Abstract
Recently we showed the in vivo relevance of chemokines in cases of bacterial peritonitis in continuous ambulatory peritoneal dialysis (CAPD) patients. Mesothelial cells, the most numerous cells in the peritoneal cavity, are hypothesized to function as a main source of chemokine production. We investigated the time- and dose-dependent expression patterns of four chemokines by mesothelial cells at the mRNA and protein level in response to stimulation with physiological doses of proinflammatory mediators that are present at the site of bacterial inflammation. Besides the chemokines huGRO-α (attractant for neutrophils), MCP-1 and RANTES (monocyte attractants), the expression and production of IP-10 was analysed. Mesothelial cells were cultured and stimulated with either IL-1β, tumour necrosis factor-alpha (TNF-α) or IFN-γ or combinations of these. The time- and dose-dependent mRNA expression of the chemokines was determined by Northern blot analysis and the protein production by ELISA. It was concluded that mesothelial cells could indeed be triggered by the mentioned stimuli to induce mRNA and protein production (huGRO-α and IP-10) or to augment constitutive protein production (MCP-1). However, RANTES mRNA and protein production could only be induced in some cases and only in small amounts. The chemokine response of mesothelial cells was regulated differentially, depending on the stimulus and the chemokine measured. In distinct cases, combination of the stimuli led to synergy in mRNA expression and protein production. The presented in vitro data support our hypothesis that mesothelial cells in vivo are the main source of relevant chemokines in response to proinflammatory mediators, suggesting an important role for mesothelial cells in host defence.
Keywords: chemokines, peritoneal cavity, inflammation, CAPD
INTRODUCTION
A recently described superfamily of specialized cytokines is the group of chemokines. These small cytokines (6–12 kD) have the ability to chemotactically attract and activate inflammatory cells [1–5]. The chemokines are divided into four subfamilies based on their genetically conserved cysteine motif, important for the structure and function of the proteins. The CXC subfamily has two conserved cysteine pairs, with one amino acid (X) between the first two cysteine residues. The best known member of this family is IL-8, a strong chemoattractant and activator of neutrophils [1,6]. In the CC subfamily, the first two cysteine residues are adjacent. MCP-1, a monocyte chemoattractant and activator, is the best described chemokine in this subfamily [1–4]. The two other subfamilies are described more recently and for both branches only one member has been characterized until now [3,5]. Lymphotactin, a lymphocyte chemoattractant, belongs to the C subfamily that is characterized by loss of the first cysteine pair. The CX3C subfamily is the most recently described branch, containing a membrane-bound molecule with a chemokine domain (first two cysteines separated by three amino acids) that can be shed and is chemotactic for monocytes and T cells. Most chemokines can be produced by a wide variety of cell types after proper stimulation with proinflammatory cytokines or even directly by stimulation with whole bacteria [7], and exert their effect on one or more target cell populations. Therefore, chemokines are important mediators in the regulation of leucocyte influx into an area of inflammation.
The study of the mechanism of inflammation and the role of chemokines in the inflammatory process in humans is rather difficult, due to the lack of relevant in vivo models. However, we recently showed the relevance of chemokines (IL-8, huGRO-α and MCP-1) in bacterial peritonitis, occurring as a complication in continuous ambulatory peritoneal dialysis (CAPD) patients [8]. The bacterial peritonitis is mainly caused by Staphylococci and is still responsible for a high morbidity in CAPD patients [9,10]. The most probable source of chemokines in this clinical model is the mesothelial cells that cover the peritoneal cavity. First, because they are the most numerous cells present in the peritoneal cavity and the first cell layer to be encountered by microorganisms that invade the peritoneal cavity via the catheter. Second, it has been demonstrated in previous studies that mesothelial cells are able to produce several immunoactive substances such as phosphatidylcholine, adhesion molecules, IL-6 and IL-8 [11–17]. Third, we demonstrated earlier that the number of mesothelial cells in the effluent of CAPD patients is inversely correlated to their peritonitis incidence [18], suggesting an important function for these cells in the prevention of inflammation.
The aim of the present study was to investigate whether mesothelial cells can indeed produce other relevant chemokines besides IL-8 and might therefore be important in the regulation of inflammatory cell influx. We focused on the clinically important chemokines huGRO-α (human melanoma-derived growth factor-α), MCP-1 and RANTES (Regulated on Activation Normal T cell-Expressed and Secreted). HuGRO-α is a CXC chemokine with neutrophils as its most important target population [4,6]. MCP-1 and RANTES are CC chemokines with a predominant chemotactic activity for monocytes [4,19,20]. In addition, we measured a fourth chemokine, IP-10 (IFN-γ-inducible protein 10) both in the expression and production patterns of stimulated mesothelial cells in vitro and in the peritonitis and stable CAPD patient effluents. IP-10 is a CC chemokine with chemotactic activity for monocytes and a particular subset of T cells [4,21].
Our findings suggest that mesothelial cells are important in the first-line defence against microorganisms by producing physiologically relevant concentrations of inflammatory mediators such as the chemokines that may induce influx of adequate populations of inflammatory cells to conquer infection.
MATERIALS AND METHODS
Mesothelial cell cultures
Mesothelial cells were isolated from samples of human omenta, obtained during elective surgery, as described earlier [22]. Briefly, pieces were washed three times in PBS (0.15 m, pH 7.4) and then incubated for 30 min in a 1.25% trypsin-containing solution at 37°C on a rock-and-roller. The suspension was then centrifuged for 10 min at 300 g. The floating fatty omentum layer and the supernatant were removed. The remaining pellet contained the mesothelial cells, which were washed twice with PBS. Cells were then resuspended in 5 ml M199 medium supplemented with Hanks' balanced salt solution (HBSS; Gibco, Uxbridge, UK), 0.35 g/l NaHCO3, 10% heat-inactivated fetal calf serum (FCS; Gibco), 50 U/ml penicillin, 50 μg/ml gentamicin and 2.4 μg/ml amphotericin B (Fungizone; Bristol-Meyers Squibb, Woerden, The Netherlands) and grown on fibronectin-coated culture flasks [23]. The culture surfaces of these flasks were incubated with 2 μg/cm2 fibronectin (Sigma, St Louis, MO) for 45 min. The medium was refreshed after 2 days of culture and 50% 3T3-supernatant was added as a growth factor [24]. When confluence was reached the cells were passed and cultured in 10% 3T3 supernatant containing medium. All experiments were performed on mesothelial cells in culture passage 1 under serum-free and 3T3 supernatant-free conditions.
Purity of the mesothelial cells was checked by immunocytochemistry with antibodies against cytokeratins and CA125 [25], by the ultrastructural evaluation of the typical appearance of mesothelial cells [23], and by their typical growth pattern [26] (data not shown).
Stimulation of cultures
To determine whether mesothelial cells are able to express chemokines, confluent mesothelial layers were incubated for 2, 4, 8, 12 and 24 h with three different proinflammatory cytokines separately: IL-1β 500 U/ml (R&D Systems, Abingdon, UK), tumour necrosis factor-alpha (TNF-α; 625 U/ml (Genzyme, Cambridge, MA)) and IFN-γ 300 U/ml (Boehringer, Ingelheim am Rhein, Germany) and combinations of these stimuli (IL-1β + IFN-γ, TNF-α + IFN-γ and IL-1β + TNF-α). All stimuli were dosed as above, unless stated otherwise. Also, mesothelial cells were incubated for 4 h with increasing doses of IL-1β (50–500 U/ml), TNF-α (62.5–6250 U/ml) or IFN-γ (30–3000 U/ml) to determine the dose-dependent mRNA expression of the described chemokines.
Furthermore, the dose-dependent protein production of chemokines was determined after an incubation period of 24 h with several different concentrations of the above mentioned cytokines: 0.5–5000 U/ml IL-1β, 0.6–6250 U/ml TNF-α, 0.3–3000 U/ml IFN-γ and combinations of these cytokines.
ELISA
HuGRO-α was measured in culture supernatants using a commercial ELISA specific for the huGRO-α form with a lower detection level of 9 pg/ml (R&D Systems). MCP-1 was determined using a sandwich ELISA, as recently described by Tekstra et al. [8], with a lower detection level of 30 pg/ml in which the catching MoAb was kindly provided by Mantovani [27] and the detecting polyclonal antibody was commercially obtained from R&D Systems.
An ELISA based on the same principles as the MCP-1 ELISA was used to detect RANTES. Both the catching anti-RANTES MoAb as well as the detecting polyclonal anti-RANTES antibody were commercially obtained from R&D Systems. The lower detection level was 30 pg/ml [8].
An ELISA was developed to detect IP-10. In this assay a polyclonal antibody against IP-10 (R&D Systems) was used as catching antibody and the same antibody in biotinylated form was used as detecting antibody, followed by peroxidase-conjugated streptavidin. The lower detection level was 100 pg/ml.
Patient material
In the same effluents of seven peritonitis episodes of five patients described earlier in detail by Tekstra et al. [8], IP-10 protein was measured during peritonitis as well as in the stable situation.
RNA isolation and Northern blot analysis
Total RNA was isolated from mesothelial cells (1.5–2.0 × 106/culture flask) using RNAzol-B (RNA isolation solvent; Cinna/Biotec Labs Inc., Houston, TX). RNA samples (15–20 μg from 1.5 to 2.0 × 106 cells) were fractionated on 1% agarose gels containing 2.2 m formaldehyde and transferred to Wathman nitrocellulose filters (Amersham, Aylesbury, UK). The blots were then hybridized with random 32P-labelled probes specific for huGRO, IP-10, MCP-1 and RANTES; a probe specific for β-actin was used as a control for constant RNA loading. The blots were analysed by densitometry of the x-ray films.
Statistical analysis
All ELISA data are expressed as means with s.e.m. Statistical analysis on the dose- and time-dependent protein production of chemokines was performed using the Wilcoxon signed rank test. P < 0.05 was considered significant.
RESULTS
mRNA expression of chemokines by mesothelial cells: Figs 1 and 2
Fig. 1.

Northern blot analysis and quantification of mRNA expression of huGRO (a), IP-10 (b) and MCP-1 (c) by human peritoneal mesothelial cells after stimulation with increasing doses of IL-1β, tumour necrosis factor-alpha (TNF-α), and IFN-γ. The graphs show the chemokine mRNA/β-actin mRNA expression ratio in densitometric units to correct for RNA loading differences, and indicate the quantity of mRNA expression.
Fig. 2.
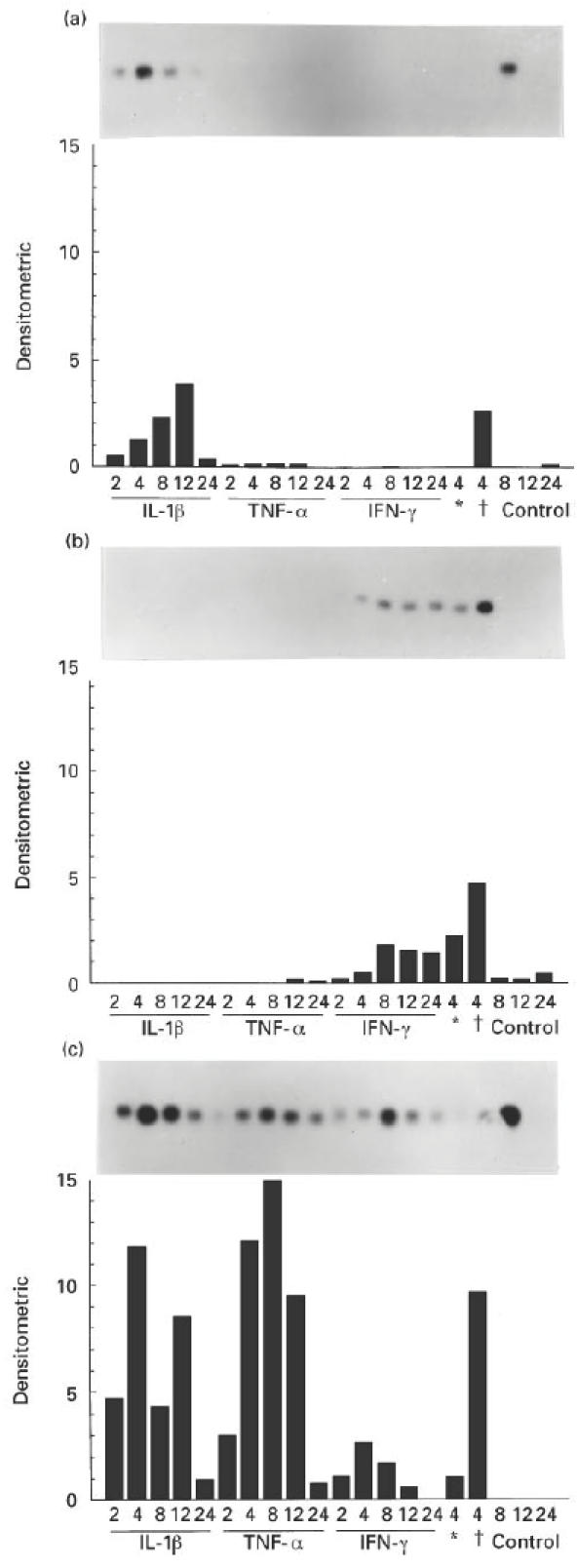
Northern blot analysis and quantification of mRNA expression of huGRO (a), IP-10 (b) and MCP-1 (c) by human peritoneal mesothelial cells after stimulation for various different periods (2, 4, 8, 12 and 24 h) with IL-1β, tumour necrosis factor-alpha (TNF-α), IFN-γ or combinations of these cytokines. *Combination of IL-1β and IFN-γ; †combination of TNF-α and IFN-γ. Control gives data of unstimulated cultures. The graphs show the chemokine mRNA/β-actin mRNA expression ratio in densitometric units to correct for RNA loading differences, and indicate the quantity of mRNA expression.
Incubation of mesothelial cells with increasing doses of IL-1β for 4 h induced an up-regulation of mRNA expression for huGRO (Fig. 1a) and MCP-1 (Fig. 1c), but no visible band was detected for IP-10 (Fig. 1b). Incubation with TNF-α for 4 h induced a dose-dependent up-regulation of the mRNA for MCP-1 only (Fig. 1c). IFN-γ induced a dose-dependent up-regulation of both MCP-1 and IP-10 expression (Fig 1b,c). MCP-1 mRNA was also expressed in cells without stimulation (Fig. 1c). Expression of mRNA for RANTES increased only very slightly after incubation with IL-1β (data not shown).
The maximum expression of mRNA for huGRO was reached after 12 h of incubation with IL-1β. The combination of TNF-α and IFN-γ induced an up-regulation of huGRO expression at 4 h of incubation (Fig. 2a). mRNA expression for IP-10 was only induced after incubation with IFN-γ and maximum expression was reached after 8 h of incubation. This could be synergistically up-regulated by adding IL-1β or TNF-α to the IFN-γ as a co-stimulus at 4 h of incubation (Fig. 2b). The maximum expression of mRNA for MCP-1 was reached after 4 h of incubation with IL-1β or IFN-γ, and after 8 h with TNF-α. None of the combinations of stimuli induced a synergistic up-regulation of mRNA expression for MCP-1 after 4 h of incubation (Fig. 2c).
Protein production of chemokines by mesothelial cells: Tables 1,2 and 3
Table 1.
The production of huGRO-α, IP-10, MCP-1 and RANTES by mesothelial cells with increasing doses of IL-1β, or IL-1β in combination with IFN-γ or tumour necrosis factor-alpha (TNF-α)

Table 2.
The production of huGRO-α, IP-10, MCP-1 and RANTES by mesothelial cells with increasing doses of tumour necrosis factor-alpha (TNF-α), or TNF-α in combination with IFN-γ or IL-1β

Table 3.
The production of huGRO-α, IP-10, MCP-1 and RANTES by mesothelial cells with increasing doses of IFN-γ, or IFN-γ in combination with IL-1β or tumour necrosis factor-alpha (TNF-α)

Stimulation of confluent monolayers of mesothelial cells with increasing doses of IL-1β induced a dose-dependent protein production of huGRO-α, MCP-1 and to a lesser extent RANTES (Table 1).
Stimulation of mesothelial cells with increasing doses of TNF-α induced a dose-dependent increase in the production of huGRO-α and MCP-1 (Table 2). None of the combinations of stimuli induced a synergistic up-regulation of the protein production of huGRO-α; the combination of IL-1β and TNF-α even seemed to have a down-regulating effect on huGRO-α production. Although TNF-α alone did not lead to the production of RANTES protein, the combination of TNF-α and IFN-γ tended to increase the production synergistically (Table 2).
Incubation of mesothelial cells with IFN-γ alone did not induce a response in huGRO-α production. MCP-1 production, however, increased significantly above background level after incubation with IFN-γ, with a maximum production at a dose of 3000 U/ml. The combinations of IL-1β with TNF-α, IL-1β with IFN-γ, and TNF-α with IFN-γ all induced a synergistic up-regulation of MCP-1 protein production (P < 0.05) (Table 3). IP-10 production could be up-regulated after stimulation with IFN-γ. Neither IL-1β nor TNF-α alone, nor the combination of both could induce IP-10 production in mesothelial cells. The combination of both IL-1β and TNF-α with IFN-γ induced a synergistic up-regulation of IP-10 production (P < 0.05) (Table 3).
Increased levels of IP-10 could be detected in the spent peritoneal effluents of seven peritonitis episodes (median 12 ng/ml) compared with the stable situation (median < 0.1 ng/ml, P < 0.05).
DISCUSSION
This study clearly illustrates the ability of mesothelial cells to significantly express and produce at least four chemokines that may contribute to the regulation of inflammatory cell influx. Next to IL-8, huGRO, IP-10, MCP-1 and to a lesser extent RANTES are all expressed and produced by mesothelial cells in a dose- and time-dependent way.
Every non-specific acute inflammatory response is characterized by a rapid influx of neutrophils followed directly by a monocyte influx [28]. Neutrophil and monocyte numbers return to normal levels after the invading microorganisms have been eliminated. The pathways through which these processes are regulated are not yet fully understood. From previous studies it can be concluded that chemokines are important mediators in these processes [1–5]. In peritoneal dialysis effluent from patients suffering from bacterial peritonitis IL-8 was shown to have a major chemoattractive activity for neutrophils. However, the chemoattractive activity for neutrophils of this effluent could not be fully blocked by a neutralizing antibody against IL-8 [29]. The leucocyte influx into an area of inflammation is not regulated by just one or two chemokines, but it is regulated by the collaboration between several different pro- and anti-inflammatory cytokines, of which chemokines are an important part.
Our data show that mRNA expression and protein production of chemokines by mesothelial cells in response to proinflammatory stimuli is a differentially regulated and complex process. When mesothelial cells responded to the single stimuli (IL-1β, TNF-α or IFN-γ) by inducing chemokine mRNA or protein, this was always in a dose-dependent manner (Fig. 1 and Tables 1,2 and 3). The highest mRNA expression level in time was dependent on the nature of the stimulus and the responding chemokine. From these differential data it seems that MCP-1 is the chemokine that is most easily induced and peaks relatively early, suggesting a regulatory role of this chemokine early in an inflammatory response. It is also the only chemokine that shows constitutive mRNA expression (Fig. 1c) and protein production (Tables 1,2 and 3). This is in accordance with the findings in the clinical situation [8], where MCP-1 was found to be present in the peritonitis as well as in the stable dialysis effluents. Also, the marginal RANTES mRNA expression and protein production (Tables 1,2 and 3, 3.8 ng/ml maximum) despite proinflammatory (co)stimulation of mesothelial cells is in agreement with the in vivo findings, where no RANTES was detected in any of the effluents.
Not only the dose of the stimulating cytokine and the time after stimulation, but also the combination of stimuli influence the level and time of expression and production of the chemokines. All combinations of stimuli lead to a clear synergistic up-regulation of MCP-1 protein production. This was not clear at the mRNA level (Fig. 2c), from which it can be concluded that post-transcriptional or post-translational processes like mRNA and/or protein stabilization are important here. IFN-γ in combination with IL-1β and TNF-α also induced a synergistic response in IP-10 production, which was in line with the increased mRNA expression at 4 h (Fig. 2b). This phenomenon could not be observed for huGRO mRNA or protein production, and only to a small degree for RANTES protein production. With respect to huGRO, the fact that only huGRO-α protein was measured, while at the mRNA level expression of the complete huGRO gene was detected, may have influenced this finding.
In addition to the earlier study by Tekstra et al. [8], in which the relevance of IL-8, huGRO-α, MCP-1 and RANTES was discussed, we analysed the IP-10 concentration in the effluents of seven peritonitis episodes in five patients. The levels of this chemokine increased significantly during peritonitis, and as shown in our data mesothelial cells are very potent producers of IP-10. Therefore, IP-10 may also be an important chemokine in the peritoneal cavity during inflammation.
IL-1β and TNF-α are definitely present during peritonitis and are probably produced by peritoneal macrophages [30,31]. IFN-γ is most likely produced by invading T lymphocytes [32,33]. These stimuli, in combination with the direct interaction between mesothelial cells and microorganisms [7], probably induce this specific chemokine production pattern by the mesothelial cells in vivo, while in inflammations of different origin, other chemokine patterns will be involved [34].
In which way these five chemokines, IL-8 included, co-operate to achieve cell influx into the peritoneal cavity is not yet clear. The distinct difference in time of incubation to reach peak expression might indicate that the successive occurrence of maximum expression and production of chemokines contribute to a distinct pattern of cell influx.
The hypothesis that mesothelial cells influence cell recruitment during peritonitis was supported by a study by Hagmolen Often Have et al. [35], in which the number of invading cells in a case of peritonitis positively correlated with mesothelial cell mass in the stable situation, as determined by CA125, which is a marker for mesothelial cell mass [25]. This indicates that loss of mesothelial cells will lead to less cell influx. This might be the result of diminished chemokine production. This, in turn, is in concordance with the hypothesis that mesothelial cells are the main producers of chemokines in the peritoneal cavity. Further studies, in particular in the animal model, may prove this hypothesis right.
Acknowledgments
The authors would like to thank Professor R. Sager (Dana-Farber Cancer Institute, Boston) for kindly providing the probe specific for huGRO, Professor A. B. Gottlieb (Rockefeller University, New York) for providing the IP-10-specific probe, Professor V. M. Dixit (Department of Pathology, University of Michigan School of Medicine, Ann Arbor) for providing the MCP-1-specific and the β-actin-specific probes, and Dr A. Krensky (Stanford University Medical Center, Stanford, CA) for providing the RANTES-specific probe. The authors would like to acknowledge Mr S. Paniry for technical assistance and Dr D. O'Brien for correction of the English.
References
- 1.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 2.Strieter RM, Koch AE, Antony VB, et al. The immunopathology of chemotactic cytokines: the role of interleukin-8 and monocyte chemoattractant protein-1. J Lab Clin Med. 1994;123:183–97. [PubMed] [Google Scholar]
- 3.Prieschl EE, Kulmberg PA, Baumruker T. The nomenclature of chemokines. Int Arch Allergy Immunol. 1995;107:475–83. doi: 10.1159/000237089. [DOI] [PubMed] [Google Scholar]
- 4.Adams DH, Lloyd AR. Chemokines: leucocyte recruitment and activation cytokines. Lancet. 1997;349:490–5. doi: 10.1016/s0140-6736(96)07524-1. [DOI] [PubMed] [Google Scholar]
- 5.Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane bound chemokine with a CX3C motif. Nature. 1997;385:640–4. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 6.Walz A, Meloni F, Clark-Lewis I, et al. [Ca2+]i changes and respiratory burst in human neutrophils and monocytes induced by NAP-1/interleukin-8, NAP-2, and gro/MGSA. J Leuk Biol. 1991;50:279–86. doi: 10.1002/jlb.50.3.279. [DOI] [PubMed] [Google Scholar]
- 7.Visser CE, Brouwer-Steenbergen JJE, Betjes MGH, et al. IL-8 production by human mesothelial cells after direct stimulation with Staphylococci. Infect Immun. 1995;63:4206–9. doi: 10.1128/iai.63.10.4206-4209.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tekstra J, Visser CE, Tuk CW, et al. Identification of the major chemokines that regulate cell influxes in peritoneal dialysis patients. J Am Soc Nephrol. 1996;7:2379–84. doi: 10.1681/ASN.V7112379. [DOI] [PubMed] [Google Scholar]
- 9.Gokal R. Recent perspectives on peritoneal dialysis. Cont Nephr. 1993;102:98–109. doi: 10.1159/000421917. [DOI] [PubMed] [Google Scholar]
- 10. 121:62–70.
- 11.Betjes MGH, Tuk CW, Struijk DG, et al. Interleukin-8 production by human peritoneal mesothelial cells in response to tumor necrosis factor-α, interleukin-1, and medium conditioned by macrophages co-cultured with Staphylococcus epidermidis. J Infect Dis. 1993;168:1202–10. doi: 10.1093/infdis/168.5.1202. [DOI] [PubMed] [Google Scholar]
- 12.Goodman RB, Wood RG, Martin ThR, et al. Cytokine-stimulated human mesothelial cells produce chemotactic activity for neutrophils including NAP-1/IL-8. J Immunol. 1992;148:457–65. [PubMed] [Google Scholar]
- 13.Topley N, Brown Z, Jörres A, et al. Human peritoneal mesothelial cells synthesize interleukin-8. Synergistic induction by interleukin-1β and tumor necrosis factor-α. Am J Pathol. 1993;142:1876–86. [PMC free article] [PubMed] [Google Scholar]
- 14.Topley N, Jörres A, Luttmann W, et al. Human peritoneal mesothelial cells synthesize interleukin-6: induction by IL1β and TNFα. Kidney Int. 1993;43:226–33. doi: 10.1038/ki.1993.36. [DOI] [PubMed] [Google Scholar]
- 15.Jonjic N, Peri G, Bernasconi S, et al. Expression of adhesion molecules and chemotactic cytokines in cultured human mesothelial cells. J Exp Med. 1992;176:1165–74. doi: 10.1084/jem.176.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanfrancone L, Boraschi D, Ghiara P, et al. Human peritoneal mesothelial cells produce many cytokines (granulocyte-colony-stimulating factor (CSF), granulocyte-monocyte-CSF, macrophage-CSF, interleukin-1 (IL-1), and IL-6) and are activated and stimulated to grow by IL-1. Blood. 1992;80:2835–42. [PubMed] [Google Scholar]
- 17.Dobbie JW. New concepts in molecular and ultrastructural pathology of the peritoneum: their significance for peritoneal dialysis. Am J Kidney Dis. 1990;15:97–109. doi: 10.1016/s0272-6386(12)80506-3. [DOI] [PubMed] [Google Scholar]
- 18.Betjes MHG, Bos HJ, Krediet RT, Arisz L. The mesothelial cells in CAPD effluent and their relation to peritonitis incidence. Perit Dial Int. 1991;11:22–26. [PubMed] [Google Scholar]
- 19.Wuyts A, Proost P, Put W, et al. Leukocyte recruitment by monocyte chemotactic proteins (MCPs) secreted by human phagocytes. J. Immunol Methods. 1994;174:237–47. doi: 10.1016/0022-1759(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 20.von Luettichau I, Nelson PJ, Pattison JM, et al. RANTES chemokine expression in diseased and normal human tissues. Cytokine. 1996;8:89–98. doi: 10.1006/cyto.1996.0012. [DOI] [PubMed] [Google Scholar]
- 21.Taub DD, Lloyd AR, Conlon K, et al. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–14. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stylianou E, Jenner LA, Davies M, et al. Isolation, culture and characterization of human peritoneal mesothelial cells. Kidney Int. 1990;37:1563–70. doi: 10.1038/ki.1990.150. [DOI] [PubMed] [Google Scholar]
- 23.Niedbala MJ, Crickard K, Bernacki RJ. Adhesion, growth and morphology of human mesothelial cells on extracellular matrix. J Cell Sci. 1986;85:133–47. doi: 10.1242/jcs.85.1.133. [DOI] [PubMed] [Google Scholar]
- 24.Wu YJ, Parker LM, Binder NE, et al. The mesothelial keratins: a new family of cytoskeletal proteins identified in cultured mesothelial cells and nonkeratinizing epithelia. Cell. 1982;31:693–703. doi: 10.1016/0092-8674(82)90324-5. [DOI] [PubMed] [Google Scholar]
- 25.Visser CE, Brouwer-Steenbergen JJE, Betjes MGH, et al. Cancer antigen 125: a bulk marker for the mesothelial mass in stable peritoneal dialysis patients. Nephrol Dial Transplant. 1995;10:64–69. [PubMed] [Google Scholar]
- 26.Pronk A, Leguit P, Hoynck van Papendrecht Aagm, et al. A cobblestone cell isolated from the human omentum: the mesothelial cell; isolation, identification, and growth characteristics. In Vitro Cell Dev Biol. 1993;29A:127–34. doi: 10.1007/BF02630943. [DOI] [PubMed] [Google Scholar]
- 27.Peri G, Milanese C, Mateucci C, et al. A new monoclonal antibody (5D3-F7): which recognizes human monocyte-chemotactic protein-1 but not related chemokines. Development of a sandwich ELISA and in situ detection of producing cells. J Immunol Methods. 1994;174:249–57. doi: 10.1016/0022-1759(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 28.Zemel D, Krediet RT, Koomen GCM, et al. Interleukin-8 during peritonitis in patients treated with CAPD; an in vivo model of acute inflammation. Nephrol Dial Transplant. 1994;9:169–74. [PubMed] [Google Scholar]
- 29.Betjes MGH, Visser CE, Zemel D, et al. Intraperitoneal interleukin-8 and neutrophil influx in the initial phase of a CAPD peritonitis. Perit Dial Int. 1996;16:385–92. [PubMed] [Google Scholar]
- 30.Betjes MGH, Tuk CW, Visser CE, et al. Analysis of the peritoneal cellular immune system during CAPD shortly before a clinical peritonitis. Nephrol Dial Transplant. 1994;9:684–92. doi: 10.1093/ndt/9.6.684. [DOI] [PubMed] [Google Scholar]
- 31.Riches DWH, Chan ED, Winston BW. TNF-α induced regulation and signalling in macrophages. Immunobiol. 1996;195:477–90. doi: 10.1016/s0171-2985(96)80017-9. [DOI] [PubMed] [Google Scholar]
- 32.Dasgupta MK, Larabie M, Halloran PF. Interferon-gamma levels in peritoneal dialysis effluents: relation to peritonitis. Kidney Int. 1994;46:475–81. doi: 10.1038/ki.1994.297. [DOI] [PubMed] [Google Scholar]
- 33.O'Garra A, Murphy K. Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol. 1994;6:458–66. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 34.Sprenger H, Meyer RG, Kaufmann A, Bussfeld D, Rischkowsky E, Gemsa D. Selective induction of monocyte and not neutrophil-attracting chemokines after influenza A virus infection. J Exp Med. 1996;184:1191–6. doi: 10.1084/jem.184.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hagmolen Often Have W, Ho-dac-Pannekeet MM, Struijk DG, et al. Mesothelial regeneration after peritonitis in peritoneal dialysis patients. Abstract of the 8th Benelux Dialyse Symposium; 1997; Brussels. [Google Scholar]


