Abstract
ANCA with specificity for myeloperoxidase (MPO) and proteinase 3 (PR3) are present in patients with systemic vasculitis. The aim of this work was to determine whether such patients have T cell responses to these antigens and whether these responses are related to disease activity. Peripheral blood lymphocytes from 45 patients and 19 controls were cultured with ANCA antigens and proliferation measured. The antigens used were heat-inactivated (HI) MPO, HI PR3, native (non-HI) PR3, HI whole α-granules, and 25 overlapping peptides covering the entire PR3 sequence. Significant responses to both whole PR3 preparations were seen from patient and control groups, and to the α-granules from the patient group. Patients responded at all stages of disease: active, remitting, treated or untreated. Only two patients responded significantly to MPO. Responses were significantly higher with the patient group than the control group to all four whole ANCA antigens. Responses to those PR3 peptides containing epitopes known to be recognized by ANCA were detected from one patient. Thus, these studies demonstrate that T cells from vasculitis patients can proliferate to PR3 and occasionally to associated ANCA antigens. Further, responses may persist even after disease remission has been achieved.
Keywords: T cells, proteinase 3, Wegener's granulomatosis, ANCA, systemic vasculitis
INTRODUCTION
ANCA are now an established marker for certain subgroups of patients with systemic vasculitis. The commonest ANCA-associated vasculitides comprise Wegener's granulomatosis (WG) and microscopic polyangiitis (MPA), including its renal-limited variant idiopathic rapidly progressive glomerulonephritis. Two major antibody specificities have emerged. The first, proteinase 3 (PR3)-ANCA gives a cytoplasmic staining pattern (C-ANCA) on ethanol-fixed neutrophils when viewed using indirect immunofluorescence; PR3-ANCA are found in the majority of patients with WG and approximately half of those with MPA. The second, myeloperoxidase (MPO)-ANCA gives a perinuclear staining pattern (P-ANCA) on ethanol-fixed neutrophils; these antibodies account for the ANCA specificities of most of the remaining MPA or WG patients (reviewed in [1]).
ANCA antibodies have the potential to induce direct pathogenic effects. In vitro studies have shown that ANCA can activate cytokine pretreated or ‘primed’ neutrophils to release reactive oxygen species and lysosomal enzymes [2]. Priming of neutrophils can be done using tumour necrosis factor-alpha (TNF-α), as may occur in vivo in the context of infection. This priming results in the increased expression of PR3 and MPO at the cell surface [3]. ANCA-activated, primed neutrophils have been shown to be cytotoxic towards cultured endothelial cells in vitro [4].
There is considerable circumstantial evidence to implicate T cells in ANCA-associated vasculitis. The granulomatous lesions of WG comprise lymphocytes and cells of the myelomonocyte lineage [5] and may be indicative of T cell hyperactivity. ANCA tend to be high-affinity IgG antibodies [6] which recognize a highly restricted epitope repertoire [7]. These observations would suggest repeated antigen stimulation (in a T cell response) driving T cell-mediated help for B cell antibody production. In addition, CD4+ T cells are present in renal biopsies [8] and the number of T cells in the renal interstitium correlates with renal function in patients with rapidly progressive glomerulonephritis [9]. Activated (CD25+) T cells have been found in cellular crescents of rapidly progressive glomerulonephritis and their numbers are increased in the peripheral blood of WG patients [10]. Serum levels of soluble IL-2 receptor, presumably derived from activated T cells, have also been shown to correlate with disease activity [11]. The persistence of the soluble IL-2 receptor in WG patients even during complete remission may be indicative of continuing background T cell activity [11]. Further, T cell-directed treatment such as cyclosporin A [12,13] and monoclonal antibody therapy [14,15] may have therapeutic benefit in some patients.
T cell reactivity to PR3 or MPO has been sought in previous studies using peripheral blood T cells from patients with systemic vasculitis [16–21]. However, only small numbers of patients with acute disease have been studied and responses to PR3 and MPO have often been relatively weak or absent [18–20]. This study has taken a large group of patients at various stages of disease and examines responses of peripheral blood T cells to a range of ANCA preparations. There is clear evidence that although normal individuals or disease controls may respond to the antigens, the responses from patients are greater and tend to persist with time even when disease is in remission.
MATERIALS AND METHODS
Patients and controls (see Tables 1 and 2)
Table 1.
Patient treatment at time of sampling
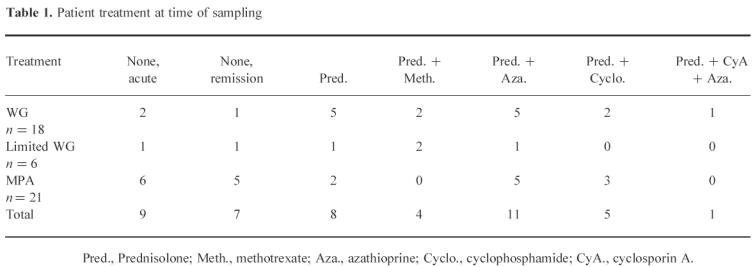
Table 2.
Patient antibody specificity: where the antibody specificity is unknown, the ANCA type has been stated
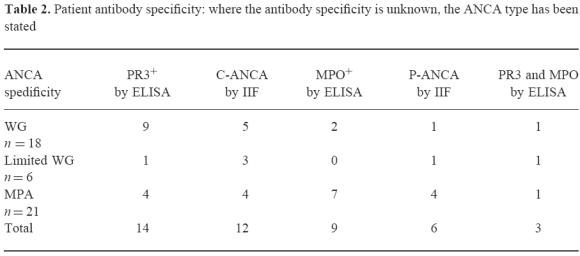
Patients with WG or MPA as described by the Chapel Hill definitions [22] were selected for study. There were 18 patients with generalized WG that included renal involvement (13 male, five female, mean age 53.2 years), six patients with limited WG which was confined to the upper and lower respiratory tract (two male, four female, mean age 50 years) and 21 patients with MPA (12 male, nine female, mean age 62 years). Sixteen patients were not on any therapy at the time of sampling (nine acute patients and seven in remission), while the others were on varying doses of prednisolone, cyclophosphamide, azathioprine or methotrexate depending on their individual stages of disease [23] (see Table 1). Disease activity was assessed for all patients at the time of sampling using the Birmingham Vasculitis Activity Score (BVAS), a validated scoring index [24]. The ANCA antigen specificity was determined for the majority of patients studied. This was done by ELISA using PR3 purified using the same method as Baslund et al. [25] and MPO (Calbiochem, La Jolla, CA). For patients who were in long-term remission and ANCA− at the time of assaying, only the result of the initial C-ANCA/P-ANCA staining pattern by indirect immunofluorescence was available (Table 2).
Controls comprised 10 healthy individuals who had not been exposed to ANCA antigens in the laboratory (six male, four female, mean age 49 years) and nine IgA nephropathy (IgAN) patients without evidence of coincident vasculitis on renal biopsy and who were on no cytotoxic therapy (five male, four female, mean age 49.5 years). Renal function in the IgAN individuals was normal or minimally impaired. All IgAN individuals were ANCA−.
Antigens
Control antigens
Purified protein derivative (PPD) of Mycobacterium tuberculosis (Seruminstitut, Copenhagen, Denmark) was used at a concentration of 10 μg/ml. Phytohaemagglutinin (PHA; Murex, Dartford, UK) was used at a concentration of 1 μg/ml for toxicity studies.
PR3
PR3 was purified from an adaptation of the method previously described by Baslund et al. [25]. Briefly, buffy coats from the Blood Transfusion Service (Birmingham, UK) were mixed with an equal volume of 20% T500 Dextran (Pharmacia, Uppsala, Sweden) for 1 h to sediment erythrocytes. The neutrophils and remaining erythrocytes were separated from the mononuclear cells by Ficoll–Hypaque (Pharmacia) and the remaining erythrocytes were then removed by hypotonic lysis with water followed by centrifugation. The resultant neutrophils were disrupted by nitrogen bomb cavitation and the nuclei removed by centrifugation. The cytoplasmic material was then separated using a Percoll gradient and the α-granule fraction removed. The Percoll was removed from α-granules by ultracentrifugation (100 000 g). The α-granules were lysed by freeze-thawing seven times followed by sonication (2 × 10 s bursts at 10 μm on ice) and the lipid membrane removed by ultracentrifugation (2 × 100 000 g). The negatively charged proteins were removed from this α-granule preparation by DEAE ion exchange chromatography using DEAE-Sepharose Fast Flow (Pharmacia). The purity of the PR3 preparation was assessed by ELISA and SDS–PAGE chromatography which was visualized using a silver stain kit (Pharmacia; Fig. 1). The ELISA demonstrated presence of PR3 with some MPO contamination but no elastase, lactoferrin, lysozyme or cathepsin G. SDS–PAGE confirmed this, showing the triplet of bands expected for PR3 at around 29 kD as well as some MPO at 113 kD. The protein contents of the PR3 and α-granule preparations were determined using the Micro-BCA assay kit (Pierce, Rockford, IL). Native PR3 and PR3 which had been heat-inactivated at 100°C for 15 min to remove enzymatic activity were both used at 10 μg/ml; as T cells recognize linear epitopes and do not rely on the conformation of the antigen to elicit a response, heat inactivation should not reduce the antigenicity of the protein. Although greater responses were seen with 40 μg/ml of heat-inactivated (HI) PR3, 10 μg/ml was chosen to preserve antigen stocks (Fig. 2). Neither form of PR3 effected a PHA response when cocultured, thereby excluding major toxicity.
Fig. 1.
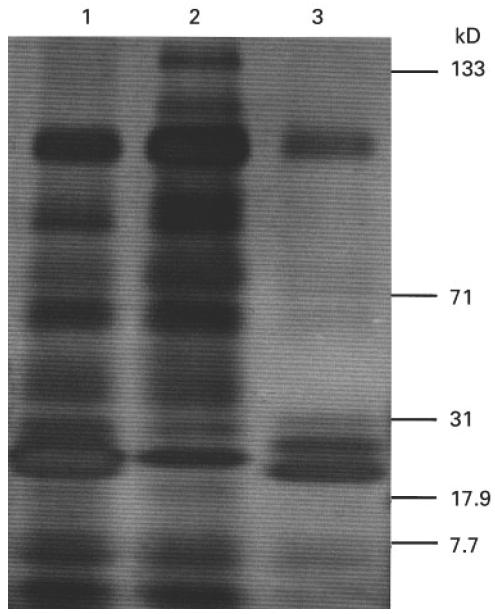
Silver-stained SDS–PAGE gel of proteinase 3 (PR3). Lane 1, whole α-granules; lane 2, DEAE-bound fraction (10 μg); lane 3, PR3 (10 μg).
Fig. 2.
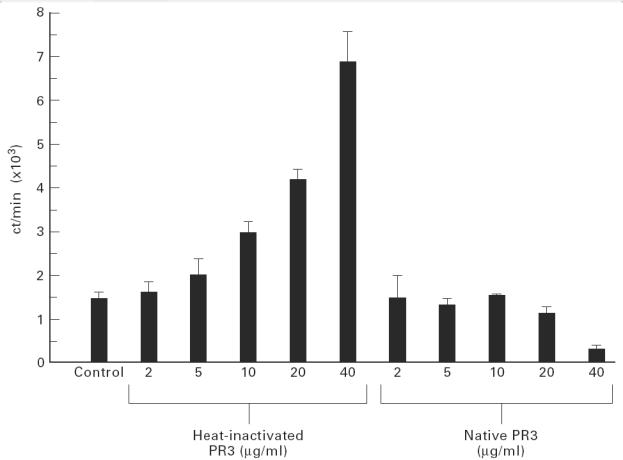
Dose response from a single patient to both heat-inactivated and native proteinase 3 (PR3): expressed as mean ct/min (of three wells) + 1 s.d. from the mean.
MPO
Heat-inactivated MPO (Calbiochem) was used at 10 μg/ml, and at this dose did not reduce responses to PHA. The native MPO was not used, as in other studies it was found to reduce PPD responses by up to 35%. We were aware of previous reports suggesting that commercial MPO may be contaminated by lactoferrin. In our hands the MPO was free of contaminating PR3, elastase, lactoferrin, lysozyme and cathepsin G by ELISA.
Alpha granules
Heat-inactivated α-granules were used at 25 μg/ml and were non-toxic to a PHA response.
PR3 peptides
Twenty-five 16mer peptides were synthesized using a BT7400 peptide synthesizer (Alta Bioscience, Birmingham, UK). These peptides were designed to cover the entire sequence of PR3, each peptide having a 6mer overlap with its adjacent peptides. The PR3 peptides were used either singly or in pools of five (1–5, 6–10, 11–15, 16–20 and 21–25) or in pools of two (1 + 2, 3 + 4, 5 + 6, 7 + 8, 9 + 10, 11 + 12, 13 + 14, 15 + 16, 17 + 18, 19 + 20, 21 + 22, 23 + 24 and 25) at concentrations of either 3 or 10 μg/ml. The peptides were dissolved in DMSO to a final concentration of not more than 0.05% in the well, a concentration of DMSO which was non-toxic to PHA responses. DMSO controls were included in all proliferation studies with peptides.
T cell proliferation assays
PBMC were isolated from heparinized blood by density gradient centrifugation on Ficoll–Hypaque and the cells washed three times. The cells were then cultured at 2 × 105 cells/well in round-bottomed 96-well plates (Becton Dickinson, Cowley, UK) with or without antigen, suspended in 200 μl of RPMI 1640 medium (Sigma, Poole, UK) containing 5% heat-inactivated male AB+ human serum (Blood Transfusion Centre), 1% non-essential amino acids (MEM; Sigma), 1% sodium pyruvate (Sigma), 15 mm HEPES buffer (Sigma), 2.5 mm glutamine (Sigma), 100 U/ml penicillin (Sigma), 100 μg/ml streptomycin (Sigma). All assays with antigen were carried out in at least triplicate. Proliferative responses were measured at day 5 for PPD, and at days 5, 8 and 10 for the test antigens. In a number of patient and control assays, IL-2 was added to boost proliferative responses. In such assays, IL-2 was added to give a final volume of 20 U/ml at day 7 and the proliferative responses were measured at day 10. Tritiated thymidine (Amersham, Aylesbury, UK) was added at a final concentration of 0.15 μCi/well for the final 16 h of culture. Cells were then harvested onto glassfibre filters using an automated cell harvester (Skatron Ltd, Newmarket, UK). Thymidine incorporation was measured using an automated beta counter (Pharmacia). Results are presented as both (i) mean ct/min + s.d. and as (ii) stimulation indices (SI) which correspond to the proliferative response in the presence of an antigen divided by the proliferative response by cells only (without antigen).
Statistical analysis
Any T cell assay which did not show a response to PPD with an SI of ≥ 5 was not included in the statistical analysis. Using this criterion seven patients and two controls were eliminated from the analysis. This step was undertaken because a number of acute patients who were on the Intensive Therapy Unit at the time of bleeding did not respond to PPD; none of these patients responded to ANCA antigens either.
The significance of a response to an individual antigen was assessed using analysis of variance allowing for variation between individual patient/control samples, and between different time points. Repeated-measures analysis of variance was carried out on the SIs to assess the difference between the responses of the patient and control groups at different times. Analysis of covariance was used to assess the effect of age, diagnosis, antibody specificity and therapy on proliferative responses.
RESULTS
Proliferative responses to control antigens
All individuals used in this study responded to PPD, indicating that T cells were viable and capable of responding to recall antigens. The control group responded significantly better to PPD than the patient group (P < 0.001): control group (mean ± s.e.m.) 36.9 ± 7.25; patient group 30.8 ± 4.35.
Proliferative responses to ANCA antigens
A typical example of an individual patient response to ANCA antigens is shown in Fig. 3. This patient responded to both native and HI PR3 and to the HI α-granule preparation, but did not respond to HI MPO. These responses reached a maximum at days 10–11. This pattern of response was seen in most patients, but some patients showed peak responses at days 5–6, therefore it was necessary to analyse the response at a range of time points.
Fig. 3.
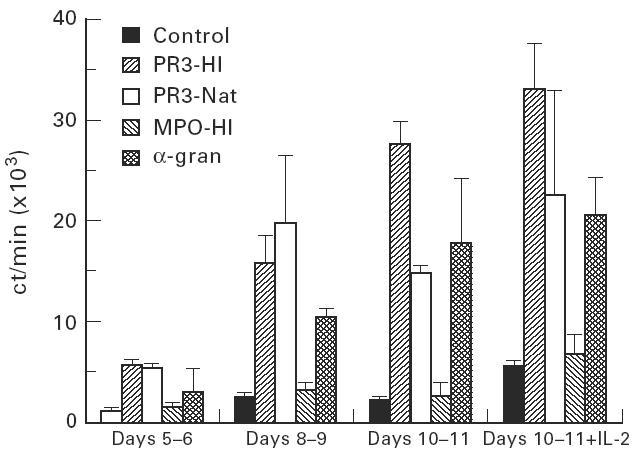
Peripheral blood mononuclear cell (PBMC) proliferative responses to ANCA antigens from a single patient: expressed as mean ct/min (of three wells) + 1 s.d. from the mean.
Although higher responses were seen in the presence of IL-2, the addition of IL-2 also increased the background and the variability within the assay. Since good responses could be achieved without the addition of IL-2, it was omitted in later assays.
Including data from all three time points and the IL-2 data, highly significant responses (P < 0.001) were seen to the HI PR3 preparation by both the patient and control groups (Fig. 4a). For example, the mean SI for the patient group at days 10–11 was 5.76 and that of the control group was 2.76. The patient group as a whole showed significantly higher responses to HI PR3 than the control group (P < 0.001).
Fig. 4.
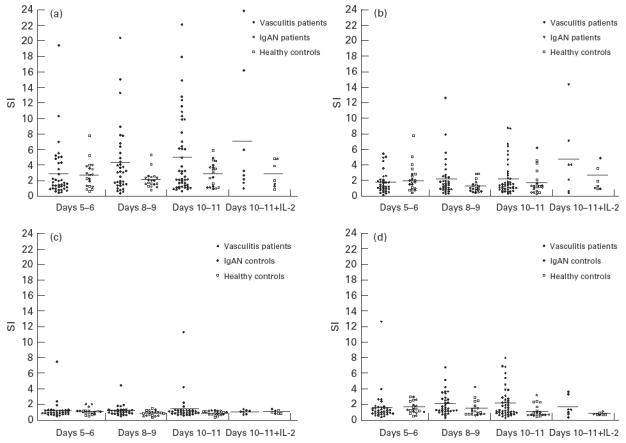
Peripheral blood mononuclear cell (PBMC) proliferative responses to ANCA antigens by ANCA+ vasculitis patients and controls (IgA nephropathy (IgAN) patients and normal controls). Proliferative responses are expressed as stimulation indices (SI), an SI = 1 is equivalent to no response, the bars represent the mean response. (a) PBMC proliferative responses to 10 μg/ml of heat-inactivated (HI) proteinase 3 (PR3). (b) PBMC proliferative responses to 10 μg/ml native PR3. (c) PBMC proliferative responses to 10 μg/ml HI myeloperoxidase (MPO). (d) PBMC proliferative responses to 25 μg/ml HI whole α-granules.
Significant responses were also seen with the native PR3 preparation, P < 0.001 for the patient group and P < 0.01 for the control group (Fig. 4b). The mean SI for the patient group at days 10–11 was 2.22 and for the control group it was 1.77. Responses to native PR3 were nearly always lower than the corresponding response to the heat-inactivated form of PR3. The patient group showed significantly higher responses than the control group to native PR3 (P < 0.01).
Responses to the HI MPO were low and failed to reach significance in either the patient or the control groups (Fig. 4c). Two patients (A and B) responded to the MPO preparation with an SI > 5 (patient A had SI = 1.16 at days 5–6, 4.47 at days 8–9 and 11.36 at days 10–11; patient B had SI = 7.45 at days 5–6 and 4.22 at days 10–11). Patient A also responded to HI PR3 (SI = 10 at days 10–11) and both responded to the α-granule preparation (SI = 8 for patient A and 12 for patient B at days 10–11). Both these patients had C-ANCA antibodies specific for PR3.
Responses to the HI α-granules by the patient group reached significance (P < 0.01), whereas those of the control group failed to reach significant levels (Fig. 4d). Responses to HI α-granules by the patient group were significantly higher than the control group (P < 0.001).
In initial experiments, the 25 PR3 peptides were pooled into five pools of 5. Twenty-four patients and 15 healthy laboratory workers were tested for responses to these five pools at days 5–6 at concentrations of 2 and 10 μg/ml. No significant responses with an SI > 3.5 were seen to any of the PR3 peptide pools (data not shown). In later experiments, it was shown that the optimal time point for responses to the whole PR3 antigen occurred at days 10–11. Therefore, a further 18 patients were tested for responses to the PR3 peptides either singularly or in pools of 2 at days 10–11 at a concentration of 10 μg/ml. Six of these patients responded to PR3 with an SI > 5 (SI of between 5.16 and 22.02), but only one of these showed any response to the PR3 peptides (Fig. 5) (SI whole, HI PR3 = 5.98; response to peptide 1 + 2 = 3.37, 17 + 18 = 3.23 and 23 + 24 = 4.18). The other five PR3-responsive patients responded well to the whole antigen, but did not respond to individual peptides.
Fig. 5.
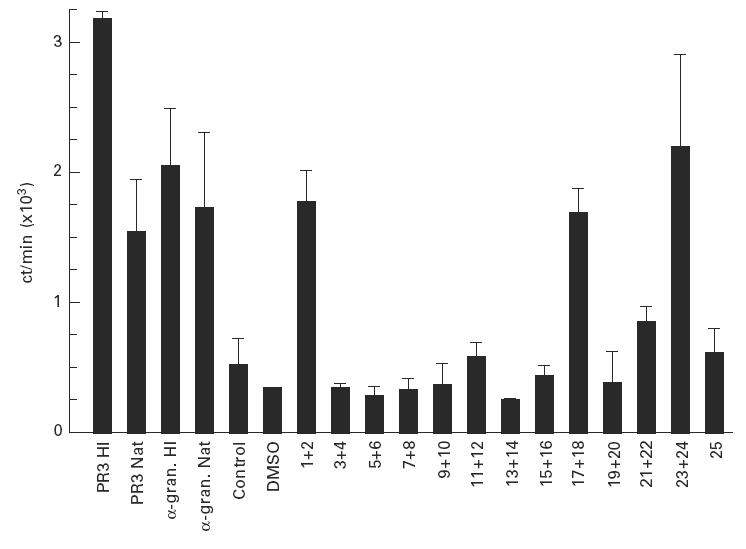
Peripheral blood mononuclear cell (PBMC) proliferative responses to ANCA antigens and proteinase 3 (PR3) peptides from a single patient assayed at days 10–11. Proliferative responses are expressed as the mean ct/min (of three assays) + 1 s.d., peptides were assayed in pools of two at a final concentration of 10 μg/ml of each peptide, control responses were measured in the presence of DMSO.
Effect of age, diagnosis, antibody specificity, therapy and disease activity on T cell responses
There was no significant effect of age on any antigen responses.
Patients with anti-PR3/C-ANCA antibodies responded better to HI PR3 than patients with anti-MPO/P-ANCA antibodies (P = 0.014), although patients with antibodies to MPO/P-ANCA were still able to respond to HI PR3. We did not test for a relation between autoantibody titre and proliferative response.
Patients diagnosed with either WG or limited WG responded significantly better to HI PR3 than patients diagnosed with MPA (P < 0.001), perhaps due to the increased frequency of positivity for anti-PR3/C-ANCA antibodies in the WG group. There were no significant differences between responses to the other ANCA antigens with respect to diagnosis.
In general, the therapy a patient was on at the time of bleeding affected T cell responses to both ANCA antigens and to PPD. Thus, responses to PPD by patients being treated with cyclophosphamide were decreased significantly. However, this did not preclude those vasculitic patients being treated with cyclophosphamide, and to a lesser extent those treated with azathioprine, from responding significantly better to HI PR3 when compared with other treated or untreated patient groups (P < 0.001 and P < 0.05, respectively). It was also noticeable that acute untreated patients responded well to HI PR3, but this did not differ significantly from other groups of patients who had received treatment or untreated patients in remission.
There was no correlation between disease activity, measured using the BVAS scoring system, and T cell proliferation to any of the ANCA antigens and PPD.
DISCUSSION
Autoantibody responses to the neutrophil enzymes PR3 and MPO are present in the small vessel vasculitic diseases WG and MPA. This is the first time T cell responses to these antigens have been investigated from a large panel of patients at different stages of disease and receiving a variety of treatment regimes. Highly significant responses were seen to PR3 from the patient group as a whole and responses to all ANCA antigens were significantly higher in the patient group compared with controls. Proliferative responses to PR3 were seen from vasculitis patients irrespective of disease activity and inclusive of patients in remission.
Several studies have previously been undertaken to examine the potential for peripheral blood T cells, obtained from patients with ANCA-associated vasculitis, to respond to putative neutrophil autoantigens [16–21]. Initial studies were promising, suggesting T cell proliferative responses to neutrophil antigens were present in patients and not controls [16,17]. However, patient numbers were small and the next report showed little or no difference between patients and controls, although this may have reflected the crude nature of the antigen preparation used [18]. Apparent responses from T cells derived from vasculitic patients to purified PR3 used at a single concentration were observed by Brouwer et al. [19], but a critical review of this and a subsequent study [20] suggested inappropriate use of statistical tests and concluded that there was no evidence for peripheral blood T cell responses to neutrophil antigens in patients with systemic vasculitis [26]. A further problem in some of these studies which may have led to low specific T cell responses was the use of detergents during the isolation and purification of PR3 [19,20]. This problem was circumvented in the next published study using purified PR3 or MPO; significant responses were observed with acute MPO-ANCA+ patients to MPO and with acute PR3-ANCA+ patients to PR3 compared with normal or disease controls [21]. Despite the statistically significant responses, responses to both antigens were seen in a proportion of healthy controls. Patients were tested only during the acute stage of the disease.
The present study did not use detergents during the purification of PR3 and so avoided the possible problems encountered previously [19,20]. Although our preparation of PR3 is not as pure as others as it did contain some MPO, it was available in relatively large quantities, allowing us to assay a large panel of vasculitis patients and controls. We have confirmed that patients with vasculitis respond to neutrophil antigens, but have also observed, as did Griffith et al. [21], that healthy individuals respond to preparations of PR3. These responses, although significant, were not as high as those seen with the vasculitis patient group. T cell responses from healthy individuals have been seen for other autoantigens such as myelin basic protein [27,28], proteolipid protein [27] and thyroid-stimulating hormone [29]. They may reflect normal responses from T cells that have previously encountered this self-antigen which, under in vivo conditions, are under regulatory control.
Higher responses were seen with the HI PR3 compared with the corresponding native preparation. There are at least three possible explanations for this. First, the enzymatic activity of PR3 could have a cytotoxic or apoptotic effect on the peripheral blood mononuclear cells (PBMC)—PR3 has previously been shown to cause apoptosis to endothelial cells [30]; this enzyme activity may be destroyed by heat inactivation. Second, an otherwise cryptic (hidden) epitope of PR3 may be revealed by the heat-inactivation process and presented to T cells by antigen-presenting cells [31]. Third, the heat-inactivation process causes precipitation of this antigen. The precipitated form of PR3 may be taken up by phagocytic macrophages and presented to T cells more readily than the native (soluble) form [32].
The lack of significant responses to MPO (in both patient and control groups) may reflect a true inability of T cells to respond to MPO. On the other hand, responses to the native form of MPO have been seen by others [21], and indeed, significant responses were seen in occasional patients in this study, suggesting that heat-inactivated MPO is antigenic. Lack of responses to MPO also suggest that the responses seen to HI PR3 and native PR3 preparations, themselves known to contain some MPO contamination, were in fact predominantly directed towards PR3 (only one patient responding to PR3 preparations also responded to MPO). Interestingly, our study suggests that the T cell proliferative response by both PR3-ANCA+ and MPO-ANCA+ vasculitis patients is predominantly directed towards PR3. Because PR3 and MPO are ‘linked molecules’ by virtue of their co-expression by phagocytes, T cells specific for PR3 may be able to provide help for MPO-specific B cells via ‘intermolecular help’ [33], a process postulated to occur in Graves' disease [34,35]. However, MPO-specific T cells may exist and provide help for MPO-specific B cells, but simply did not proliferate to MPO in our system.
Only one patient responded significantly to the PR3 peptides, despite good responses being seen by many patients to the whole antigen. Responses were seen from pools 1 + 2 (aa 1–26), 17 + 18 (aa 161–186) and 23 + 24 (aa 221–246). These three sequences contain three of the six important regions recognized by C-ANCA (aa 11–16 recognized by 5/8 C-ANCA, and aa 121–136 and 215–229 both recognized by 7/8 C-ANCA using the SPOTS system; personal communication from Professor C. Pusey, Hammersmith Hospital, London, UK). The other three sequences recognized by 7/8 C-ANCA sera but not by T cells in the current study were: aa 43–56, 121–136 and 197–210. The concurrence of recognition of these same peptides by antibodies and T cells supports the likelihood that these epitopes are major autoantigens. Studies with PR3-specific T cell lines are in progress to test this further.
In the present study T cell responses were obtained from both active and remitting patients and there was no significant correlation between disease activity (derived from the BVAS scores) and T cell responses to any of the ANCA antigens. At first this may seem surprising. However, T cell responses by patients in remission may reflect the continued tendency that these patients have to relapse. Further, the continued expression of elevated circulating soluble IL-2 receptor levels in patients in remission supports the proposition that there is on-going background T cell activity [11]. The T cells may maintain immunological memory which may trigger disease relapse at any time when the balance between immunological activity and unresponsiveness is upset.
Acknowledgments
We thank the Medical Research Council for their financial support of this work, Dr Anne Ben-Smith for reviewing the manuscript and Dr A.Wiik, his laboratory, and Anne McTiernan for their help with the PR3 purification.
Appendix 1
The PR3 peptide sequences were as follows:
1 (1–16) MAHR PPSP ALAS VLLA, 2 (11–26) ASVL LALL LSGA ARAA, 3 (21–36) GAA R AAEI VGGH EAQP, 4 (31–46) GHEA QPHS RPYM ASLQ, 5 (41–56) YMAS LQMR GNPG SHFC, 6 (51–66) PGSH FCGG TLIH PSFV, 7 (61–76) IHPS FVLT AAHC LRDI, 8 (71–86) HCLR DIPQ RLVN VVLG, 9 (81–96) VNVV LGAH NVRT QEPT, 10 (91–106) RTQE PTQQ HFSV AQVF, 11 (101–116) SVAQ VFLN NYDA ENKL, 12 (111–126) DAEN KLND I LLI QLSS, 13 (121–136) LIQL SSPA NLSA SVTS, 14 (131–146) SASV TSVQ LPQQ DQPV, 15 (141–156) QQDQ PVPH GTQC LAMG, 16 (151–166) QCLA MGWG RVGA HDPP, 17 (161–176) GAHD PPAQ VLQE LNVT, 18 (171–186) QELN VTVV TFFC RPHN, 19 (181–196) FCRP HNIC TFVP RRKA, 20 (191–206) VPRR KAGI CFGD SGGP, 21 (201–216) GDSG GPLI CDGI IQGI, 22 (211–226) GIIQ GIDS FVIW GCAT, 23 (221–236) IWGC ATRL FPDF FTRV, 24 (231–246) DFFT RVAL YVDW IRST, 25 (241–256) DWIR STLR RVEA KGPX.
References
- 1.Kallenberg CGM, Mulder AHL, Cohen Tervaert JW. Antineutrophil cytoplasmic antibodies: a still-growing class of autoantibodies in inflammatory disorders. Am J Med. 1992;93:675–82. doi: 10.1016/0002-9343(92)90202-m. [DOI] [PubMed] [Google Scholar]
- 2.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–9. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charles LA, Caldas ML, Falk RJ, Terrell RS, Jennette JC. Antibodies against granule proteins activate neutrophils in vitro. J Leuk Biol. 1991;50:539–46. doi: 10.1002/jlb.50.6.539. [DOI] [PubMed] [Google Scholar]
- 4.Savage COS, Pottinger BE, Gaskin G, Pusey CD, Pearson JD. Autoantibodies developing to myeloperoxidase and proteinase 3 in systemic vasculitis stimulate neutrophil cytotoxicity towards cultured endothelial cells. Am J Pathol. 1992;141:335–42. [PMC free article] [PubMed] [Google Scholar]
- 5.Gephardt G, Ahmad N, Tubbs R. Pulmonary vasculitis (Wegener's granulomatosis) immunohistochemical study of T and B cell markers. Am J Med. 1983;74:700–4. doi: 10.1016/0002-9343(83)91030-6. [DOI] [PubMed] [Google Scholar]
- 6.Mellbye OJ, Mollnes TE, Slettevoll Steen L. IgG subclass distribution and complement activation ability of autoantibodies to neutrophil cytoplasmic antigens. Clin Immunol Immunopathol. 1994;70:32–39. doi: 10.1006/clin.1994.1007. [DOI] [PubMed] [Google Scholar]
- 7.Bini P, Gabay JE, Teitel A, Melchior M, Zhou J, Elkon KB. ANCA in WG recognise conformational epitope(s) on Pr3. J Immunol. 1992;149:1409–15. [PubMed] [Google Scholar]
- 8.Brouwer E, Cohen Tervaert J, Weening J, et al. Immunohistology of renal biopsies in Wegener's granulomatosis (WG): clues to its pathogenesis. Kidney Int. 1991;39:1055. [Google Scholar]
- 9.Hooke D, Gee D, Atkins R. Leukocyte analysis using monoclonal antibodies in human glomerulonephritis. Kidney Int. 1987;31:961–72. doi: 10.1038/ki.1987.93. [DOI] [PubMed] [Google Scholar]
- 10.Stegeman CA, Cohen-Tervaert JW, Huitema MG, Kallenberg CGM. Serum markers of T cell activation in relapses of Wegener's granulomatosis. Clin Exp Immunol. 1993;91:415–20. doi: 10.1111/j.1365-2249.1993.tb05918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt W, Heesen C, Csernok E, Rautmann A, Gross WL. Elevated serum levels of soluble interleukin-2 receptor in patients with Wegener's granulomatosis. Arthritis Rheum. 1992;35:1088–96. doi: 10.1002/art.1780350914. [DOI] [PubMed] [Google Scholar]
- 12.Schollmeyer P, Grotz W. Cyclosporin treatment of Wegener's granulomatosis (WG) and related diseases. APMIS. 1990;98(Suppl. 19):54–55. doi: 10.1111/j.1600-0463.1990.tb05738.x. [DOI] [PubMed] [Google Scholar]
- 13.Allen N, Caldwell D, Rice J, McCullum R. Cyclosporin: a therapy for Wegener's granulomatosis. In: Gross W, editor. ANCA-associated vasculitides. London: Plenum Press; 1993. pp. 473–6. [DOI] [PubMed] [Google Scholar]
- 14.Mathieson PW, Cobbold SP, Hale G, Clark MR, Oliviera DBG, Lockwood CM, Waldmann H. Monoclonal-antibody therapy in systemic vasculitis. N Engl J Med. 1990;323:250–4. doi: 10.1056/NEJM199007263230407. [DOI] [PubMed] [Google Scholar]
- 15.Lockwood CM, Thiru S, Isaacs J, Hale G, Waldmann H. Long-term remission of intractable systemic vasculitis with monoclonal antibody therapy. Lancet. 1993;341:1620–2. doi: 10.1016/0140-6736(93)90759-a. [DOI] [PubMed] [Google Scholar]
- 16.van der Woude FJ, van Es LA, Daha MR. The role of the c-ANCA antigen in the pathogenesis of Wegener's granulomatosis. A hypothesis based on both humoral and cellular mechanisms. Neth J Med. 1990;36:169–71. [PubMed] [Google Scholar]
- 17.Peterson J, Rasmussen N, Szpirt W, Hermann E, Mayet W. T lymphocyte proliferation to neutrophil cytoplasmic antigen(s) in Wegener's granulomatosis (WG) Am J Kid Dis. 1991;18:205. (Abstr.) [Google Scholar]
- 18.Mathieson PW, Lockwood CM, Oliveira DBG. T and B cell responses to neutrophil cytoplasmic antigens in systemic vasculitis. Clin Immunol Immunopathol. 1992;63:135–41. doi: 10.1016/0090-1229(92)90005-9. [DOI] [PubMed] [Google Scholar]
- 19.Brouwer E, Stegeman CA, Huitema G, Limburg PC, Kallenberg CGM. T cell reactivity to proteinase 3 and myeloperoxidase in patients with Wegener's granulomatosis (WG) Clin Exp Immunol. 1994;98:448–53. doi: 10.1111/j.1365-2249.1994.tb05511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballieux BEPB, van der Burg SH, Hagen EC, van der Woude FJ, Melief CJM, Daha MR. Cell-mediated autoimmunity in patients with Wegener's granulomatosis (WG) Clin Exp Immunol. 1995;100:186–93. doi: 10.1111/j.1365-2249.1995.tb03651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffith ME, Coulthart A, Pusey CD. T cell responses to myeloperoxidase (MPO) and proteinase 3 (PR-3) in patients with systemic vasculitis. Clin Exp Immunol. 1996;103:253–8. doi: 10.1046/j.1365-2249.1996.d01-629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides: the proposal of an International Consensus Conference. Arthritis Rheum. 1994;37:187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 23.Savage COS, Harper L, Adu D. Primary systemic vasculitis. Lancet. 1997;349:553–8. doi: 10.1016/s0140-6736(97)80118-3. [DOI] [PubMed] [Google Scholar]
- 24.Luqmani RA, Bacon PA, Moots RJ. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotising vasculitis. Q J Med. 1994;87:671–8. [PubMed] [Google Scholar]
- 25.Baslund B, Petersen J, Permin H, Wiik A, Weislander J. Measurements of proteinase 3 and its complexes with α1-proteinase inhibitor ANCA in plasma. J Immunol Methods. 1994;175:215–25. doi: 10.1016/0022-1759(94)90364-6. [DOI] [PubMed] [Google Scholar]
- 26.Mathieson PW, Oliviera DBG. The role of cellular immunity in systemic vasculitis. Clin Exp Immunol. 1995;100:183–5. doi: 10.1111/j.1365-2249.1995.tb03650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Markovic-Plese S, Lacet B, Raus J, Weiner HL, Hafler DA. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1994;179:973–84. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liblau R, Tournier-Lasserve E, Maciazek J, et al. T cell responses to myelin basic protein in multiple sclerosis patients and healthy subjects. Eur J Immunol. 1991;21:1391–5. doi: 10.1002/eji.1830210610. [DOI] [PubMed] [Google Scholar]
- 29.Tandon N, Freeman MA, Weetman AP. T cell responses to synthetic TSH receptor peptides in Grave's disease. Clin Exp Immunol. 1992;89:468–73. doi: 10.1111/j.1365-2249.1992.tb06982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang JJ, Kettritz R, Falk RJ, Jennette JC, Gaido ML. Apoptosis of endothelial cells induced by the neutrophil serine proteases proteinase 3 and elastase. Am J Pathol. 1996;149:1617–26. [PMC free article] [PubMed] [Google Scholar]
- 31.Lanzavecchia A. How can cryptic epitopes trigger autoimmunity. J Exp Med. 1995;181:1945–8. doi: 10.1084/jem.181.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dresser DW, Mitchison NA. The mechanism of immunological paralysis. Adv Immunol. 1968;8:129–81. doi: 10.1016/s0065-2776(08)60466-6. [DOI] [PubMed] [Google Scholar]
- 33.Mitchison NA. An exact comparison between the efficiency of two and three-cell type clusters in mediating helper activity. Eur J Immunol. 1990;20:699–702. doi: 10.1002/eji.1830200335. [DOI] [PubMed] [Google Scholar]
- 34.Dayan CM, Londei M, Corcoran AE, Grubeck-Loebenstein B, James RFL, Rapoport B, Feldman M. Autoantigen recognition by thyroid-infiltrating T cells in Graves disease. Immunol. 1991;88:7415–9. doi: 10.1073/pnas.88.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullins RJ, Cohen SBA, Webb LMC, Chernajovsky Y, Dayan CM, Londei M. Identification of TSHR-specific T cells in Grave's disease thyroid using autoantigen-transfected EBV transformed B cell lines. J Clin Invest. 1995;96:30–37. doi: 10.1172/JCI118034. [DOI] [PMC free article] [PubMed] [Google Scholar]


