Abstract
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease of unknown aetiology. Recent studies have shown that genetic factors and both cellular and humoral immunological abnormalities are important in the pathogenesis of PSC. The most prominent autoantibodies in PSC are anti-neutrophil cytoplasmic antibodies (ANCA). The autoepitopes of ANCA in PSC are not well defined. The aim of this study was to identify corresponding ANCA autoantigens in patients with PSC. A biochemical approach with enrichment and partial purification of soluble neutrophil proteins, detection of autoantibodies by Western blot and partial amino acid sequencing were used. Two new autoantigen/autoantibody systems in patients with PSC were detected: catalase and α-enolase. The presence of catalase autoantibodies in 9/15 (60%) and α-enolase autoantibodies in 4/15 (27%) was confirmed by ELISA and Western blot. Furthermore, we showed immunoreactions of PSC sera with human biliary epithelial cells, showed the reduction of fluorescence in anti-catalase absorption experiments and observed partial co-localization of anti-catalase antibodies and PSC sera in double-staining experiments on biliary epithelial cells. The anti-catalase antibody-positive PSC patients had a more severe course of disease with a significantly higher alkaline phosphatase compared with the anti-catalase-negative PSC patients (P < 0.06). All ulcerative colitis control sera were anti-catalase antibody-negative. The identified antigens catalase and α-enolase can partly explain the ANCA fluorescence on ethanol-fixed and formaldehyde-fixed granulocytes in patients with PSC. Catalase is an important anti-oxidant enzyme and prevents cell damage from highly reactive oxygen-derived free radicals. Catalase autoantibodies might play a pathogenic role in patients with PSC. Our findings support the hypothesis that oxidative stress is one of the pathogenic mechanisms in patients with PSC.
Keywords: primary sclerosing cholangitis, autoantibody, catalase, α-enolase, ANCA
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease of unknown aetiology and characterized by chronic inflammation and fibrosis of the entire biliary tree. The disease usually leads to bile duct obliteration, biliary cirrhosis, hepatic failure and frequently to cholangiocarcinoma. There is a close association with ulcerative colitis (UC), ≈ 70% of patients with PSC having coexisting UC. As yet there is no specific medical therapy, and the treatment of choice for end-stage PSC is liver transplantation. Recent studies have shown that genetic factors and both cellular and humoral immunological abnormalities are important in the pathogenesis of PSC [1,2]. PSC has been strongly associated with HLA DR52a, moreover associations with HLA B8, DR2 and DR3 have been found [2]. Low titres of antinuclear antibodies (ANA) and smooth muscle antibodies (SMA) have been detected in patients with PSC [3]. Further studies identified multiple autoantibodies in patients with PSC against nuclear and cytoplasmic antigens [4]. The most prominent autoantibodies in PSC are anti-neutrophil cytoplasmic antibodies (ANCA). They have been found in sera of patients with PSC in up to 87% [5–19]. The PSC and UC associated P-ANCA yield a perinuclear staining pattern on alcohol-fixed neutrophils. Formalin fixation of granulocytes leads to a cytoplasmic ANCA pattern, therefore a cytoplasmic localization of the antigen/s was concluded [19–21]. The antigens of P-ANCA in PSC and UC are different from proteinase 3 (PR3) and myeloperoxidase (MPO). It was shown that neutrophil granule components are not the major PSC and UC specific antigens [17–21]. Despite extensive work in a number of laboratories, the antigens recognized by P-ANCA found in sera from patients with PSC and UC are still unclear. Antigenic determinants characterized from sonicated neutrophils by Western blot have been described for UC [20] and PSC [10,18], but other authors [22] and we were unable to detect specific bands in PSC and UC using crude neutrophil extract. Up to now, there is no clear evidence for a pathogenic role of ANCA in PSC.
We analysed 15 sera of patients with PSC for the presence of autoantibodies against purified human granulocyte proteins. The results of the present study identified two new autoantigen/antibody systems in patients with PSC.
PATIENTS AND METHODS
Patients
We studied sera from 15 PSC patients (median age 33 years, range 20–52 years). PSC was diagnosed on the basis of a cholestatic liver enzyme profile, a typical cholangiogram and a liver biopsy specimen demonstrating bile duct obliteration [1,2]. The clinical and autoantibody features are shown in Table 1. We included sera from 20 P-ANCA+ patients with mildly to moderate active UC; diagnoses were made on conventional clinicopathologic criteria. All patients were diagnosed and treated in our Department. They underwent a complete physical examination and concomitant immunological disorders were recorded. Additional studies were performed if clinical history, physical examination or laboratory values showed abnormalities besides the liver or bowel diseases. Treatment was administered in accordance with pre-established and internationally accepted protocols. Sera from patients with autoimmune hepatitis (AIH) type 1 (n = 20) and PBC (n = 20) were included, diagnoses were made according to accepted criteria. Sera from 10 patients with chronic hepatitis B virus (HBV), sera from 10 patients with chronic hepatitis C virus (HCV) and sera from 50 healthy blood donors were studied. All blood donors had no history of liver disease or autoimmune disorder, were negative for hepatitis B surface antigen (HBsAg), anti-HBc, anti-HCV, anti-HIV antibodies and showed normal aspartate aminotransferase levels.
Table 1.
Clinical, laboratory and histological data of 15 primary sclerosing cholangitis (PSC) patients

For ANCA testing, C-ANCA+ sea with PR3 specificity from patients with Wegener's granulomatosis (WG) and P-ANCA+ sera with MPO specificity from patients with microscopic polyarteritis were included. Sera were aliquoted and stored at −20°C.
Methods
Human granulocyte extract
Buffy coats were obtained from 40 healthy blood donors, mixed with an equal volume of 2% Dextran T-500 (Pharmacia Biotech, Freiburg, Germany) and sedimented for 1 h at room temperature. The granulocyte-rich upper layer was collected and centrifuged at 200 g for 10 min. To disrupt remaining erythrocytes the pellet (20 ml) was resuspended in 100 ml ice-cold H2O. After mixing for 30 s, 40 ml 0.6 m NaCl were added to achieve an isotonic solution. After another centrifugation at 150 g for 10 min the supernatant was discarded. The pellet was washed twice with PBS, and each washing step was followed by centrifugation at 150 g for 10 min. The pellet was resuspended in buffer A (20 mm Tris–HCl pH 9.0, 5 mm CaCl2, 5 mm ZnCl2, 0.05% PMSF, 0.05% DFP, 0.05% benzamidine hydrochloride) and transferred into a nitrogen bomb (Parr Instruments, Frankfurt, Germany) for 20 min at 3450 kPa. The granulocytes were disrupted after decompression. Another centrifugation step at 48 000 g for 2 h was added. The pellet contained enriched cytoplasmic material including granulocyte granules. The pellet was resuspended in buffer B (20 mm Tris–HCl pH 8.5, 5 mm CaCl2, 5 mm ZnCl2, 0.02% NaN3, 0.005% DFP), homogenized with an Ultraturrax homogenizer (Braun, Melsungen, Germany) and centrifuged at 48 000 g for 30 min. The resulting supernatant contained soluble granulocyte cytoplasmic enzymes and was subjected to SDS–PAGE and the following chromatographic steps.
Chromatography of human granulocyte extract
Human granulocyte extract containing soluble cytoplasmic enzymes (F1) was subjected to Zinc-Chelate-Sepharose CL-6B (Pharmacia Biotech) and washed with buffer C (20 mm Tris–HCl pH 8.5, 5 mm CaCl2, 0.5 mm ZnCl2, 0.02% NaN3). The unretarded fraction (F2) was collected. The yellow eluate (F3) was transferred to DEAE-Sepharose (Pharmacia Biotech) and washed with buffer C. The unretarded fraction after DEAE-Sepharose (F4) was dialysed against buffer C and centrifuged at 48 000 g for 30 min. The resulting pellet (F5) was resuspended in buffer C [23]. The DEAE-Sepharose was eluted with 2 m NaCl and the eluate was collected (F6). Protein concentrations were determined according to Bradford [24] and the fractions were subjected to SDS–PAGE.
SDS–PAGE and Western blot
SDS–PAGE was performed according to Laemmli [25] under denaturing and reducing conditions (25 μg protein/lane, 4% SDS stacking gel, 4–20% SDS resolving gel; Mini-Protean II, BioRad, Munich, Germany). Western blot analysis was performed as described [26]. Briefly, proteins were transferred electrophoretically (Fastblot; Biometra, Göttingen, Germany) from the gel to nitrocellulose sheets (Schleicher & Schuell, Dassel, Germany) at 2.5 mA/cm2 for 1 h at room temperature (buffer, 25 mm Tris–HCl, 192 mm glycine, 10% methanol). The remaining binding sites were blocked with PBS containing 1.5% (w/v) liquid gelatin overnight at 4°C. The blots were incubated with sera diluted with PBS containing 1.5% (w/v) liquid gelatin for 2 h at room temperature (Mini-Protean II Multiscreen Apparatus; BioRad). After five washings with PBS containing 1.5% (w/v) liquid gelatin, peroxidase-conjugated, γ-chain-specific goat anti-human IgG (A-6029; Sigma, Deisenhofen Germany) diluted 1:5000 in PBS containing 1.5% (w/v) liquid gelatin was added and incubated for 2 h at room temperature. The staining reaction was performed with 4-chloro-1-naphthol and stopped after 5 min with distilled water.
Protein sequence analysis
For N-terminal sequencing approaches the proteins were separated in 1D SDS–PAGE and electroblotted onto PVDF membranes (Sartorius, Göttingen, Germany). After staining with coomassie blue the bands were excised and subjected to automated Edman degradation using a protein sequencer (model 477A; Applied Biosystems, Foster City, CA) connected to an on-line PTH-amino acid analyser (model 120; Applied Biosystems). In cases of N-terminally blocked proteins identification was achieved by internal fragmentation either in the gel matrix or on the PVDF membrane [27]. Briefly, coomassie blue-stained bands were excised after SDS–PAGE and washed with water and water/acetonitrile 1:1, and after shrinkage of the gel piece with acetonitrile it was rehydrated by 0.1 m ammonium bicarbonate buffer pH 8.1 containing 1 μg protease. Enzymatic digestion was performed overnight at 37°C, extraction of peptide fragments was done using three volumes of water/trifluoroacetic acid (TFA) 1:1 and TFA/acetonitrile 1:1 each. The supernatant was evaporated to dryness. The peptide fragments were re-dissolved in high performance liquid chromatography (HPLC) starting buffer and isolated by RP-HPLC (Vydac 218TP, 1.6 × 250 mm) using a TFA/acetonitrile gradient. Fractionated samples were directly loaded onto the sequencer. For in situ fragmentation the PVDF membranes were first quenched applying 0.2% PVP-40 for 30 min at room temperature. Digestion and extraction were performed as described above. Computer sequence analysis was carried out performing a blast search at the National Center for Biotechnology Information Blast network server [28]. The actual versions of the protein and genomic databases were searched online for homology.
ELISA of human granulocyte proteins
The protein antigens (catalase, 219008, Calbiochem, Bad Soden, Germany; α-enolase, E-6126, Sigma) were added to 96-well polystyrol microtitre plates (1 μg/well; Maxisorb, Nunc, Wiesbaden, Germany) in buffer (34 mm Na2CO3–16 mm NaHCO3, pH 10.6) and incubated overnight at 4°C. The plates were then incubated for 30 min at 37°C. Remaining binding sites were blocked with PBS containing 1.5% (w/v) liquid gelatin for 1 h at 37°C. After washing three times with PBS containing 0.05% (v/v) Tween 20, sera diluted with PBS containing 1.5% (w/v) liquid gelatin were added and incubated for 2 h at 37°C. The plates were washed three times with PBS/Tween 20. Peroxidase-conjugated, γ-chain-specific goat anti-human IgG diluted 1:5000 in PBS containing 1.5% (w/v) liquid gelatin was added and incubated for 2 h at 37°C. The plates were washed twice with PBS/Tween and once with PBS. The staining reaction was performed with o-phenylenediamine as substrate and was stopped after 30 min at room temperature with 1 m H2SO4. The optical density (OD) was measured by a BioRad ELISA reader at 492 nm. Anti-catalase antibodies were obtained from Calbiochem (219010). All sera were tested in duplicate on the same day to avoid day-to-day variation.
Removing catalase antibodies from ANCA+ PSC sera
To remove catalase antibodies, anti-catalase-positive PSC sera as determined by Western blot and ELISA (dilution 1:5) were added to catalase (5 mg/ml) and incubated for 30 min at 37°C. After centrifugation at 30 000 g for 30 min, the supernatant was used for indirect immunofluorescence and in parallel read to unabsorbed sera from the same patient. In another set of experiments a catalase affinity column was prepared. Catalase (5 mg/ml) was coupled to cyanogen bromide (CNBr)-activated Sepharose 4B (Pharmacia). Patient sera were diluted 1:5 in coupling buffer (0.1 m NaHCO3, 0.5 m NaCl, pH 8.0), applied to the column and elution was achieved by change in pH. The unbound material (serum without anti-catalase antibodies) and eluate (anti-catalase antibodies) were collected and subjected to indirect immunofluorescence and ELISA.
Culture of human gallbladder epithelial cells
Culture of human gallbladder epithelial cells (HGBEC) was established according to the method described by Auth et al. [29] with minor modifications. Gallbladders or parts of gallbladders obtained from cholecystectomies because of cholecystolithiasis were turned inside out. During incubation with 0.125% (w/v) collagenase type 4 (Sigma) under sterile conditions mechanical abradation of the mucosa was performed every 5 min four times. The cell suspension was centrifuged (85 g for 5 min) and cells were grown in Dulbecco's modified Eagles’ medium (DMEM)/Ham's F12 1:1 (Seromed, Biomed, Berlin, Germany) in a humidified 5% CO2 gas mixed atmosphere at 37°C. Initially HGBEC formed characteristic clusters, each consisting of 20–250 cells. After cell adhesion in the first 24–72 h, the HGBEC spread out and formed a confluent monolayer. Cell characterization was achieved with immunohistochemical methods; the cells showed a positive staining pattern for cytokeratin and a negative pattern for vimentin and factor VIII, indicating their epithelial origin.
Indirect immunofluorescence on HGBEC and human neutrophil granulocytes (ANCA)
HGBEC were passaged on eight-well chamber slides (Lab-Tec; Nunc) and incubated in a humidified atmosphere with 5% CO2 and 37°C for 24 h. The cells were incubated with PSC sera (1:100), control sera (1:100) and anti-catalase antibodies (1:100) for 30 min at room temperature. After extensive washing with PBS cells were incubated with anti-human IgG FITC-labelled secondary antibodies (Dako, Hamburg, Germany) diluted 1:100 in PBS, with FITC-labelled mouse anti-rabbit IgG (Dako) diluted 1:100 in PBS for 30 min at room temperature. Detection of ANCA was performed according to the standard procedure with minor modifications as described in detail [21]. Cells were analysed with a Zeiss Axiophot microscope, data were documented with a Sony video camera, video processing unit and video printer.
Statistical analysis
OD values exceeding 5 s.d. of healthy blood donors were considered to be positive. Mann–Whitney rank sum test was computed when appropriate (Primer of Biostatistics, 3rd edn, 1992; Singapore: McGraw-Hill). P < 0.06 was considered significant.
RESULTS
Chromatography of human neutrophil extract
Human granulocyte extract containing soluble granulocyte cytoplasmic enzymes was separated by Zinc-Chelate-Sepharose and DEAE-Sepharose chromatography. The resulting fractions were subjected to SDS–PAGE. An enrichment and partial purification of soluble granulocyte enzymes was achieved (Fig. 1).
Fig. 1.

SDS–PAGE of human granulocyte extract. Granulocyte proteins (25 μg) were separated on SDS–PAGE (4–20% polyacrylamide), silver-staining. Lane 1, low molecular weight standard; lane 2, human neutrophil extract containing soluble granulocyte cytoplasmic enzymes (F1); lane 3, unretarded fraction of Zinc-Chelate-Sepharose CL-6B (F2); lane 4, eluate of Zinc-Chelate-Sepharose CL-6B (F3); lane 5, unretarded fraction of DEAE-Sepharose (F4); lane 6, unretarded fraction of DEAE-Sepharose after dialysis and centrifugation (F5); lane 7, 2 m NaCl eluate of DEAE-Sepharose (F6).
Characterization of immunoreactive proteins by Western blot
Granulocyte extract (F1) and the fractions F2–F6 obtained by chromatography were loaded on SDS–PAGE and transferred to nitrocellulose sheets. The sheets were incubated with sera from PSC patients and control sera (sera dilution 1:200). In fraction 3 (eluate after Zinc-Chelate-Sepharose CL-6B) immunoreactivity was found for 10/15 PSC sera with a 60-kD protein and for 7/15 PSC sera with a 48-kD protein. In fraction 5 (unretarded fraction of DEAE-Sepharose) weak immunoreactivity was found for 10/15 PSC sera with a 43-kD protein. In fractions 2, 4 and 6 no dominant immunoreactive proteins with PSC sera were found.
Identification of catalase, α-enolase and actin as antigens in patients with PSC
The immunoreactive proteins detected by Western blot were identified by partial protein sequencing. At least two tryptic peptides were sequenced per gel band to identify unambiguously catalase, α-enolase and actin (Table 2).
Table 2.
Identification of immunoreactive proteins with sera from primary sclerosing cholangitis (PSC) patients by amino acid sequencing

Autoantibody detection against catalase and α-enolase by Western blot and ELISA
The content of catalase in fraction 3 was demonstrated by positive reactions with anti-catalase antibodies. The specificity of catalase autoantibodies in sera from patients with PSC was confirmed by Western blot and ELISA. In Western blot with human catalase 11/15 (73%) PSC sera were anti-catalase autoantibody-positive (Fig. 2), 9/15 (60%) sera were positive by ELISA. Moreover, CNBr fragments of human catalase where shown to be immunoreactive with sera from patients with PSC. The catalase autoantibody was restricted to patients with PSC and AIH, sera from 5/20 (25%) patients with AIH type 1 contained catalase autoantibodies. Patients with primary biliary cirrhosis (PBC), HBV, HCV and UC were anti-catalase antibody-negative (Fig. 3a). For α-enolase a positive Western blot was obtained for 5/15 (33%) PSC sera (Fig. 4), in ELISA we found 4/15 (27%) anti-α-enolase antibody-positive PSC sera (Fig. 3b). Of sera from patients with AIH type 1, 12/20 (60%), PBC 6/20 (30%), HBV 1/10 (10%), HCV 2/10 (20%) and UC 1/20 (5%) were found to be anti-α-enolase antibody-positive by ELISA (Fig. 3b).
Fig. 2.
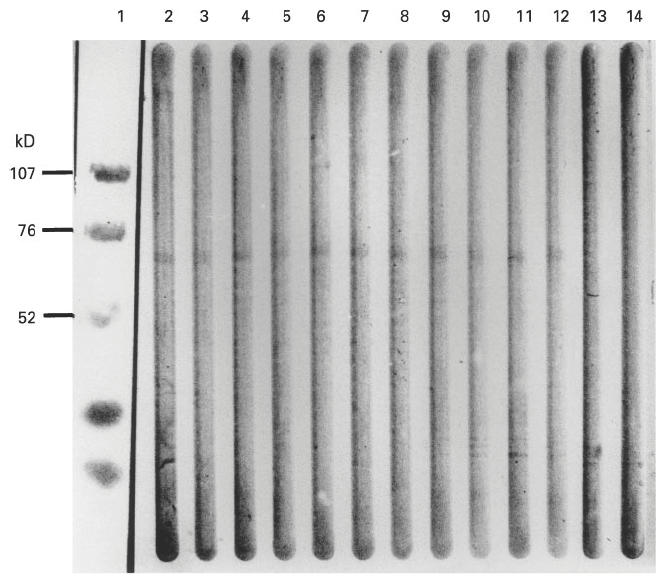
Western blot showing the reactivity of primary sclerosing cholangitis (PSC) sera with purified human catalase. Catalase (1 μg/lane) was subjected to SDS–PAGE (4–20% polyacrylamide) and transferred to nitrocellulose sheets. Lane 1, prestained low molecular weight standard; lanes 2–12, Western blot with PSC sera (dilution 1:200) showing immunoreactivity against catalase in lanes 2–12; lanes 13 and 14, normal human sera.
Fig. 3.

(a) Results of the ELISA for catalase autoantibodies. Fifteen patients with primary sclerosing cholangitis (PSC), 20 patients with autoimmune hepatitis (AIH), 20 patients with primary biliary cirrhosis (PBC), 10 patients with hepatitis B virus (HBV), 10 patients with hepatitis C virus (HCV), 20 patients with ulcerative colitis (UC) and 50 healthy blood donors (HBD) (20 are shown) were analysed. Mean of healthy blood donors was 0.17 ± 0.04, cut off was set at 0.4. (b) Results of the ELISA for α-enolase autoantibodies. Same patients as above. Mean of healthy blood donors was 0.14 ± 0.05, cut off was set at 0.4. OD, Optical density.
Fig. 4.

Western blot showing the reactivity of primary sclerosing cholangitis (PSC) sera with purified human α-enolase. α-enolase (1 μg/lane) was subjected to SDS–PAGE (4–20% polyacrylamide) and transferred to nitrocellulose sheets. Lane 1, prestained low molecular weight standard; lanes 2–6, Western blot with PSC sera (dilution 1:200) showing immunoreactivity against α-enolase in lanes 2–6.
Immunofluorescence studies on human gallbladder epithelial cells and granulocytes
Immunofluorescence studies with PSC sera on HGBEC showed a fine granular cytoplasmic immunofluorescence pattern in 9/15 (60%) sera which could be reduced after catalase absorption of sera (Fig. 5). The same result was obtained when anti-catalase antibodies were removed from sera by affinity chromatography with the ligand catalase. When the eluted anti-catalase antibodies were subjected to indirect immunofluorescence on HGBEC, again a fine granular cytoplasmic fluorescence pattern was observed. Staining of the cells with anti-catalase antibodies led to an indistinguishable fine granular cytoplasmic pattern. Double-staining studies of cells with PSC sera and anti-catalase antibodies with different labelled secondary antibodies showed a partial but not complete co-localization of positive immunofluorescence. Anti-catalase antibodies on ethanol-fixed granulocytes showed a fine granular cytoplasmic staining pattern, on formaldehyde-fixed granulocytes a weak fine granular cytoplasmic pattern was observed (Fig. 6).
Fig. 5.

Immunofluorescence studies on human gallbladder epithelial cells (HGBEC). (A) Primary sclerosing cholangitis (PSC) serum produces a fine granular cytoplasmic staining pattern on HGBEC. (B) Anti-catalase antibodies on HGBEC lead to a fine granular cytoplasmic staining pattern. (C) PSC serum after removing anti-catalase antibodies with affinity chromatography. The cytoplasmic staining pattern is reduced.
Fig. 6.

Immunofluorescence studies on human neutrophils with anti-catalase antibodies. (A) Anti-catalase antibodies on ethanol-fixed neutrophils lead to a fine granular cytoplasmic staining pattern. (B) Anti-catalase antibodies on formaldehyde-fixed neutrophils produce a weaker fine granular cytoplasmic pattern compared with ethanol-fixed neutrophils. (C) Negative control on formaldehyde-fixed neutrophils.
Correlation of catalase autoantibodies and ANCA to clinical parameters in patients with PSC
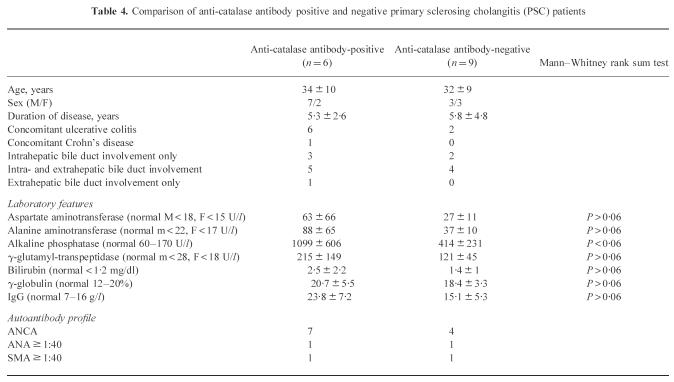
Eleven of 15 (73%) sera were positive for perinuclear ANCA, 9/15 (60%) of PSC sera were positive by ELISA for catalase autoantibodies. Five of the nine anti-catalase antibody-positive PSC sera were also P-ANCA+, two anti-catalase-positive sera showed a cytoplasmic pattern on ethanol-fixed granulocytes. Three ANCA+ PSC sera were anti-catalase- and anti-α-enolase-negative. We did not observe either a significant difference between catalase autoantibodies or a significant difference in ANCA prevalence between PSC alone and PSC + UC. All ANCA+ PSC patients with concomitant inflammatory bowel disease had an intra- and extrahepatic bile duct involvement (Table 3). The catalase autoantibody and ANCA positivity was not affected by age or duration of disease. There was an association between anti-catalase and elevated cholestatic liver enzymes. Values for alkaline phosphatase (AP) were significantly higher in the anti-catalase antibody-positive PSC group (P < 0.06). Values for AST, ALT, γ-GT, bilirubin and IgG tended to be higher in the anti-catalase antibody-positive PSC patients, but the differences were not statistically significant (Table 4).
Table 3.
Comparison of autoantibody profiles in patients with primary sclerosing cholangitis (PSC)
Table 4.
Comparison of anti-catalase antibody positive and negative primary sclerosing cholangitis (PSC) patients
DISCUSSION
We and others [22] were not able to generate PSC-specific bands by Western blotting using crude neutrophil extract. Therefore in the present study an approach with partial purification of soluble granulocyte proteins was chosen. Enrichment and partial purification of soluble cytoplasmic granulocyte proteins, Western blot and subsequent partial amino acid sequencing were successful in identifying new autoantibodies in patients with PSC: catalase and α-enolase. We found anti-catalase antibodies in 9/15 (60%) of our PSC patients. They were restricted to sera from patients with PSC and AIH.
Catalase is an oxidoreductase and a classical peroxisomal marker in mammalian cells, including hepatocytes and biliary epithelial cells [30]. The localization is cytoplasmic. It has been shown that there is a shift from the granule fraction in immature human leukaemic HL-60 promyelocytes to the cytosolic fraction in mature neutrophils during the course of differentiation [31]. Catalase is an important anti-oxidant enzyme and prevents cell damage from highly reactive oxygen-derived free radicals [32].
α-enolase is a glycolytic enzyme, α-enolase autoantibodies have been detected in patients with vasculitis and α-enolase has been considered as a minor ANCA antigen [33]. More recently, α-enolase autoantibodies have been found in patients with PBC and autoimmune hepatitis [34]. We detected α-enolase autoantibodies by ELISA in our PSC patients in 27%, and in PBC in 30%, a similar percentage to the 28.6% observed by Akisawa et al. [34]. In our AIH type 1 patients the frequency of α-enolase autoantibodies was higher than reported by Akisawa et al. (60% versus 31.6%). This discrepancy might be due to different patient populations. However, the titres of anti-α-enolase antibodies were low, and this result is in line with the reported data [34].
Ischaemic injury to the bile ducts may contribute to the pathogenesis of PSC [1,35]. Catalase autoantibodies might have a direct effect on biliary epithelial cells like PR3 antibodies in patients with WG [36,37]. We studied HGBEC as a model for the biliary tree and observed cytoplasmic immunofluorescence with PSC sera and partial co-localization with anti-catalase antibodies. The idea of biliary lesions due to oxidative stress is further supported by the observation of liver transplant recipients with ischaemic type bile duct lesions which can resemble the lesions seen in patients with PSC [38]. To test this idea we examined five sera from patients with ischaemic type bile duct lesions after undergoing liver transplantation (PSC patients were not included), and found anti-catalase antibodies in 3/5 patients. Moreover, the subgroup of anti-catalase-positive PSC patients had significantly higher values for AP and higher values for γ-GT, bilirubin, AST, ALT and IgG. Two interesting results have to be discussed: first, all PBC sera were anti-catalase antibody-negative, second, all UC sera were anti-catalase antibody-negative. These findings have two important implications: first, the different autoantibody profile of PSC and PBC might reflect different pathogenic mechanisms; second, in patients with PSC and concomitant UC the UC is not responsible for development of anti-catalase autoantibodies.
In conclusion, we have shown that PSC sera react with gallbladder epithelial cells and contain different autoantibodies against cytoplasmic proteins. We have demonstrated reactions of catalase autoantibodies with human biliary epithelial cells and granulocytes. The anti-catalase antibody-positive PSC patients had a more severe course of disease with a significant higher alkaline phosphatase compared with the anti-catalase-negative PSC patients. All UC sera were anti-catalase antibody-negative. These findings support the hypothesis that oxidative stress of biliary epithelial cells contributes to the pathogenesis of PSC. The suggested pathogenic role of anti-catalase antibodies has to be investigated. Whether the autoantibodies against α-enolase are due to inflammation or are just an epiphenomenon must be established. The identified antigens catalase and α-enolase cannot account for all ANCA fluorescence in patients with PSC.
Acknowledgments
The authors gratefully acknowledge the technical assistance of A. Müller, S. Kloss, N. Weidner and I. Heidemann. The authors are indebted to Professor H. Tschesche (Faculty of Chemistry, University of Bielefeld, Germany) for his support with the preparation and chromatography of human neutrophil extracts. The authors would like to thank Professor G. Otto (Department of Surgery, University of Heidelberg, Germany) for the sera from patients with ischaemic type bile duct lesions, and Dr A. Höfelin (Department of Surgery, Hildegardis Hospital, Mainz, Germany) for the gallbladder specimens. The authors are grateful to Professor G. Gerken (I Department of Internal Medicine, University of Mainz, Germany) for his critical reading of the manuscript.
References
- 1.Wiessner RH. Current concepts in primary sclerosing cholangitis. Mayo Clin Proc. 1994;69:969–82. doi: 10.1016/s0025-6196(12)61822-9. [DOI] [PubMed] [Google Scholar]
- 2.Boberg KM, Lundin KEA, Schrumpf E. Etiology and pathogenesis in primary sclerosing cholangitis. Scand J Gastroenterol. 1994;29(Suppl 20):47–58. doi: 10.3109/00365529409103625. [DOI] [PubMed] [Google Scholar]
- 3.Chapman RW, Arborgh BA, Rhodes JM, Summerfield JA, Dick R, Schuer PJ, Sherlock S. Primary sclerosing cholangitis—A review of its clinical features, cholangiography and hepatic histology. GUT. 1990;21:870–7. doi: 10.1136/gut.21.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gur H, Shen G, Sutjita M, et al. Autoantibody profile of primary sclerosing cholangitis. Pathobiology. 1995;63:76–82. doi: 10.1159/000163937. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen H, Wiik A, Elmgreen J. Granulocyte specific antibodies in ulcerative colitis. APMIS. 1983;91:23–26. [PubMed] [Google Scholar]
- 6.Snook JA, Chapman RW, Fleming K, Jewell DP. Anti-neutrophil nuclear antibody in ulcerative colitis, Crohn's disease and primary sclerosing cholangitis. Clin Exp Immunol. 1989;76:30–33. [PMC free article] [PubMed] [Google Scholar]
- 7.Saxon A, Shanahan F, Lander C, Ganz T, Targan SR. A subset of antineutrophil cytoplasmic antibodies is associated with inflammatory bowel disease. J Allergy Clin Immunol. 1990;86:202–10. doi: 10.1016/s0091-6749(05)80067-3. [DOI] [PubMed] [Google Scholar]
- 8.Rump JA, Schölmerich J, Gross V, et al. A new type of perinuclear antineutrophil cytoplasmic antibody (p-ANCA) in active ulcerative colitis but not in Crohn's disease. Immunobiology. 1990;181:406–13. doi: 10.1016/S0171-2985(11)80509-7. [DOI] [PubMed] [Google Scholar]
- 9.Duerr RH, Targan SR, Landers CJ, Larusso NF, Lindsay K, Wiesner RH, Shanahan F. Neutrophil cytoplasmic antibodies: a link between sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1991;100:1385–91. [PubMed] [Google Scholar]
- 10.Klein R, Weisenburg J, Weber P, Seibold F, Berg PA. Significance and specificity of antibodies to neutrophils detected by Western blotting for the serological diagnosis of primary sclerosing cholangitis. Hepatology. 1991;14:1147–52. [PubMed] [Google Scholar]
- 11.Seibold F, Weber P, Klein R, Berg PA, Wiedmann KH. Clinical significance of autoantibodies against neutrophils in patients with inflammatory bowel disease and primary sclerosing cholangitis. Gut. 1992;33:657–62. doi: 10.1136/gut.33.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo SK, Fleming KA, Chapman RW. Prevalence of antineutrophil antibody in primary sclerosing cholangitis and ulcerative colitis using an alkaline phosphatase technique. Gut. 1992;33:1370–5. doi: 10.1136/gut.33.10.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cambridge G, Rampton DS, Stevens TRJ, McCarthy DA, Kamm M, Leaker B. Anti-neutrophil antibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1992;33:668–74. doi: 10.1136/gut.33.5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halbwachs-Mecarelli L, Nusbaum P, Noel LH, Reumaux D, Erlinger S, Grünfeld JD, Lesavre P. Antineutrophil cytoplastic antibodies (ANCA) directed against cathepsin G in ulcerative colitis, Crohn's disease and primary sclerosing cholangitis. Clin Exp Immunol. 1992;90:79–84. doi: 10.1111/j.1365-2249.1992.tb05835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardarson S, LaBrecque DR, Mitros FA, Neil GA, Goeken JA. Antineutorphil cytoplasmic antibody in inflammatory bowel and hepatobiliary diseases. Am J Clin Pathol. 1993;99:277–81. doi: 10.1093/ajcp/99.3.277. [DOI] [PubMed] [Google Scholar]
- 16.Pool MO, Ellerbroek PM, Ridwan BU, et al. Serum antineutrophil cytoplasmic antibodies in inflammatory bowel disease are mainly associated with ulcerative colitis. A correlation study between perinuclear antineutrophil cytoplasmic autoantibodies and clinical parameters, medical and surgical treatment. Gut. 1993;34:46–50. doi: 10.1136/gut.34.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peen E, Almer S, Bodemar G, Ryden BO, Sjölin C, Tejle K, Skogh T. Anti-lactoferrin antibodies and other types of ANCA in ulcerative colitis, primary sclerosing cholangitis, and Crohn's disease. Gut. 1993;34:56–62. doi: 10.1136/gut.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulder AHL, Horst G, Haagsma EB, Limburg PC, Kleibeuker JH, Kallenberg CGM. Prevalence and characterization of neutrophil cytoplasmic autoantibodies in autoimmune liver disease. Hepatology. 1993;17:411–7. [PubMed] [Google Scholar]
- 19.Ellerbroek PM, Oudkerk Pool M, Ridwan BU, Dolman KM, von Blomberg BME, Kr von dem Borne AEG, Meuwissen SGM, Goldschmeding R. Neutrophil cytoplasmic antibodies (p-ANCA) in ulcerative colitis. J Clin Pathol. 1994;47:257–62. doi: 10.1136/jcp.47.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulder AHL, Broekroelofs J, Horst G, Limburg PC, Nelis GF, Kallenberg CGM. Anti-neutrophil cytoplasmic antibodies (ANCA) in inflammatory bowel disease: Characterization and clinical correlates. Clin Exp Immunol. 1994;95:490–7. doi: 10.1111/j.1365-2249.1994.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orth T, Gerken G, Meyer zum Büschenfelde KH, Mayet WJ. Antineutrophil nuclear antibodies (ANNA) in patients with primary biliary cirrhosis: their prevalence and antigen specificity. Z Gastroenterol. 1997;35:113–21. [PubMed] [Google Scholar]
- 22.Lo SK, Fleming KA. Investigation of the specific autoantigen of primary sclerosing cholangitis by Western blotting and immunoprecipitation. Hepatology. 1993;18:469–71. (letter) [PubMed] [Google Scholar]
- 23.Tschesche H. Human neutrophil collagenase. Methods Enzymol. 1995;248:431–49. doi: 10.1016/0076-6879(95)48028-5. [DOI] [PubMed] [Google Scholar]
- 24.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye bending. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli UK. Cleavage of structural proteins during the assembly of the bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kellner R, Orth T, Mayet WJ. Characterization of target antigens from anti-neutrophil cytoplasmic antibodies in autoimmune hepatitis type-1. Electrophoresis. 1997;18:507–10. doi: 10.1002/elps.1150180328. [DOI] [PubMed] [Google Scholar]
- 28.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 29.Auth MKH, Keitzer R, Scholz M, Blaheta RA, Hottenrott EC, Herrmann G, Encke A, Markus BH. Establishment and immunological characterization of cultured human gallbladder epithelial cells. Hepatology. 1993;18:546–55. [PubMed] [Google Scholar]
- 30.Van den Munckhof RJ. In situ heterogeneity of peroxisomal oxidase activities: an update. Histochem J. 1996;28:401–29. doi: 10.1007/BF02331433. [DOI] [PubMed] [Google Scholar]
- 31.Ballinger CA, Mendis-Handagama SMLC, Kalmar JR, Arnold RR, Kinkade JM. Changes in the localization of catalase during differentiation of netrophilic granulocytes. Blood. 1994;83:2654–68. [PubMed] [Google Scholar]
- 32.Michiels C, Raes M, Tossaint O, Rmacle J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med. 1994;17:235–48. doi: 10.1016/0891-5849(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 33.Moodie FDL, Leaker B, Cambrifge G, Totty NF, Segal AW. Alpha-enolase: a novel cytosolic autoantigen in ANCA positive vasculitis. Kidney In. 1993;43:675–81. doi: 10.1038/ki.1993.97. [DOI] [PubMed] [Google Scholar]
- 34.Akisawa N, Maeda T, Iwasaki S, Onishi S. Identification of an autoantibody against alpha-enolase in primary biliary cirrhosis. J Hepatol. 1997;26:845–51. doi: 10.1016/s0168-8278(97)80251-6. [DOI] [PubMed] [Google Scholar]
- 35.Sherlock S. Pathogenesis of sclerosing cholagnitis: the role of nonimmune factors. Semin Liver Dis. 1991;11:5–10. doi: 10.1055/s-2008-1040416. [DOI] [PubMed] [Google Scholar]
- 36.Mayet WJ, Csernok E, Szymkowiak C, Gross WL, Meyer zum Büschenfelde KH. Human endothelial cells express proteinase 3, the target antigen of anticytoplasmic antibodies in Wegener's granulomatosis. Blood. 1993;82:1221–9. [PubMed] [Google Scholar]
- 37.Mayet WJ, Meyer zum Büschenfelde KH. Antibodies to proteinase 3 increase adhesion of neutrophils to human endothelial cells. Clin Exp Immunol. 1993;94:440–6. doi: 10.1111/j.1365-2249.1993.tb08215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ludwig J, Batts KP, MacCarthy RL. Ischemic cholangitis in hepatic allografts. Mayo Clin Proc. 1992;67:519–26. doi: 10.1016/s0025-6196(12)60457-1. [DOI] [PubMed] [Google Scholar]