Abstract
The low-affinity IgE receptor (FcεRII/CD23) plays a role in IgE production. Cytokines participating in IgE synthesis also modulate CD23 expression on lymphocytes, but whether this modulation is different in atopic subjects remains unclear. We studied CD23 expression on B and T lymphocytes in 10 asthmatic patients with Dermatophagoides pteronyssinus hypersensitivity and 10 healthy non-atopic subjects. Studies were performed by flow cytometry, in phytohaemagglutinin (PHA) or IL-4-stimulated mononuclear cell cultures, alone or in the presence of IFN-γ. Soluble CD23 (sCD23) released in the culture supernatants was measured by enzyme-linked immunoassay. Both PHA and IL-4 induced the expression of CD23 on lymphocytes of atopic and non-atopic subjects. Whereas PHA increased both the percentage and mean fluorescence intensity of CD23+ B and T cells, IL-4 alone did not increase the percentage of CD23+ T cells. The effects of IFN-γ were different in both groups, since it was able to reduce the percentage of PHA-stimulated CD23+ T cells only in non-atopic individuals. In non-atopic subjects more than atopic, levels of sCD23 were increased in the supernatants of PHA and IL-4 cultures. These results show that the modulation of CD23 expression is different on B and T cells, and that IFN-γ acts differently in atopic and non-atopic individuals.
Keywords: atopy, B lymphocytes, CD23, interferon-gamma, IL-4, soluble CD23, T lymphocytes
INTRODUCTION
The low-affinity receptor for IgE or CD23 (FcεRII/CD23) is a 45-kD type II transmembrane glycoprotein expressed in many haematopoietic cells. The role of CD23 in the regulation of IgE synthesis has been demonstrated [1–3], but the modulation of its expression on many cellular types and the functions exerted are controversial. The expression of CD23 by B lymphocytes and its modulation by cytokines such as IL-4 and IFN-γ are well known [4–6]. However, questions regarding CD23 modulation on T cells remain unclear [7,8]. The function of released fragments of CD23 (sCD23) is also under discussion, because it has been proved that certain fragments may display either suppressive [9] or enhancing activity on IgE synthesis [10,11].
It is likely that CD23 expression is a dynamic process depending on the ratio between synthesis and proteolysis. This process could be influenced in part by the profile and the concentration of produced cytokines that modulated CD23 expression and by the levels of IgE, since it has been shown that IgE induces CD23 stabilization on cellular membrane. Thus, atopic patients may present differences in both the modulation of receptor expression and in the levels of soluble forms contributing to a different regulation of IgE synthesis.
In order to test this hypothesis we assessed CD23 expression on B and T cells from either phytohaemagglutinin (PHA) or recombinant human IL-4-stimulated mononuclear cell cultures and the effect of the addition of recombinant human IFN-γ. In order to evaluate the role of CD23 proteolysis in relation to the degree of CD23 expression, the levels of sCD23 in the supernatants of these cultures were also measured.
MATERIALS AND METHODS
Mononuclear cells from 10 asthmatic patients with Dermatophagoides pteronyssinus hypersensitivity (positive prick test, D. pteronyssinus-specific IgE radioallergosorbant test (RAST) of class 3 or 4 and high IgE levels (648 ± 627 kU/l)) and from 10 healthy non-atopic donors (negative clinical history and prick test and low IgE values (111 ± 109 kU/l)) were obtained from peripheral blood samples using a Ficoll density gradient method.
Cell cultures
Peripheral blood mononuclear cells (PBMC) (1 × 106cells/ml) were cultured in RPMI 1640 supplemented with 2 mm glutamine, antibiotics (100 U/ml penicillin, 100 mg/ml streptomycin) and 10% heat-inactivated fetal calf serum (FCS) in presence of optimal concentrations of PHA (2.5 μg/ml; Gibco, Grand Island, NY) or recombinant human IL-4 (100 U/ml; Genzyme Corp., Boston, MA), with or without the presence of 500 U/ml recombinant human IFN-γ (Genzyme). The cells were incubated at 37°C in a water-saturated atmosphere containing 5% CO2 for 72 h (duration considered optimal after previous observations). Afterwards, the cells were collected by centrifugation, and CD23 expression on B and T cells was analysed. Culture supernatants were stored at −70°C until sCD23 measurement.
Flow cytometry analysis
Cultured PBMC were stained with the combination of the following MoAbs (Becton Dickinson, Mountain View, CA): PE-labelled anti-CD23 MoAb (Leu-20) plus either FITC-labelled anti-CD20 MoAb (Leu-16) as B cell marker or FITC-labelled anti-CD3 MoAb (Leu-4) as T cell marker. The cells were analysed in a FACScan device (Becton Dickinson). Electronic gate of cells was set based on side light scatter and FL1 fluorescence parameter (CD20+ cells or CD3+ cells). The percentage of CD23+ B or T lymphocytes was calculated from immunofluorescence cytograms. The mean channel fluorescence intensity (MFI) due to CD23 expression (this parameter is a measure of the density of receptors per cell) was determined with FL2 single histograms. The values corresponding to specific fluorescence were obtained by subtraction of the background staining produced by an irrelevant FITC- and PE-conjugated MoAb of the same isotype as the corresponding specific MoAb used.
Soluble CD23
The concentration of soluble CD23 released into culture supernatants was quantified using a ‘sandwich’ enzyme immunoassay kit (The Binding Site, Birmingham, UK) according to the manufacturer's instructions. The concentration was expressed in μg/l. This technique measures within the range 0.79–50 μg/l.
Statistical analysis
Data were analysed by the statistical package SPSS. Wilcoxon's signed rank test for matched pairs was performed to compare the results within the same group. Mann–Whitney U-test was used to determine differences between groups. Pearson correlation analyses were also applied. Statistically significant differences were considered P values < 0.05.
RESULTS
CD23 expression
PHA stimulation of PBMC of both atopic and non-atopic subjects resulted in a significant increase in the percentage of CD23+ B and T cells in comparison with unstimulated cultures (P < 0.01). Similar results were obtained when the MFI due to CD23 expression was analysed on either B or T cells in these cultures (P < 0.01) (Figs 1 and 2).
Fig. 1.
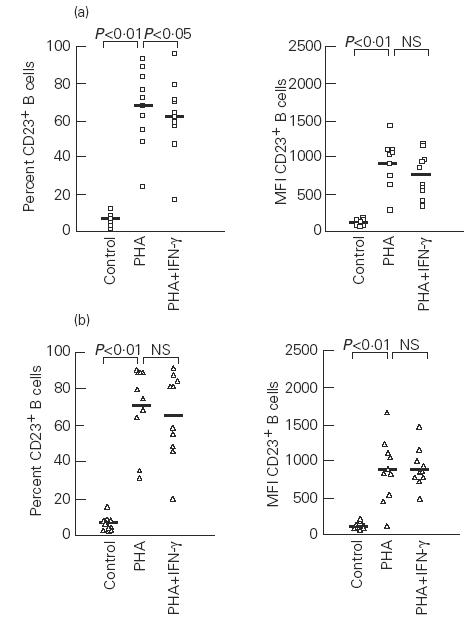
Effects of phytohaemagglutinin (PHA) and IFN-γ on the percentage and mean fluorescence intensity (MFI) of CD23+ B cells in atopic (a) and non-atopic (b) subjects. Control, Unstimulated cells; NS, not significant. Median values are represented by the solid bars.
Fig. 2.
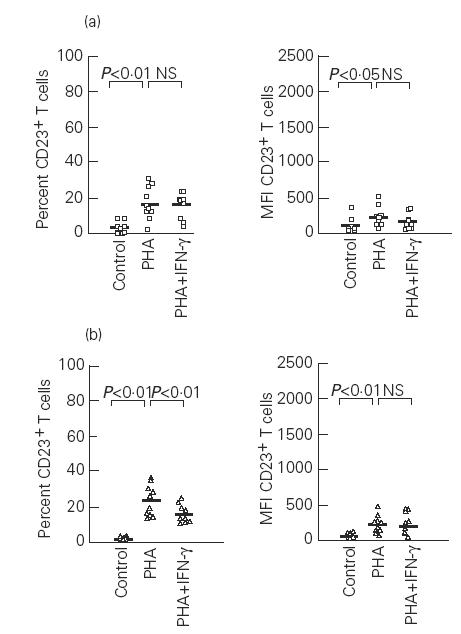
Effects of phytohaemagglutinin (PHA) and IFN-γ on the percentage and mean fluorescence intensity (MFI) of CD23+ T cells in atopic (a) and non-atopic (b) subjects. Control, Unstimulated cells; NS, not significant. Median values are represented by the solid bars.
The presence of IFN-γ at the onset of PHA-stimulated cultures produced a reduction in the percentage of CD23+ B cells which was only statistically significant in atopic subjects (P < 0.05) (Fig. 1). In these cultures, the MFI of CD23+ B cells decreased in atopic patients, but without reaching statistical significance, while in non-atopic subjects IFN-γ failed to modify the MFI of CD23+ B cells (Fig. 1). The effect of IFN-γ on T cells was different, since it lowered the percentage of CD23+ T cells only in non-atopic individuals (P < 0.01). However, the MFI due to CD23 expression on T cells was not reduced by IFN-γ in any group (Fig. 2).
In both atopic and non-atopic subjects IL-4 induced a significant increase in the percentage of B cells expressing CD23 in comparison with unstimulated cells (P < 0.01) (Fig. 3), but on T cells, IL-4 did not change the percentage of CD23+ T cells (Fig. 4). The MFI of both CD23+ B and T cells was up-regulated by IL-4 in both groups (P < 0.01) (Figs 3 and 4).
Fig. 3.
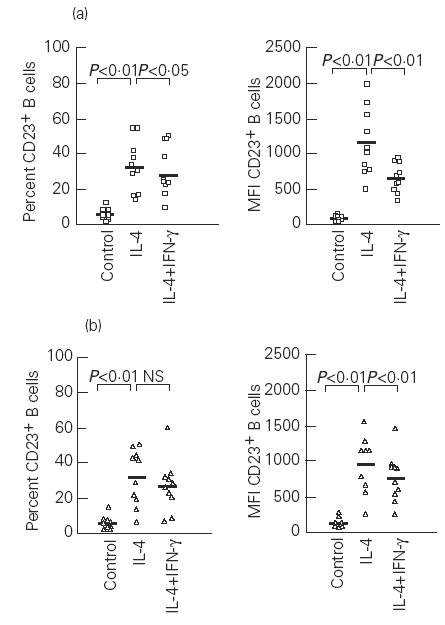
Effects of IL-4 and IFN-γ on the percentage and mean fluorescence intensity (MFI) of CD23+ B cells in atopic (a) and non-atopic (b) subjects. Control, Unstimulated cells; NS, not significant. Median values are represented by the solid bars.
Fig. 4.
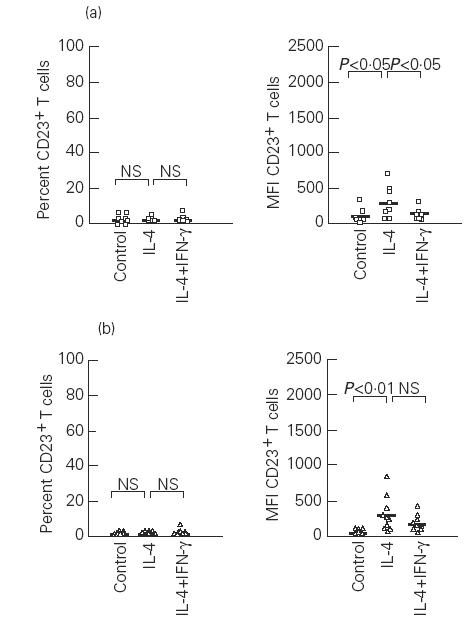
Effects of IL-4 and IFN-γ on the percentage and mean fluorescence intensity (MFI) of CD23+ T cells in atopic (a) and non-atopic (b) subjects. Control, Unstimulated cells; NS, not significant. Median values are represented by the solid bars.
In IL-4-stimulated cultures the addition of IFN-γ decreased the percentage of CD23+ B cells, mainly in atopic patients (P < 0.05), and resulted in a significant inhibition of CD23+ B cell expression (MFI) in both groups (P < 0.01) (Fig. 3). As was the case with PHA-stimulated cultures, the addition of IFN-γ did not cause significant alterations of the degree of CD23 expression on T cells in non-atopic subjects. However, T cells from atopic patients exhibited reduced MFI values (P < 0.05) (Fig. 4).
Soluble CD23
Supernatants from PHA- and IL-4-stimulated cultures of both groups showed an increase in sCD23 in comparison with unstimulated cultures (P < 0.02) (Fig. 5). However, the concentration of sCD23 in stimulated cultures was higher in non-atopic subjects than in atopic patients (PHA, P = 0.04; IL-4, P = 0.028). Both stimuli (PHA and IL-4) induced similar sCD23 concentrations in healthy subjects. In contrast, in atopic patients IL-4 induced higher concentrations of sCD23 than PHA (P = 0.018). The presence of IFN-γ in the cultures increased the concentration of sCD23 mainly in allergic patients, but without reaching statistical significance.
Fig. 5.
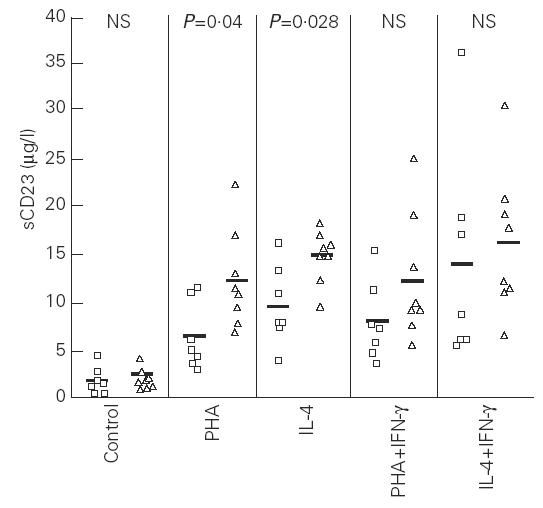
Levels of soluble CD23 in the supernatants of peripheral blood mononuclear cell-stimulated cultures of atopic (□) and non-atopic (▵) subjects. Control, Unstimulated cells; NS, not significant. Statistical comparison in each group: control versus phytohaemagglutinin (PHA), P < 0.02; control versus IL-4, P < 0.02 (atopic and non-atopic group); PHA versus PHA + IFN-γ and IL-4 versus IL-4 + IFN-γ, NS (atopic and non-atopic group).
Correlation studies
The effects of PHA, IL-4 and IFN-γ in the induction/proteolysis of CD23 expression on lymphocytes were also evaluated through studies correlating the concentration of sCD23 in culture supernatants with the percentage and MFI of CD23+ B or T cells in these cultures. The results showed that in PHA-stimulated cultures of atopic patients a significant correlation was obtained between the levels of sCD23 and the percentage of CD23+ B cells (r = 0.81; P < 0.05) as well as between the levels of sCD23 and the percentage of CD23+ T cells (r = 0.86; P < 0.01). When IFN-γ was present, a significant correlation was found between the percentage of CD23+ B cells and the levels of sCD23 (r = 0.76; P < 0.05). In contrast, non-atopic subjects did not show any correlation between CD23 expression on B or T cells from PHA-stimulated cultures and the concentrations of sCD23.
We did not obtain any correlation between CD23 expression on B or T lymphocytes and the levels of sCD23 in IL-4-stimulated cultures of both groups. However, the addition of IFN-γ in IL-4-stimulated cultures of non-atopic individuals yielded a negative correlation between the percentage of CD23+ B cells and the levels of sCD23 (r = –0.82; P < 0.01).
DISCUSSION
The results obtained in this study show that in vitro PHA and IL-4 cause similar effects on the expression of CD23 on B and T cells in both atopic and non-atopic individuals, since both substances up-regulate the expression of CD23. However, while PHA raised the percentage of both B and T cells expressing CD23, IL-4 did not on T cells at the concentrations used in this study. Both substances increased CD23 expression on B and T cells in both groups and induced the release of CD23 in the supernatants of cell cultures.
The observed increase in CD23 expression on B lymphocytes from PHA- or IL-4-stimulated PBMC cultures is in agreement with the results reported in other studies [5,6,12]. In our experiments, the increase in CD23+ B lymphocyte percentage was higher in PHA-stimulated cultures than in IL-4-stimulated cultures. This can be explained, because PHA induces T lymphocyte activation, and this results in the production of cytokines by these cells, which in turn can induce [4,13] or inhibit [5] the expression of CD23 on B cells. We believe that the increase in the percentage of CD23+ B lymphocytes in PHA-stimulated cultures might be due to the synergistic action of endogenous IL-4 and other cytokines synthesized by T lymphocytes, for as our results show, IL-4 alone induces CD23 expression on B lymphocytes.
The expression of CD23 on T cells, on the other hand, has been controversial. Some studies failed to show CD23 expression on peripheral blood T lymphocytes either in resting T cells or following activation with PHA [7,14]. Others reported that the presence of B cells was necessary for CD23 expression on activated T cells [8], and finally, it has been definitively proven that this receptor is produced by T cells [15,16].
We saw that PHA produces an increase, though less than on B lymphocytes, in the percentage of CD23+ T cells in both groups, which did not occur when IL-4 was the only stimulated substance. This suggests that the activation of T cells is necessary for CD23 expression, and that cytokines other than IL-4 must also be involved [17].
Our results show that IL-4 boosts CD23 expression only on the cells where it was previously expressed, since IL-4 raises receptor density, but does not modify the percentage of CD23+ T cells. It is likely that IL-4 acts only on activated T cells. These findings agree with some reports showing an increase of CD23+ T cells when PBMC were stimulated with PHA or antigen plus IL-4, but not with IL-4 alone [8,18].
Other important data concern the differential effects of IFN-γ on CD23 expression on B and T lymphocytes and their differences in atopic and non-atopic subjects. It is possible that the observed increase in the percentage of CD23+ B and T lymphocytes from PHA-stimulated cultures was caused differently in atopic and non-atopic subjects, perhaps due to a different pattern of cytokine production [19,20]. This would explain why the effects of IFN-γ on CD23 expression were different in both groups.
CD23 shedding is produced, in part, by a membrane receptor proteolysis, although it has been shown that IFN-γ can modulate this release.
The fact that the addition of IFN-γ to PHA-stimulated cultures from atopic patients results in a decrease in the percentage of CD23+ B cells, directly related to a decrease in sCD23 levels, implies that in these subjects IFN-γ acts primarily on CD23 synthesis, which agrees with the results of molecular studies suggesting that in B cells, IFN-γ could down-regulate the expression of the CD23 gene induced by IL-4 at post-transcriptional level [21].
However, in non-atopic subjects IFN-γ might induce an increase of receptor breakdown [22], such as the results obtained in IL-4-stimulated cultures, where the decrease of percentage of CD23+ B cells is correlated to an increase of sCD23 levels, suggest. This effect was not observed in atopic patients. Thus, greater IgE levels might induce CD23 receptor stabilization. This could also explain, in part, why IFN-γ does not down-regulate the percentage of CD23+ T cells from PHA-stimulated cultures of atopic patients, as opposed to what occurs with non-atopic patients.
In our study, atopic patients showed lower sCD23 levels than healthy individuals, in agreement with Batterman et al. [23]. This is in contrast with former reports [24–26], but the differences in methods used and the overlap between the ranges of sCD23 concentrations in atopic and non-atopic individuals may explain the discrepancies.
It is likely that CD23 expressed on cellular membrane plays a more important role in the regulation of IgE production than soluble forms [27]. We failed to find statistically significant differences between atopic and non-atopic individuals in CD23 expression on lymphocytes induced by PHA or IL-4. However, interestingly, IFN-γ produced a distinct modulation of CD23 expression in both groups, which could contribute to a distinct regulation of IgE synthesis. Along these lines, it is known that atopic patients present a higher percentage of CD23+ B cells in peripheral blood [6], probably due to an unbalanced IL-4/IFN-γ ratio and higher IgE levels than non-atopics. The higher number of CD23+ B cells may improve the antigen presentation to T cells and their activation, and consequently increase the percentage of CD23+ T cells, which in atopic individuals, as has been demonstrated in this study, would not be down-regulated by IFN-γ. The high expression of CD23 on T cells would then favour the interaction between T and B cells by means of CD23 expressed on T cells and its receptor (CD21) on B cells [3,28]. This would lead to IgE production.
In conclusion, while PHA and IL-4 induce the expression of CD23 on B and T lymphocytes of atopic and non-atopic subjects in similar ways, the effects of IFN-γ are different in both groups. These differences in the modulation of CD23 expression could contribute to altering the regulation of IgE synthesis in atopic individuals.
Acknowledgments
This study was supported by a grant from the ‘Fondo de Investigaciones Sanitarias’ exp 90/0642. We thank Caita Mané and Jordi Bonete for their technical support.
References
- 1.Kehry MR, Yamashita LC. Role of the low-affinity Fcε receptor in B-lymphocyte antigen presentation. Res Immunol. 1990;141:77–81. doi: 10.1016/0923-2494(90)90106-9. [DOI] [PubMed] [Google Scholar]
- 2.Santamaria LF, Bheekha R, Van-Reijsen FC, et al. Antigen focusing by specific monomeric immunoglobulin E bound to CD23 on Epstein–Barr virus-transformed B cells. Hum Immunol. 1993;37:23–30. doi: 10.1016/0198-8859(93)90139-r. [DOI] [PubMed] [Google Scholar]
- 3.Aubry JP, Pochon S, Graber P, Jansen KU, Bonnefoy JY. CD21 is a ligand for CD23 and regulates IgE production. Nature. 1992;358:505–7. doi: 10.1038/358505a0. [DOI] [PubMed] [Google Scholar]
- 4.Defrance T, Aubry JP, Rousset F, et al. Human recombinant interleukin 4 induces Fcε receptors (CD23) on normal human B lymphocytes. J Exp Med. 1987;165:1459–67. doi: 10.1084/jem.165.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galizzi JP, Cabrillat H, Rousset F, Ménétrier C, de Vries JE, Banchereau J. IFN-γ and prostaglandin E2 inhibit IL-4-induced expression of FcεR2/CD23 on B lymphocytes through different mechanisms without altering binding of IL-4 to its receptor. J Immunol. 1988;141:1982–8. [PubMed] [Google Scholar]
- 6.Corominas M, Mestre M, Bas J, et al. CD23 expression on B-lymphocytes and its modulation by cytokines in allergic patients. Clin Exp Allergy. 1993;23:612–7. doi: 10.1111/j.1365-2222.1993.tb00902.x. [DOI] [PubMed] [Google Scholar]
- 7.Armitage RJ, Goff LK, Berveley PCL. Expression and functional role of CD23 on T cells. Eur J Immunol. 1989;19:31–35. doi: 10.1002/eji.1830190106. [DOI] [PubMed] [Google Scholar]
- 8.Kawabe T, Maekawa N, Maeda Y, Hosoda M, Yodoi J. Induction of FcεRII/CD23 on phytohemagglutinin-activated human peripheral blood T lymphocytes. I. Enhancement by IL-2 and IL-4. J Immunol. 1991;147:548–53. [PubMed] [Google Scholar]
- 9.Sarfati M, Bettler B, Letellier M, et al. Native and recombinant soluble CD23 fragments with IgE suppressive activity. Immunology. 1992;76:662–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Sarfati M, Delespesse G. Possible role of human lymphocyte receptor for IgE (CD23) or its soluble fragments in the in vitro synthesis of human IgE. J Immunol. 1988;141:2195–9. [PubMed] [Google Scholar]
- 11.Gordon J. CD23 and B cell activation. Clin Exp Allergy. 1992;22:199–204. doi: 10.1111/j.1365-2222.1992.tb03073.x. [DOI] [PubMed] [Google Scholar]
- 12.Kicza K, Fischer A, Pfeil T, Bujanowski-Weber J, König W. Cell–cell interactions for CD23 expression and soluble CD23 release from peripheral lymphocytes of atopic donors. Immunology. 1989;68:532–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Punnonen J, Aversa G, Cocks BG, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;30:3730–4. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carini C, Pini C, DiFelice GC, Fattorossi A, Fratazzi C. CD23/Fc epsilon RII expression on phytohemagglutinin-A- or phorbol-12myristate-13-acetate -Ca(2+)-activated human tonsil T cells. Int Arch Allergy Immunol. 1993;101:31–38. doi: 10.1159/000236495. [DOI] [PubMed] [Google Scholar]
- 15.Maekawa N, Kawabe T, Sugie K, et al. Induction of FcεRII/CD23 on PHA-activated human peripheral blood T lymphocytes and the association of Fyn tyrosine kinase with FcεRII/CD23. Res Immunol. 1992;143:422–5. doi: 10.1016/s0923-2494(05)80075-6. [DOI] [PubMed] [Google Scholar]
- 16.Nunez R, Matsui M, Yodoi J, Lynch RG. Identification of novel CD23 transcripts on human T and B lymphocytes and eosinophil cell line. Immunol Letters. 1995;44:169–74. doi: 10.1016/0165-2478(95)00210-v. [DOI] [PubMed] [Google Scholar]
- 17.Fratazzi C, Carini C. Interleukin-7 modulates intracitoplasmatic CD23 production and induces adhesion molecule expression and adhesiveness in activated CD4+CD23+ T cell subsets. Clin Immunol Immunopathol. 1996;81:261–70. doi: 10.1006/clin.1996.0187. [DOI] [PubMed] [Google Scholar]
- 18.Prinz JC, Baur X, Mazur G, Rieber EP. Allergen-directed expression of Fc receptors for IgE (CD23) on human T lymphocytes is modulated by interleukin 4 and interferon-γ. Eur J Immunol. 1990;20:1259–64. doi: 10.1002/eji.1830200610. [DOI] [PubMed] [Google Scholar]
- 19.Corominas M, Mestre M, Bas J, Verdaguer J, Buendia E. Expression of lymphocyte receptors and cytokine production in atopic patients. Rev Esp Alergol Immunol Clin. 1994;9:15–22. [Google Scholar]
- 20.Jung T, Lack G, Schauer U, et al. Decreased frequency of Interferon-γ and interleukin-2-producing cells in patients with atopic diseases measured at the single cell level. J Allergy Clin Immunol. 1995;96:515–27. doi: 10.1016/s0091-6749(95)70296-2. [DOI] [PubMed] [Google Scholar]
- 21.Lee CE, Yoon SR, Pyun K. Mechanism of interferon-gamma down regulation of the interleukin-4 induced CD23/Fc epsilon RII expression in human B cells: posttranscriptional modulation by interferon-gamma. Mol Immunol. 1993;30:301–7. doi: 10.1016/0161-5890(93)90058-j. [DOI] [PubMed] [Google Scholar]
- 22.Conrad DH, Keegan AD, Kalli KR, Van Dusen R, Rao M, Levine AD. Superinduction of low affinity receptors on murine B lymphocytes by lipopolysaccharide and IL-4. J Immunol. 1988;141:1091–7. [PubMed] [Google Scholar]
- 23.Batterman J, Mazza DS, Meriney DK, Cleveland WL, Reddy MM, Grieco MH. In vitro release of soluble CD23 by human lymphocytes in ragweed-sensitive versus nonatopic patients following stimulation with ragweed antigen E. Ann Allergy Asthma Immunol. 1996;76:359–62. doi: 10.1016/S1081-1206(10)60038-5. [DOI] [PubMed] [Google Scholar]
- 24.Bujanowski-Weber J, Brings B, Knöller I, Pfeil T, König W. Expression of low-affinity receptor for IgE (FcεRII, CD23) and IgE-BF (soluble CD23) release by lymphoblastoid B cell-line RPMI-8866 and human peripheral lymphocytes of normal and atopic donors. Immunology. 1989;66:505–11. [PMC free article] [PubMed] [Google Scholar]
- 25.Monteseirin J, Llamas E, Muñoz F, Bandrés F, Bono MJ, Conde J. Production of soluble CD23 from peripheral blood lymphocytes of asthmatic patients. Allergol Immunopathol. 1993;21:75–78. [PubMed] [Google Scholar]
- 26.Pène J, Rivier A, Lagier B, Becker WM, Michel FB, Bousquet J. Differences in IL-4 release by PBMC are related with heterogeneity of atopy. Immunology. 1994;81:58–64. [PMC free article] [PubMed] [Google Scholar]
- 27.Bonnefoy JY, Lecoanet-Henchoz S, Aubry JP, Gauchat JF, Graber P. CD23 and B cell activation. Curr Opin Immunol. 1995;7:355–9. doi: 10.1016/0952-7915(95)80110-3. [DOI] [PubMed] [Google Scholar]
- 28.Gagro A, Rabatic S. Allergen-induced CD23 on CD4+ T lymphocytes and CD21 on B lymphocytes in patients with allergic asthma: evidence and regulation. Eur J Immunol. 1994;24:1109–14. doi: 10.1002/eji.1830240515. [DOI] [PubMed] [Google Scholar]


