave 33 amino acid residues. In most cycles two equimolecular signals were obtained. After an exhaustive analysis, the sequences corresponded exactly with components C1 and C3 of subunit F of prostatein, also known as rat prostatic steroid binding protein or prostate α-protein.
Fig. 3.
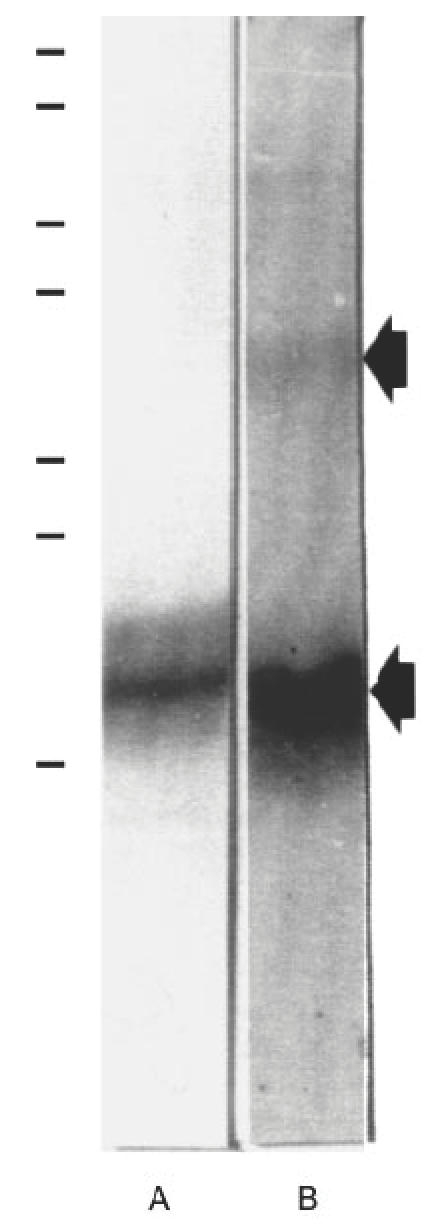
Isolation of the 20-kD rat prostate antigen by electroelution. Electroeluted protein (20 μg) was separated by SDS–PAGE under non-reducing conditions and stained with coomassie blue (lane A) or electrotransferred onto nitrocellulose filter and incubated with an autoimmune serum (lane B). The molecular weights of the markers from top to bottom are: 195, 112, 84, 63, 35, 32 and 18 kD.
Cellular immune response against the 20-kD autoantigen identified as prostatein
The DTH response against the purified protein of 20 kD identified as prostatein was evaluated by challenging animals on day 7 after one immunization with MRAG-FCA. The DTH response against RP was evaluated as a positive control. As shown in Fig. 4a, normal animals did not react against prostatein, while a positive footpad reaction was observed in the immunized animals. Also, the cellular immune response against prostatein was measured by an in vitro assay. Lymph node cells (LNC) obtained from immunized animals were tested in an in vitro proliferative assay. The results shown in Fig. 4b clearly show that LNC from immunized animals were able to proliferate against RP and also exhibited a positive proliferative response against prostatein. These results show that prostatein is not only recognized by the humoral autoimmune response elicited in this experimental model, but is also recognized by the cellular immune response measured in vivo and in vitro.
Fig. 4.
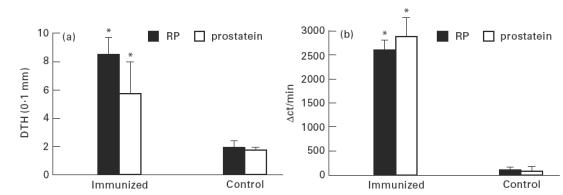
(a) DTH response against prostatein studied 7 days after immunization with modified rat accessory gland saline extract (MRAG)–Freund's complete adjuvant (FCA). Bars represent the mean ± s.d. of at least four rats. *P < 0.001, Student's t-test for grouped samples. (b) In vitro proliferative response of lymph node cells from immunized male Wistar rats against prostatein and rat prostate (RP). Animals were killed 21 days after immunization with MRAG–FCA and lymph nodes removed. Proliferation was determined by uptake of 3H-thymidine and expressed as the average ct/min of replicates with antigen minus the average ct/min of replicates without antigen (Δct/min) ± s.e.m. In each experiment lymph node cells from one to three rats in each group were assayed separately. *P < 0.05, Student's t-test for grouped samples.
Induction of experimental autoimmune prostatitis by i.d. injection of prostatein–FCA
We next examined the ability of prostatein to induce the autoimmune disease in male Wistar rats. Prostatein was chemically modified as was previously done with RAG [14] and intradermally injected emulsified in FCA (150 μg/ml per immunization) on days 0 and 30. Later, the humoral and cellular response to prostatein and the lesion in the prostate gland were studied.
The DTH response against prostatein was evaluated on day 7 after the first immunization. Animals were challenged in their left footpads either with RP or prostatein. As shown in Table 1, immunization with prostatein–FCA was able to induce DTH reactivity against the purified autoantigen at similar levels as those observed in animals immunized with MRAG–FCA (Pprosta tein–FCA−control < 0.001; PMRAG–FCA−control < 0.001). However, the DTH response against RP was positive but lower when the immunizing antigen was prostatein (Pprostatein–FCA–control < 0.05; PMRAG–FCA–control < 0.001; Pprostatein-FCA–MRAG–FCA < 0.001). These findings suggest that it is unlikely that a single antigen is solely responsible for the induction of the pathology.
Table 1.
Induction of experimental autoimmune prostatitis (EAP) in male Wistar rats by i.d. injection of prostatein–Freund's complete adjuvant (FCA)
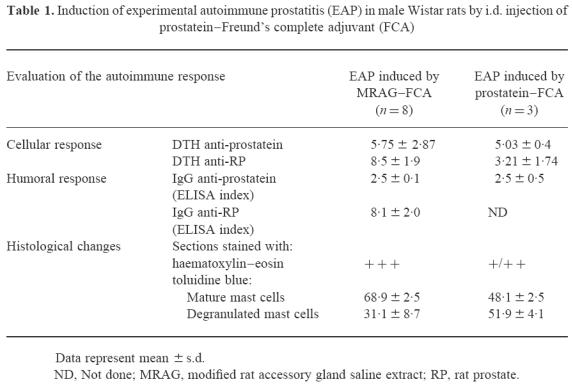
The humoral response against prostatein was then evaluated by ELISA. The immunized animals were bled on day 37 after two immunizations with prostatein–FCA. The results shown in Table 1 indicate that two immunizations with prostatein–FCA elicited autoantibodies against rat prostatein at similar levels to those observed in animals immunized with MRAG–FCA. However, in these latter animals the ELISA binding index against RP was much higher than the index observed for prostatein, suggesting once again that more that one autoantigen was involved.
To perform the histopathological studies, prostates from normal and immunized animals were removed on day 37, after two immunizations with prostatein–FCA. Lesions of varying degrees (+ and + +) were consistently observed in the glands of almost all experimental animals. The most remarkable alteration took place in the interstitium of the prostate gland, being the presence of mast cells, the most abundant type of inflammatory cells. Sections of prostates were also stained with toluidine blue. Mast cells, easily recognized by their dark violet granules against the faint blue background, were observed in normal and experimental groups, but were preponderant in the immunized animals. The proportion of mast cells appearing degranulated was significantly larger in this latter group (Pprostatein–FCA–control < 0.001) (Table 1). Mast cells were observed dispersed or in clusters and sometimes in rows in the prostate of the immunized animals (Fig. 5). Interestingly, animals immunized with the whole MRAG extract also exhibited a higher proportion of degranulated mast cells compared with normal animals (PMRAG–FCA–control < 0.001), but infiltration of mononuclear cells was the main alteration present.
Fig. 5.
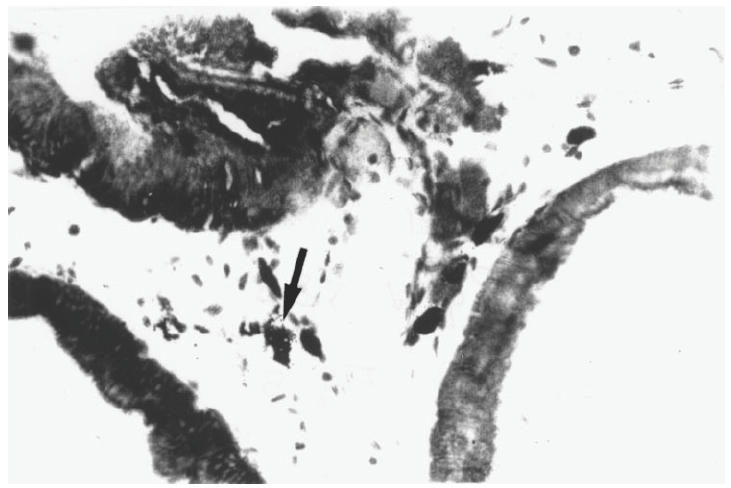
Section of rat prostates stained with toluidine blue from rats immunized on days 0 and 30 and killed 7 days after the last immunization. Infiltration of mast cells is observed. The arrow indicates a degranulating mast cell. (×450.)
DISCUSSION
In this study we have identified and characterized for the first time a prostatic antigen recognized by an autoimmune response. Here we report that prostatein, also known as rat prostatic steroid binding protein or prostate α-protein is an autoantigen in our model of experimental autoimmune prostatitis (EAP) in Wistar rats.
Many experimental models of non-bacterial prostatitis have been proposed in the last few years, and in most of them an autoimmune compromise has been suggested: experimental studies in 1-year-old rats have shown that certain strains of rats (Lewis and Wistar) developed prostatitis spontaneously with advancing age [19]. It has also been shown that administration of 17β-oestradiol increases the incidence of prostatitis in aged rats [20]. Seethalakshmi et al. [21] demonstrated by adoptive transfer experiments that this prostatitis is an autoimmune disease. However, the antigens involved in the induction of prostatitis in this model have not been identified yet. Keetch et al. [3] described a mouse model of prostatitis by immunizing male mice with syngeneic prostate antigens. Adoptive transfer studies demonstrated that this prostatic inflammation was at least in part immune-mediated. By Western blot analysis, the authors identified a 25-kD protein, which they suggest may play a role in the induction of the autoimmunity. However, further studies are needed to identify and characterize the inducing antigen(s) in this model.
In the present work, we identify and characterize a target antigen of the autoimmune response in prostatitis: prostatein. This antigen is recognized not only by the autoantibodies, but also by the cellular autoimmune response elicited in this model. Prostatein is the major secretory protein in the rat ventral prostate, and so far it has not been found in any other organ. It binds steroid hormones and its synthesis is stimulated by testosterone. The mature protein is a tetramer of molecular weight of about 40 000 kD, composed of two different dimeric subunits: subunits S and F. In subunit S, C3, a polypeptide of 12 000 kD, is linked by disulphide bridges to C2, a polypeptide of 10 000 kD. In subunit F, C3 is linked by disulphide bridges to C1, a polypeptide of 8000 kD [22,23]. Our experimental data agree with these facts, since the autoantibodies obtained after two immunizations of MRAG–FCA recognized a polypeptide band of molecular weight ≈ 20 000 kD. This polypeptide band is highly specific for prostate tissue, since no antigen from any other organ was identified in the immunoblotting experiments. The antibody binding site is highly dependent on disulphide bonds, since treatment of the sample with reductor agents such as 2-ME resulted in a great loss of antigenicity. Moreover, N-terminal amino acid sequence gave 33 amino acid residues, with two equimolecular signals in most cycles, which after an exhaustive analysis corresponded exactly with the sequences of components C1 and C3 of subunit F of prostatein.
Amino acid sequence homology between prostatein and rabbit uteroglobin (rUg) has been demonstrated [24]. Moreover, it has been suggested that prostatein, together with mammaglobin, human Clara cell 10-kD protein (the human homologue of the rUg gene), rUg and major cat allergen Fel dI (chain 1) and mouse salivary androgen binding protein (subunit α) belong to the uteroglobin family of proteins [25,26]. Although the in vivo function of these proteins has not been elucidated yet, it has been demonstrated that uteroglobin is a multifunctional, cytokine-like protein with potent phospholipase A2-inhibitory activity. As prostatein is secreted to the seminal fluid, it presumably performs a similar anti-inflammatory role in the feminine reproductive tract.
Prostatein is not only the target of the autoimmune response in animals immunized with MRAG–FCA, but is also an inducing antigen of the disease. In fact, when prostatein is chemically modified and intradermally injected incorporated in FCA, it is able to elicit prostatitis. The autoimmune response is observed in a positive DTH, presence of autoantibodies and the increased degranulation of mast cells in the prostate gland. Although prostatein is able to induce autoimmune prostatitis, the stronger autoimmune response and the differences in the lesion observed in animals immunized with a wider spectrum of prostate antigens indicate that more than one autoantigen is involved, as it usually occurs in other autoimmune diseases. In spite of the small number of animals analysed, the differences found were conclusive.
In addition to lymphocyte infiltration, degranulated mast cells have been described in the prostatitis models mentioned above [3,20,21]. Moreover, mast cell activation has been reported in humans with non-bacterial prostatitis [27]. Mast cells and their potent chemical mediators are known to initiate and modulate a number of important inflammatory cascades. Powell et al. [28] and Brosnan et al. [29] documented mast cell degranulation within the nerves during experimental allergic neuritis. A significant increase in the extent of mast cell degranulation in the accessory glands was already described in EAP induced by i.p. injection of RAG autoantigens associated to liposomes [6,12]. Further studies of the histological alterations at different times after immunization will be useful in understanding the role of mast cells in the pathogenesis of autoimmune prostatitis.
The identification of the target antigens in autoimmune prostatitis has provided a further refinement and characterization of our model, which could serve for a better understanding of the aetiology, pathogenesis and pathophysiology of non-bacterial prostatitis, and lead to more definite diagnostic procedures and more effective treatment regimens.
Addendum
While this work was under revision, Liu et al. published a paper entitled ‘Identification of rat prostatic steroid binding protein as a target antigen of experimental autoimmune prostatitis’ (J Immunol 1997; 159:472–80), in which very similar results are described.
Acknowledgments
This work was supported in part by grants from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Consejo de Investigaciones Científicas y Tecnológicas de la Provincia de Córdoba (CONICOR) and Secretaría de Ciencia y Técnica de la Universidad Nacional de Córdoba (SeCyT-UNC), A. J. Roemmers Fund, Argentina. M.M. and V.E.R. are fellows, and C.M.R. career investigators from CONICET.
References
- 1.Shortliffe LM, Sellers RG, Schachter J. The characterization of nonbacterial prostatitis: a search for an etiology. J Urol. 1992;148:1461–6. doi: 10.1016/s0022-5347(17)36940-9. [DOI] [PubMed] [Google Scholar]
- 2.Schaeffer AJ, Wendel EF, Dunn JK, et al. Prevalence and significance of prostatic inflammation. J Urol. 1981;125:215–9. doi: 10.1016/s0022-5347(17)54976-9. [DOI] [PubMed] [Google Scholar]
- 3.Keetch DW, Humphrey P, Ratliff TI. Development of a mouse model for nonbacterial prostatitis. J Urol. 1994;152:247–50. doi: 10.1016/s0022-5347(17)32871-9. [DOI] [PubMed] [Google Scholar]
- 4.Pacheco-Rupil B, Depiante-Depaoli M, Romero M, et al. Experimental autoimmune damage to rat male accessory glands (MAG) Am J Reprod Immunol. 1981;1:255–61. doi: 10.1111/j.1600-0897.1984.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 5.Galmarini M, Serra H, Pistoresi-Palencia C, et al. Production of rat male accessory gland lesions by transfer of spleen mononuclear cells. Cell Mol Biol. 1986;32:293–301. [PubMed] [Google Scholar]
- 6.Correa S, Riera CM. Adjuvant effect of liposomes in the autoimmune response to rat male accessory glands. Immunol Letters. 1991;28:39–46. doi: 10.1016/0165-2478(91)90125-t. [DOI] [PubMed] [Google Scholar]
- 7.Pacheco-Rupil B, Depiante-Depaoli M, Casadio B. Experimental autoimmune damage to rat male accessory glands. II. T cell requirement in adoptive transfer of specific tissue damage. Am J Reprod Immunol. 1984;5:15–19. doi: 10.1111/j.1600-0897.1984.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 8.Galmarini M, Riera CM, Pesoa S, et al. Induction of humoral response to male accessory glands. Med (Bs. As.) 1981;41:349–53. [PubMed] [Google Scholar]
- 9.Maccioni M, Rivero V, Riera CM. Autoantibodies against rat prostate antigens. Association of specific IgG2b and IgG2c with the DTH response. J Autoimmun. 1996;9:485–91. doi: 10.1006/jaut.1996.0065. [DOI] [PubMed] [Google Scholar]
- 10.Casas-Ingaramo A, Depiante-Depaoli M, Pacheco-Rupil B. Activation of cytotoxic cells by syngeneic prostate antigens in experimental vesiculo-prostatitis. Autoimmunity. 1991;9:151–7. doi: 10.3109/08916939109006751. [DOI] [PubMed] [Google Scholar]
- 11.Galmarini M, Ferro ME, Riera CM. Delayed hypersensitivity and lesions following isoimmunization with modified rat male accessory glands: kinetics of induction. J Reprod Immunol. 1988;13:147–57. doi: 10.1016/0165-0378(88)90058-7. [DOI] [PubMed] [Google Scholar]
- 12.Rivero VE, Iribarren P, Riera CM. Mast cells in accessory glands of experimentally induced prostatitis in male Wistar rats. Clin Immunol Immunopathol. 1995;74:236–42. doi: 10.1006/clin.1995.1035. [DOI] [PubMed] [Google Scholar]
- 13.Meares EM, Jr, Stamey TA. Bacteriological localization patterns in bacterial prostatitis and urethritis. Invest Urol. 1968;5:492. [PubMed] [Google Scholar]
- 14.Vottero-Cima E, Vides MA, Yantorno C. Humoral autoimmune response against rabbit male accessory glands elicited by untreated and chemically modified rabbit seminal plasma. Ann Immunol Inst Pasteur. 1973;124C:273–84. [PubMed] [Google Scholar]
- 15.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Towbim H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrilamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunkapiller MW, Luján E, Ostrander F, et al. Isolation of microgram quantities of protein from polyacrilamide gels for amino acid sequence analysis. Methods Enzimol. 1983;91:227–36. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- 18.Matsudaira P. Sequence of picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–8. [PubMed] [Google Scholar]
- 19.Lundgren R, Holmquist B, Hesselvik M, et al. Treatment of prostatitis in the rat. Prostate. 1984;5:277–84. doi: 10.1002/pros.2990050305. [DOI] [PubMed] [Google Scholar]
- 20.Naslund MJ, Strandberg JD, Coffey DS. The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J Urol. 1988;140:1049–53. doi: 10.1016/s0022-5347(17)41924-0. [DOI] [PubMed] [Google Scholar]
- 21.Seethalakshmi L, Bala RS, Malhotra RK, et al. 17β-estradiol induced prostatitis in the rat is an autoimmune disease. J Urol. 1996;156:1838–42. [PubMed] [Google Scholar]
- 22.Chen C, Schilling K, Hiipakka RA, et al. Prostate α-protein. Isolation and characterization of the polypeptide components and cholesterol binding. J Biol Chem. 1982;257:116–21. [PubMed] [Google Scholar]
- 23.Parker M, Needham M, White R. Prostatic steroid binding protein: gene duplication and steroid binding. Nature. 1982;298:92–94. doi: 10.1038/298092a0. [DOI] [PubMed] [Google Scholar]
- 24.Baker ME. Amino acid sequence homology between rat prostatic steroid binding protein and rabbit uteroglobin. Biochem Biophys Res Commun. 1983;114:325–9. doi: 10.1016/0006-291x(83)91631-5. [DOI] [PubMed] [Google Scholar]
- 25.Watson MA, Fleming TP. Mammaglobin, a mammary specific member of the uteroglobin gene family, is overexpressed in human breast cancer. Cancer Res. 1996;56:860–5. [PubMed] [Google Scholar]
- 26.Dominguez P. Cloning of a Syrian hamster cDNA related to sexual dimorphism: establishment of a new family of proteins. FEBS Letters. 1995;376:257–61. doi: 10.1016/0014-5793(95)01294-4. [DOI] [PubMed] [Google Scholar]
- 27.Tehoharides TC, Flaris N, Cronin CT, et al. Mast cell activation in sterile bladder and prostate inflammation. Int Arch Appl Immunol. 1990;92:281–5. doi: 10.1159/000235190. [DOI] [PubMed] [Google Scholar]
- 28.Powell H, Braheny S, Myers R, et al. Early changes in experimental allergic neuritis. Lab Invest. 1983;48:332–8. [PubMed] [Google Scholar]
- 29.Brosnan C, Lyman W, Tansey F, et al. Quantitation of mast cells in experimental allergic neuritis. J Neuropathol Exp Neurol. 1985;44:196–203. doi: 10.1097/00005072-198503000-00008. [DOI] [PubMed] [Google Scholar]


