Abstract
This investigation was conducted to detect Fcγ receptors (FcγR) on cytokine-stimulated human endothelial cells (EC) by measuring anti-FcγR MoAb binding with an ELISA. TNF-α and IFN-γ significantly increased the expression of FcγR type II (FcγRII) and type III (FcγRIII) on aortic EC. Simultaneous treatment with both cytokines had a synergistic effect and pretreatment of EC with IFN-γ augmented the effect of TNF-α. The greatest effect was the increase (up to four-to-six-fold) in expression of FcγRII found by the simultaneous treatment of aortic EC with both cytokines. The receptors were expressed on the cell surface and showed receptor capping after incubation at 37°C. This study showed that the inflammatory cytokines TNF-α and IFN-γ enhanced low-affinity FcγR expression on human EC in vitro. The expression of FcγR may contribute to the specific localization of circulating immune complexes on blood vessels in areas of vasculitis.
Keywords: endothelial cells, Fcγ receptors, tumour necrosis factor-alpha, interferon-gamma, vasculitis
INTRODUCTION
The formation and deposition of immune complexes (IC) in blood vessels is generally believed to be important in the pathogenesis of certain types of vasculitis [1]. However, the mechanism of IC targeting to specific areas of blood vessels is largely unknown. Elevated circulating levels of cytokines such as TNF-α and IFN-γ have been reported in those types of vasculitic diseases [2,3].
TNF-α induces or increases endothelial expression of chemotactic mediators [4], tissue factors, cytokines, growth factors, vasomotor factors [5], and adhesion molecules [6,7]. TNF-α also causes endothelial morphologic change [8], increases vascular permeability [9], and inhibits endothelial cell (EC) growth [10]. The actions of IFN-γ on EC include an induction of MHC class II expression [11] and morphologic change, an increase in the expression of MHC class I antigens and adhesion molecules vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), and inhibition of growth [11]. An important feature of IFN-γ activity is its synergistic effect on the activity of other cytokines. Synergistic effects of IFN-γ with TNF-α or IL-1 on EC include the expression of ICAM-1, MHC class I and II [12], and changes in morphology [8] and permeability [9]. IFN-γ also enhances the TNF-α-induced expression of FcγR on monocyte cell line U937 [13]. TNF-α and IFN-γ have been known to modulate the expression and function of FcγR [14–16].
There are three types of FcγR, CD64/FcγRI, CDw32/FcγRII, and CD16/FcγRIII. FcγRII is the most broadly distributed FcγR. It preferentially binds complexed or aggregated IgG [17]. The role of leucocyte FcγR in the pathogenesis of IC-associated vasculitis has been recognized [18]. However, the mechanism for IC deposition on specific areas of blood vessels is poorly understood. The appearance of Fcγ receptors on traumatized bovine pulmonary circulation has been documented and is postulated to contribute to the accumulation of IC in the pulmonary circulation [19]. To date, FcγR receptor expression on cytokine-stimulated EC has not been reported. We suggest that induced or enhanced FcγR expression on the EC under vasculitic conditions may actively contribute to the IC localization. Therefore, using MoAbs, FcγR expression on cytokine-stimulated human EC was studied.
MATERIALS AND METHODS
Human EC cultures
Human aortic endothelial cells (HAEC) were purchased from Clonetics Corp. (San Diego, CA). The cells were grown in EC basal medium, with 10 ng/ml recombinant human epidermal growth factor, 1 μg/ml hydrocortisone, 50 μg/ml amphotericin-B, 12 μg/ml bovine brain extract, and 2% fetal bovine serum (FBS) (all from Clonetics), in a 5% CO2 and fully humidified atmosphere at 37°C. EC were harvested before confluence and seeded at 2500 cells/cm2. EC from passages 4–8 were used for the experiments. The maintenance of EC characteristics was confirmed by morphology, positive staining for von Willebrand factor, binding of Ulex europaeus agglutinin-1 and DiI-LDL uptake.
Monocyte cell line U937 cell culture
Human monocyte cell line U937 cells were purchased from ATCC (Rockville, MD). They were cultured with RPMI 1640 medium (Life Technologies, USA) supplemented with 10% FBS (Harlen, USA), 2 mm glutamine, MEM vitamins, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Life Technologies).
Human fibroblast cell culture
Third passage normal human skin fibroblasts were purchased from ATCC and grown in Eagle's minimum essential medium with Eagle's salts and 0.1 mm non-essential amino acids, 1 mm MEM sodium pyruvate solution, and 10% FBS.
Antibodies and reagents
MoAbs including anti-FcγRI (10.1, mouse IgG1), anti-FcγRII (8.26, mouse IgG2b) [20], anti-FcγRIII (3G8, mIgG1), and negative isotype controls mIgG1 and mIgG2b all came from Pharmingen (Los Angeles, CA). Other reagents were obtained from Sigma (St Louis, MO), except those specifically indicated.
Recombinant human cytokines and treatment
TNF-α with a specific activity of 2 × 107 U/mg and granulocyte-macrophage colony-stimulating factor (GM-CSF) with 2.05 × 108 U/mg were purchased from Genzyme (Cambridge, MA); IFN-γ (specific activity > 2 × 107 U/mg) from Boehringer Mannheim (Indianapolis, IN). EC were seeded in 96-well plates with 40 000 cells/cm2 and allowed to grow to confluence. Cytokines were then added to the medium with final concentrations of IFN-γ, TNF-α, and GM-CSF to 100 U/ml, 200 U/ml, and 205 U/ml, respectively. The medium with or without cytokines was changed every day. The pretreatment of cells with IFN-γ lasted for 24 h, 48 h, or 72 h. Transcription and translation inhibitors were included in the medium 4 h before cytokine treatment at 2 μg/ml of actinomycin D, 30 μg/ml of cycloheximide and puromycin.
ELISA
Treated and control cells were blocked with filtered 2% bovine serum albumin (BSA) at 37°C for 1 h and then incubated with different MoAbs at 5 μg/ml. After three washings with PBS, optimum concentrations of anti-mouse IgG F(ab′)2 fragments labelled with horseradish peroxidase (HRP; Southern Biotechnology, USA) were added and incubated for an additional 1.5 h. Following several washings, HRP substrate TMB was added, incubated for 20 min, and then stopped with 4 n H2SO4. The bound enzyme activity was measured spectrophotometrically at 450 nm on a microtitre plate reader (Molecular Devices, USA).
Immunofluorescent and confocal microscopy
Treated (72 h) and control cells were harvested and washed. The cells were then stained using unlabelled mouse MoAbs (1:50) in 2% BSA and FITC-labelled F(ab′)2 fragments of anti-mIgG (1:50; Sigma) at 4°C and washed with cold PBS. Stained cells were resuspended in cold medium, half of them were kept in 4°C, the other half were incubated at 37°C for 15 min. Finally, the cells were fixed in 1% paraformaldehyde and mounted in 90% glycerol. Immunoglobulin isotype controls were run in parallel and U937 cells and human fibroblasts were used to serve as positive and negative controls, respectively. The cells were photographed under excitation wavelength of 488 nm using an Olympus fluorescent microscope. The location of FcγR was further examined under a BioRad MRC 1024 confocal microscope with a Nikon inverted scope oil objective 40× Fluor DIC.
Statistical analysis
Data are summarized as the mean ± s.d. of replicate optical density (OD) values. Significant differences between groups were calculated using Student's t-test. P < 0.05 was considered significant.
RESULTS
Cytokine stimulation and the binding of monoclonal anti-FcγR antibodies
The binding of anti-FcγR type I, II, III and effect of cytokines have been examined and confirmed on FcγRI- and FcγRII-bearing cells U937 (data not shown). The binding of all antibodies including anti-FcγRI, anti-FcγRII, anti-FcγRIII, negative isotype controls, and secondary antibody to non-stimulated human aortic EC was low (Fig. 1). Compared with negative control cells and antibody isotype controls, non-stimulated human EC may express low levels of FcγRII and FcγRIII, but not FcγRI. However, the binding of anti-FcγRII to EC was increased significantly after 72 h simultaneous stimulation with TNF-α and IFN-γ (P < 0.0001, four-to-six-fold). The difference in anti-FcγRIII binding between stimulated and non-stimulated EC was also significant (P < 0.01, 1.5–2-fold). However, the difference in anti-FcγRI binding before and after stimulation was not significant when compared with antibody controls (P > 0.2, Fig. 1).
Fig. 1.
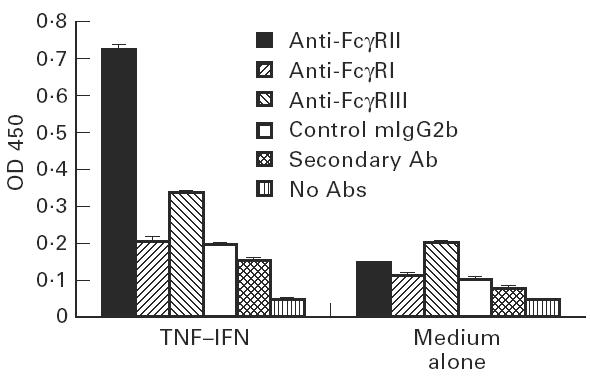
Enhanced FcγR expression on human endothelial cells (EC) by TNF-α and IFN-γ. Human aortic EC were stimulated with TNF-α and IFN-γ for 3 days. The binding of anti-FcγR MoAbs was determined using an ELISA. Data are presented as mean ± s.d. of four replicates. *P < 0.01; **P < 0.0001, compared with negative isotype control mIgG2b.
Cytokine dose and time course of stimulation
A dose-dependent increase in FcγRII expression was observed on human aortic EC incubated with TNF-α for 72 h (Fig. 2a). Significant (P < 0.001) induction occurred at TNF-α concentrations as low as 1 U/ml (50 pg/ml) and was further enhanced by coincubation with 100 U/ml IFN-γ. The effect of IFN-γ on the expression of FcγRII was less marked than that of TNF-α and dose-dependence of IFN-γ was not as significant as that of TNF-α (Fig. 2b). However, IFN-γ drastically augmented the effect of TNF-α at low concentrations of 15 U/ml.
Fig. 2.
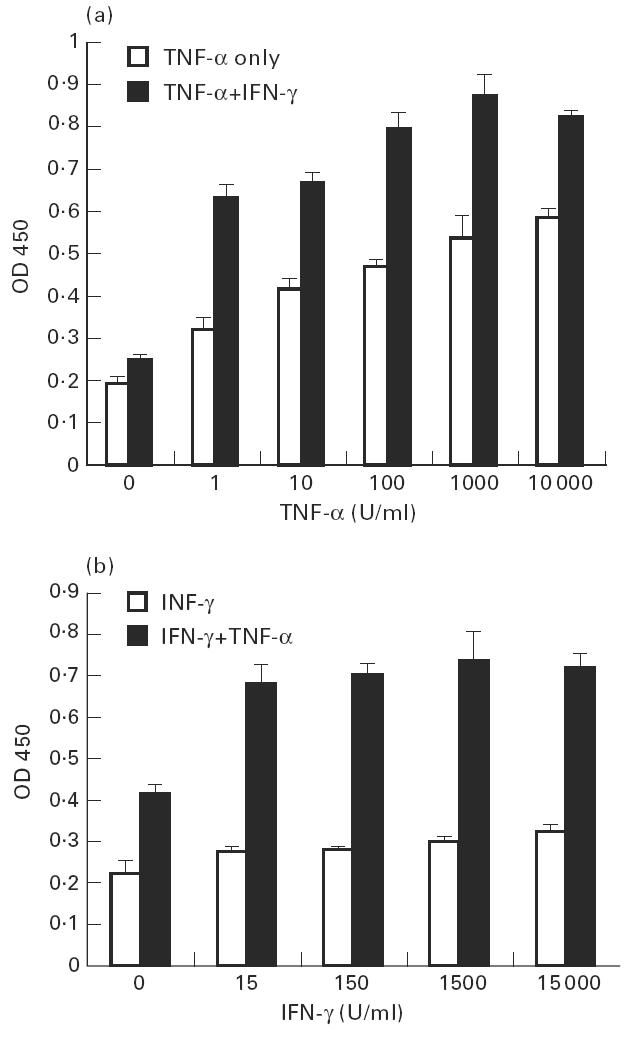
Dose–response relationships for the effect of TNF-α or IFN-γ on FcγRII expression. (a) Confluent endothelial cell (EC) monolayers were incubated with different concentrations of TNF-α alone or mixed with 100 U/ml IFN-γ for 3 days. (b) EC were incubated with different concentrations of IFN-γ alone or with TNF-α (200 U/ml). Data are presented as mean ± s.d. of four replicates.
In time course experiments (Fig. 3), significant induction of FcγRII began after 24 h treatment with TNF-α alone (P < 0.01) and began after 48 h treatment with IFN-γ alone (P < 0.05). Enhanced FcγRII expression peaked at 3 day stimulation with TNF-α (P < 0.0005) or a combination of TNF-α and IFN-γ (P < 0.0001) and declined slowly thereafter when cytokines were maintained in the medium and renewed every day. The effect of IFN-γ was more persistent than that of TNF-α, with a continuous level of activity plateau from 3 days to 1 week (P < 0.005). However, human recombinant GM-CSF did not show any effect on endothelial cell FcγRII expression (Fig. 3).
Fig. 3.
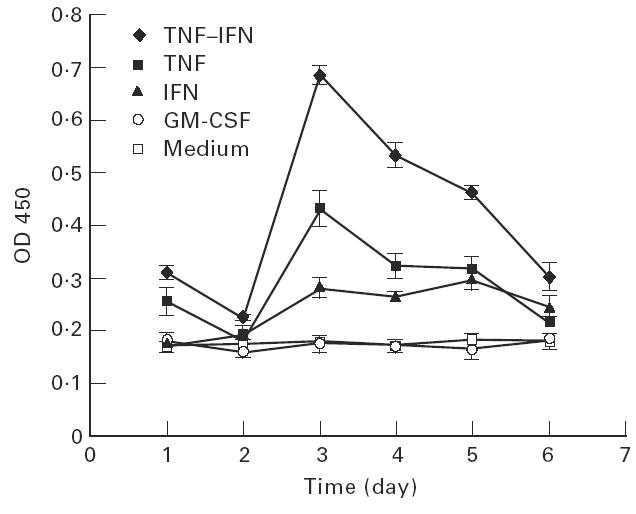
Time-dependency of cytokine-triggered FcγRII expression on human endothelial cells (EC). EC were incubated with 200 U/ml TNF-α, 100 U/ml IFN-γ, the combination of both cytokines, 205 U/ml granulocyte-macrophage colony-stimulating factor (GM-CSF), or medium only. The binding of 5 μg/ml MoAbs was measured every day.
Synergistic effect of IFN-γ and TNF-α
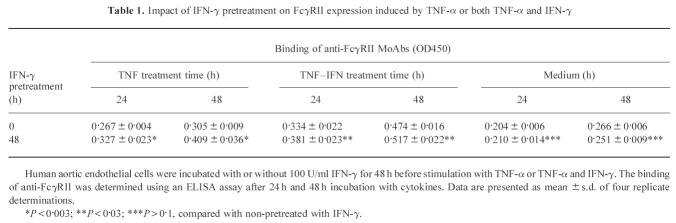
As shown in Figs 2 and 3, the synergistic effect of TNF-α and IFN-γ on endothelial cell FcγRII expression was obvious, since the OD values resulting from the simultaneous stimulation were greater than the summation of TNF-α and IFN-γ alone when the background absorbency was subtracted from each group and under the same cell number. The data from time course experiments showed similar results (Fig. 3). The modulation of the receptors for one cytokine by another cytokine has been reported and this was considered as one of the mechanisms of the synergistic effect of TNF-α and IFN-γ. We found that pretreatment of EC with IFN-γ for 24 h, 48 h, or 72 h augmented the FcγRII expression that was induced by TNF-α (compared with non-pretreated, P < 0.002 for 48 h and 72 h pretreatment and P < 0.05 for 24 h, data not shown). The pretreatment of EC with IFN-γ also enhanced FcγRII expression induced by coincubation with TNF-α and IFN-γ (P < 0.05, Table 1). EC that were pretreated with IFN-γ for 48 h, then washed and grown in cytokine-free medium did not exhibit enhanced FcγRII expression after 24 h and thereafter (P > 0.1).
Table 1.
Impact of IFN-γ pretreatment on FcγRII expression induced by TNF-α or both TNF-α and IFN-γ
Gene regulation by cytokines
FcγRII expression on cytokine-stimulated EC was significantly lower after withdrawal of cytokines from medium for 24 h and afterward. EC stimulated with cytokines for 1, 2, 3, 4 or 5 days and then incubated with medium only had similar results (data not shown). As the FcγRII expression declined after the elimination of cytokines, the morphology of stimulated EC also changed from elongated fibroblastoid to polygonal epithelioid. In addition, the blockade of FcγRII expression by transcription and translation inhibitors indicated that cytokine-enhanced FcγRII expression on EC requires gene activation at both the mRNA and protein level (Fig. 4). The effect of those inhibitors was dose- and time-dependent.
Fig. 4.
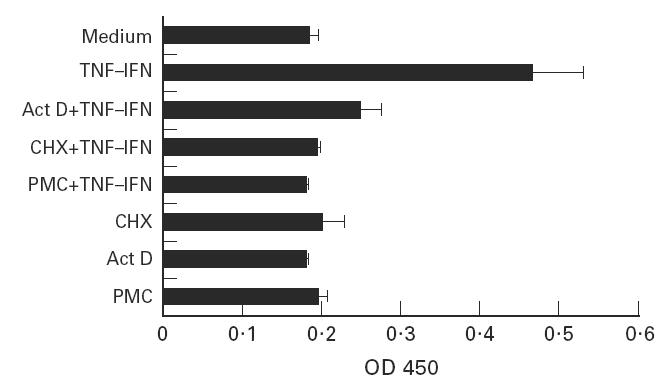
Inhibition of cytokine-induced FcγRII expression by RNA or protein synthesis inhibitors. Endothelial cells were treated with 2 μg/ml actinomycin D (Act D), 30 μg/ml cycloheximide (CHX), or puromycine (PMC) for 4 h prior to addition of the cytokines. The binding of 5 μg/ml anti-FcγRII MoAbs was determined after 24 h.
Fluorescent and confocal microscopy
To characterize the location of FcγRII expression on stimulated EC, the cells were stained with anti-FcγRII. Fluorescent staining was found on the cell surface under fluorescent microscope (Figures not shown) and under confocal microscope when cells were incubated at 4°C (Fig. 5a). The fluorescence on stimulated EC shifted from distribution over the whole surface to one or two small, condensed areas (receptor capping) after incubation at 37°C (Fig. 5b).
Fig. 5.
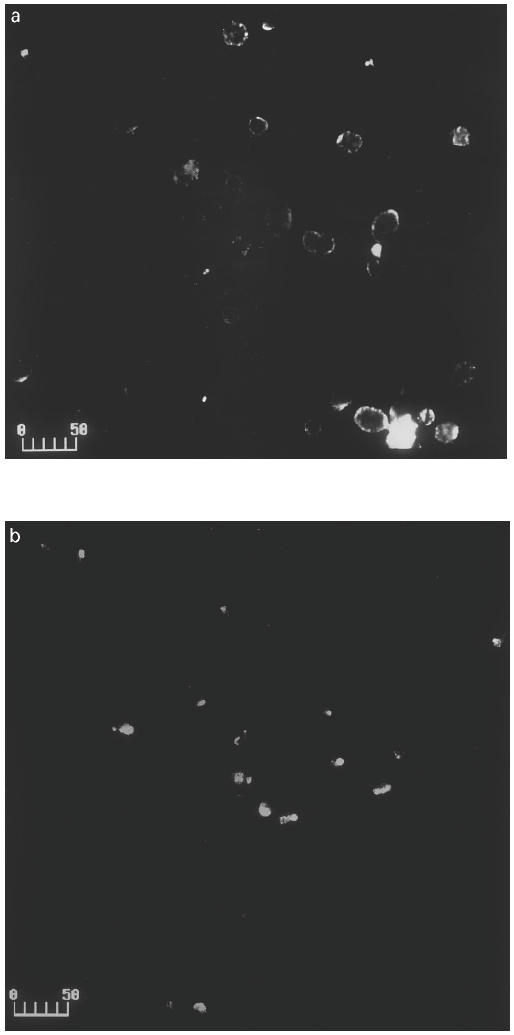
Confocal microscopy visualization and receptor capping. Endothelial cells (EC) were cultured with TNF-α and IFN-γ for 3 days, and then stained with anti-FcγRII and FITC-labelled F(ab′)2 fragments of anti-mouse IgG at 4°C. After washings, the cells were incubated at 4°C or 37°C for 15 min, and photographed under × 20 objective of a BioRad MRC 1024 confocal microscope. (a) Stimulated EC at 4°C. (b) Stimulated EC at 37°C. Scale bar, 50 μm.
DISCUSSION
Fcγ receptors type II and III have been demonstrated in EC in placenta [21], lymphoid tissues, and nerves [22]. The presence of Fcγ receptors or FcγR-like bindings has been reported on virus-infected EC [23], neutrophil lysate injured EC [19], as well as EC in transplant hearts undergoing rejection [24]. However, several studies failed to confirm the presence of FcγR on other normal vascular EC [21]. These data suggest that the expression of FcγR on EC may accompany a state of activation of the cells. We demonstrated that TNF-α or IFN-γ stimulated human EC to express FcγRII on the cell surface. Simultaneous treatment of both cytokines had a synergistic effect. The same phenomenon was reported in FcγR expression on U937 cells [12]. The time course of cytokine-induced FcγR expression on EC in this study was also close to that on U937, which peaked after 3–5 days stimulation and declined thereafter.
TNF-α has been shown to increase FcγR expression on rat microglia and peritoneal macrophages [16] and enhance human neutrophil FcγR-mediated phagocytosis. TNF-α also regulates EC gene expression and causes EC morphology change [7,11]. IFN-γ caused a slower, longer lasting, and less pronounced change in FcγRII expression on EC which was similar to the change in the expression of MHC class II on EC induced by IFN-γ [25]. Like TNF-α, IFN-γ also regulates EC gene expression [11] and FcγR expression [15,16]. In this study, IFN-γ not only up-regulated FcγR expression on EC, but also augmented the action of TNF-α. The synergistic effect of IFN-γ with TNF-α on EC gene expression has been reported on adhesion molecules, MHC antigens, and morphology [8,12]. The enhancement of TNF-α action by IFN-γ pretreatment suggests that up-regulation of one cytokine receptor by another cytokine may partially contribute to the synergistic effect [26,27].
The bindings of anti-FcγRII and anti-FcγRIII to non-stimulated EC are very low, but they are higher than those of negative controls mIgG2b and anti-FcγRI. Therefore, non-stimulated human EC may express low levels of FcγRII and FcγRIII. According to reports, anti-FcγRII and anti-FcγRIII bound to endothelium of nerve vessels [28], placental villi [29], and endocardium of hearts undergoing rejection [24].
The importance of FcγR in the pathogenesis of such diseases as rheumatoid arthritis, systemic lupus erythematosus (SLE), and immune vasculitis began to be recognized following the research work on Arthus reaction [30]. A growing body of evidence indicates that endothelial injury is a fundamental step in the development of all types of vasculitis. Among the proposed mechanisms of endothelial injury, the most important and best studied one is IC-mediated injury. FcγR expressed on inflammatory cytokine-stimulated EC may contribute to the specific localization of circulating IC to blood vessel walls and further damage to the endothelium itself under vasculitic conditions.
Acknowledgments
This work was supported by a grant from American Heart Association. We thank Ms Monica Shively and Mr Sam Royer for their assistance with photographs.
References
- 1.Somer T, Finegold SM. Vasculitides associated with infections, immunization, and anti-microbial drugs. Clin Infect Dis. 1995;20:1010–36. doi: 10.1093/clinids/20.4.1010. [DOI] [PubMed] [Google Scholar]
- 2.Matsubara T, Furukawa S, Yabuta K. Serum levels of TNF, IL-2 receptor, and IFN-γ in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol. 1990;56:29–36. doi: 10.1016/0090-1229(90)90166-n. [DOI] [PubMed] [Google Scholar]
- 3.Grau G, Lomband P, Gysler C, et al. Serum cytokine changes in systemic vasculitis. Immunol. 1989;68:196–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Vane JR, Botting RM. Mediators from the endothelial cell and their participation in inflammation. Int J Tiss Reac. 1994;16:19–49. [PubMed] [Google Scholar]
- 5.Cotran RS. Endothelial cells. In: Kelley WN, editor. Textbook of rheumatology. Philadelphia: Saunders; 1993. pp. 327–36. [Google Scholar]
- 6.Batten P, Yacoub MH, Rose ML. Effect of human cytokines (IFN-γ, TNF-α, IL-1β, IL-4) on porcine endothelial cells: induction of MHC and adhesion molecules and functional significance of these changes. Immunol. 1996;87:127–33. [PMC free article] [PubMed] [Google Scholar]
- 7.Wyble CW, Desai TR, Clark ET, et al. Physiologic concentrations of TNF-α and IL-1β released from reperfused human intestine upregulate E-selectin and ICAM-1. J Surg Res. 1996;63:333–8. doi: 10.1006/jsre.1996.0271. [DOI] [PubMed] [Google Scholar]
- 8.Stolpen AH, Guinan EC, Fiers W, et al. Recombinant tumor necrosis factor and immune interferon act singly and in combination to reorganize human vascular endothelial cell monolayers. Am J Pathol. 1986;123:16–24. [PMC free article] [PubMed] [Google Scholar]
- 9.Beynon HLC, Haskard DO, Davies KA, et al. Combinations of low concentration of cytokines and acute agonists synergize in increasing the permeability of endothelial monolayers. Clin Exp Immunol. 1993;91:314–9. doi: 10.1111/j.1365-2249.1993.tb05901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frater-Schroder M, Risau W, Hallman R, et al. Tumor necrosis factor type α, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Pro Natl Acad Sci USA. 1987;84:5277–81. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992;6:2591–9. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- 12.Gerritsen ME, Niedbala MJ, Szczepanski A, et al. Cytokine activation of human macro- and microvessel-derived endothelial cells. Blood Cells. 1993;19:325–42. [PubMed] [Google Scholar]
- 13.Gessl A, Willheim M, Spittler A, et al. Influence of tumor necrosis factor-α on the expression of Fc IgG and IgA receptors, and other markers by cultured human blood monocytes and U937 cells. Scand J Immunol. 1994;39:151–6. doi: 10.1111/j.1365-3083.1994.tb03354.x. [DOI] [PubMed] [Google Scholar]
- 14.Boltz-Nitulescu G, Willheim M, Spittler A, et al. Modulation of IgA, IgE, and IgG Fc receptor expression on human mononuclear phagocytes by 1-alpha, 25-dihydroxyvitamin D3 and cytokines. J Leukoc Biol. 1995;58:256–62. doi: 10.1002/jlb.58.2.256. [DOI] [PubMed] [Google Scholar]
- 15.de Andrés B, Cárdaba B, del Pozo V, et al. Modulation of the FcγRII and FcγRIII induced by GM-CSF, IFN-γ and IL-4 on murine eosinophils. Immunol. 1994;83:155–60. [PMC free article] [PubMed] [Google Scholar]
- 16.Loughlin AJ, Woodroofe MN, Cuzner ML. Regulation of Fc receptor and major histocompatibility complex antigen expression on isolated rat microglio by tumor necrosis factor, interleukin-1, and lipopolysaccharide: effects on interferon-gamma induced activation. Immunol. 1992;75:170–5. [PMC free article] [PubMed] [Google Scholar]
- 17.Kimberly RP, Salmon JE, Edberg JC. Receptors for immunoglobulin G: molecular diversity and implications for disease. Arthritis Rheum. 1995;38:306–14. doi: 10.1002/art.1780380303. [DOI] [PubMed] [Google Scholar]
- 18.Sylvestre DL, Ravetch JV. Fc receptors initiate the Arthus reaction: redefining the inflammatory cascade. Science. 1994;265:1095–8. doi: 10.1126/science.8066448. [DOI] [PubMed] [Google Scholar]
- 19.Ryan US, Schulz DR, Ryan JW. Fc and C3b receptors on pulmonary endothelial cells: induction by injury. Science. 1981;214:557–8. doi: 10.1126/science.6270789. [DOI] [PubMed] [Google Scholar]
- 20.Ierino FL, Hulett MD, McKenzie IFC, et al. Mapping epitopes of human FcγRII CDw32 with monoclonal antibodies and recombinant receptors. J Immunol. 1993;150:1794–803. [PubMed] [Google Scholar]
- 21.Sedmak DD, Davis DH, Singhman U, et al. Expression of IgG Fc receptor antigens in placenta and on endothelial cells in humans: an immunohistochemical study. Am J Pathol. 1991;138:175–81. [PMC free article] [PubMed] [Google Scholar]
- 22.Tuijnman WB, van Wichen DF, Schurman HJ. Tissue distribution of human IgG Fc receptors CD16, CD32 and CD64: a immunohistochemical study. APMIS. 1993;101:319–29. doi: 10.1111/j.1699-0463.1993.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 23.Friedman HM. Infection of endothelial cell by common human viruses. Rev Infect Dis. 1989;11:S700–4. doi: 10.1093/clinids/11.supplement_4.s700. [DOI] [PubMed] [Google Scholar]
- 24.Tuijnman WB, Wijngaard PLJ, van Winchen D, et al. Presence of CD16 on endothelial cells in heart transplant rejection: an immunohistochemical study. Scand J Immunol. 1992;35:569–73. doi: 10.1111/j.1365-3083.1992.tb03256.x. [DOI] [PubMed] [Google Scholar]
- 25.Mantovani A, Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989;10:370–5. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal BB, Essalu TE, Hass PE. Characterization of receptors for human tumor necrosis factor and their regulation by gamma interferon. Nature. 1986;318:665–7. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- 27.Raitano AB, Scuderi P, Korc M. Upregulation of interferon-γ binding by tumor necrosis factor and lymphotoxin: disparate potencies of cytokines and modulation of their effects by phorbol esters. J Interferon Res. 1991;11:61–67. doi: 10.1089/jir.1991.11.61. [DOI] [PubMed] [Google Scholar]
- 28.Vedeler CA, Matre R, Kristoffersen EK, et al. IgG Fc receptor heterogeneity in human peripheral nerves. Acta Neurol Scand. 1991;84:177–80. doi: 10.1111/j.1600-0404.1991.tb04933.x. [DOI] [PubMed] [Google Scholar]
- 29.Lang I, Hartmann M, Blaschitz A, et al. Immunohistochemical evidence for the heterogeneity of maternal and fetal vascular endothelial cells in human full-term placenta. Cell Tissue Res. 1993;274:211–8. doi: 10.1007/BF00318740. [DOI] [PubMed] [Google Scholar]
- 30.Ierino FL, Powell MS, McKenzie IFC, et al. Recombinant soluble human FcγRII: production, characterization, and inhibition of the Arthus reaction. J Exp Med. 1993;178:1617–28. doi: 10.1084/jem.178.5.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]