Abstract
Although in cord blood (CB) transplantation graft-versus-host disease (GVHD) is reported to be less severe, GVHD may occur even in patients with HLA-identical sibling donors. This result shows that HLA typing can not entirely predict GVHD. The standard MLR with CB cells was either normal or slightly reduced compared with adult peripheral blood (PB) cells. We used two manipulations to increase the responses of CB cells to allo-antigens. The first was to treat the stimulator cells with cytokines, and the second to amplify weak proliferative responses by adding exogenous cytokines to MLR cultures (modified MLR). The stimulator cells were treated with both interferon-gamma (IFN-γ) and IL-4. The responder cells were treated with both IL-2 and tumour necrosis factor-alpha (TNF-α). It is still to be determined whether or not this cytokine-enhanced MLR could be a possible predictor of GVHD. However, using these cytokines, 90% of CB could recognize allo-antigens, even if the standard MLR was negative.
Keywords: cord blood, MLR, cytokine, graft-versus-host disease
INTRODUCTION
Graft-versus-host disease (GVHD), mediated primarily by T cells and natural killer (NK) cells, is a leading cause of morbidity and mortality after allogeneic bone marrow transplantation (BMT) [1]. Recently, reports of successful haematopoietic reconstitution by transplantation with umbilical cord blood (CB) cells in haematologic disorders have increased [2,3]. Clinical results suggest a lower incidence of severe GVHD in children receiving HLA-matched or HLA-1 antigen-disparate CB transplantation, compared with those receiving allogeneic BMT [4]. However, a study in allogeneic sibling umbilical CB transplantation in children showed that grade II GVHD may occur even in patients with HLA-identical sibling donors [5]. The explanation may be a mismatch of minor histocompatibility antigens [6].
To detect alloreactivity in vitro, DNA typing for HLA class I (A and B) and II (DRB1) antigen has almost replaced the mixed lymphocyte culture (MLC) in BMT patients. Some studies have identified certain HLA antigens associated with increased rates of GVHD, but individual variations have been observed in BMT [7].
Bishara et al. reported that the modified MLR by using cytokines was useful in selecting the most compatible BMT donor [8]. The present study describes attempts to increase the sensitivity in the standard MLR test. We used two manipulations to increase the responses of CB cells to allo-antigens by treatment of the stimulator cells with cytokines, and to amplify weak proliferative responses by addition of exogenous cytokines to MLR cultures.
MATERIALS AND METHODS
Collection of samples
Human CB was obtained from normal deliveries by the Ob/Gyn nursing staff. CB was collected in an acid citrate dextrose (ACD) syringe following delivery of the infant and clamping and transecting of the umbilicus. ACD peripheral blood (PB) was obtained from informed consenting healthy volunteers. Mononuclear cells (MNC) were obtained from all samples by centrifugation over Ficoll–Hypaque gradients.
MLR
Standard MLR assay
The one-way standard MLR test was performed: PB versus PB or CB versus PB. Responder cells (5 × 104) were incubated with the same number of 3000 cGy-irradiated stimulator cells for 120 h in round-bottomed 96-well microtitre plates in a humidified 5% CO2 incubator. The cells were pulsed with 1 mCi 3H-thymidine for 16 h, then harvested and counted in a beta scintillation counter. Allogeneic stimulator cells were chosen from an unrelated adult PBMC donor, although HLA typing was not done.
Modified MLR assay
MLRs were performed between samples which were not reactive in the standard MLR test.
Pretreatment of stimulator cells with cytokines. Stimulator cells (5 × 105/ml) were incubated in a humidified 37°C, 5% CO2 incubator for 72 h with the following recombinant human cytokines alone or two at a time: IL-1α (Genzyme, Cambridge, MA; 3 U/ml), IL-3 (Genzyme; 100 U/ml), IL-4 (Genzyme; 1000 U/ml), IL-6 (Genzyme; 1000 U/ml), interferon-gamma (IFN-γ; Genzyme; 1000 U/ml), tumour necrosis factor-alpha (TNF-α; Genzyme; 500 U/ml). The cells were washed three times, irradiated and then added to the MLRs.
Addition of cytokines to MLR cultures. The following recombinant human cytokines were added alone or two at a time to the cultures: IL-1α (15 U/ml), IL-2 (Takeda Pharmaceutical Co. Ltd, Osaka, Japan; 18 U/ml), IL-4 (1000 U/ml), IL-6 (1000 U/ml), and TNF-α (500 U/ml), and the tests were performed.
Combinations of (i) and (ii).
Cytokines at the above mentioned concentrations were added to MLR cultures (ii), performed with cytokine-pretreated stimulator cells (i). The MLR tests were performed in the standard manner.
Every MLR culture was performed in triplicate. The standard MLR was considered positive when the stimulation index (SI) ((allogeneic MLR ct/min)/(autologous MLR ct/min)) exceeded 3 [9]. Results are provided in mean ct/min or as the relative response index (RRI), which was calculated according to the formula: RRI (%) = ((test ct/min − autologous MLR ct/min)/standard MLR ct/min) × 100. Any relative response index > 100% was considered positive.
FACS analysis
Expression of class II antigens (HLA-DP, DQ, DR) on the surface of PBMC from volunteer stimulator cells was investigated by fluorescence analysis. The mononuclear cells were incubated with FITC-conjugated IgG-negative control MoAb, FITC-conjugated anti-HLA-DP (Leinco Technologies, Inc., Manchester, UK), anti-HLA-DQ (Becton Dickinson Immunocytometry Systems, San Jose, CA), and PE-conjugated anti-HLA-DR (Becton Dickinson). HLA-DQ expression was followed by FITC-conjugated goat (Fab′2) anti-mouse IgG (Becton Dickinson) for 15 min. After two washes cells were resuspended in 0.5 ml 1% bovine serum albumin (BSA)/ PBS and analysed using a Becton Dickinson FACScan flow cytometer. A minimum of 5000 events were analysed for each sample.
Modified MLR using clinical samples from matched sibling
We reported a patient with pure red cell aplasia, who was successfully transplanted with umbilical cord cells from his newborn sibling [10]. HLA typing was identical to the patient and the standard MLR was negative. We performed the pretreatment of recipient samples with cytokines (IL-3, IL-4 or IFN-γ).
Statistical analysis
Statistical analysis was performed using Student's t-tests; P < 0.05 was considered significant.
RESULTS
Standard MLR of CB and PB
When the proliferative response was expressed as the SI, there was no difference between CB and PB: (3.1 ± 2.8 in CB (n = 74) versus 5.4 ± 4.5 in PB (n = 16)).
Modified MLR in standard MLR-negative cases
Fifty-five out of 74 cases in CB were standard MLR-negative. A modified MLR was performed using cytokines in these cases (Fig 1).
Fig. 1.
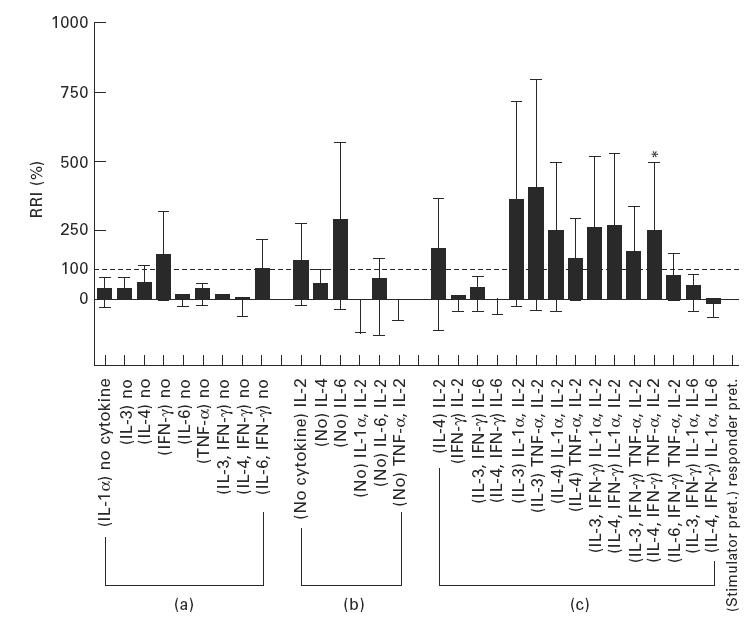
Up-regulation of the MLR in standard MLR-negative cases using cytokines. (a) The MLR was not up-regulated by pretreating the stimulator cells. (b) Pretreatment of the responder cells did not up-regulate the MLR response. (c) Combination of (a) and (b). The changes noted with cytokine treatment of cells for MLR analysis are very small and have a large deviation. *Exclusively, when the stimulator cells were treated with IL-4 + IFN-γ and the responder cells were treated with tumour necrosis factor-alpha (TNF-α) + IL-2, the relative response index (RRI) was up-regulated to 245 ± 130% (mean ± s.e.m., n = 10) > 100%.
The MLR was not up-regulated by pretreating the stimulator cells.
Similarly, pretreatment of the responder cells did not up-regulate the MLR response.
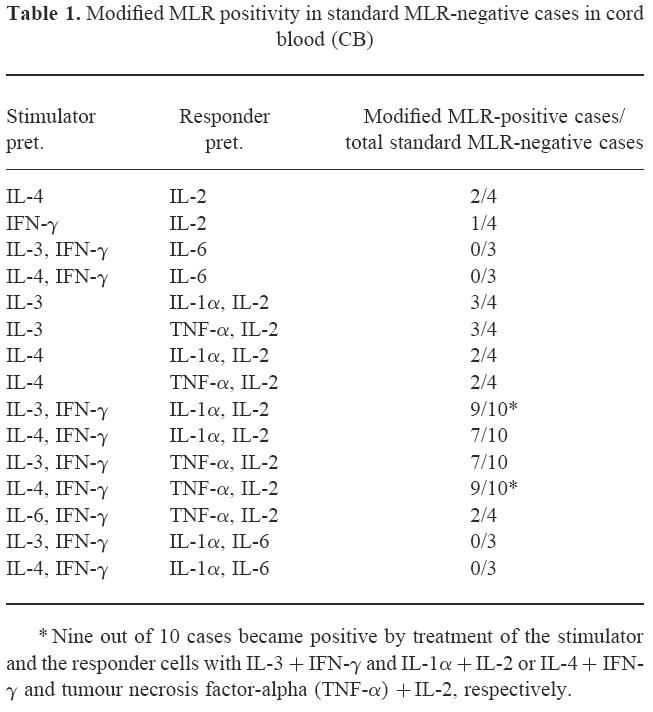
Combinations of (i) and (ii): as presented, the changes noted with cytokine treatment of cells for MLR analysis were very small and had large deviation. Exclusively, when the stimulator cells were treated with IL-4 + IFN-γ and the responder cells were treated with TNF-α+ IL-2, RRI was up-regulated to 245 ± 130% (mean ± s.e.m., n = 10) > 100%. Moreover, modified MLR by using this powerful combination could significantly up-regulate the SI (P < 0.01, n = 10) (Fig. 2). Nine out of 10 standard MLR-negative cases in CB became positive by treatment of the stimulator and the responder cells with IL-3 + IFN-γ and IL-1α + IL-2 or IL-4 + IFN-γ and TNF-α + IL-2 (Table 1).
Fig. 2.
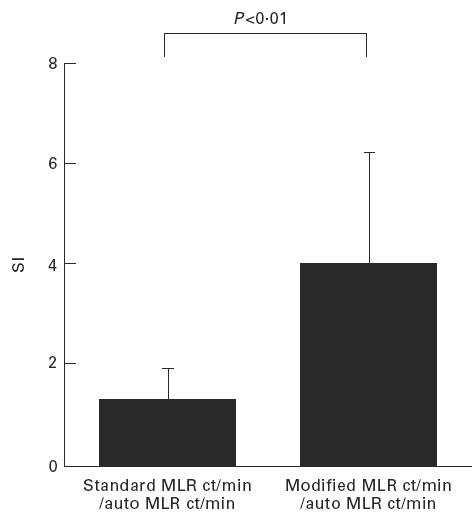
Modified MLR using the most powerful combination with IL-4 + IFN-γ and tumour necrosis factor-alpha (TNF-α) + IL-2 can significantly up-regulate the stimulation index (SI) (P < 0.01).
Table 1.
Modified MLR positivity in standard MLR-negative cases in cord blood (CB)
Class II MHC expression by the stimulator PB cells treated with cytokines
One specific volunteer's PB was used in an MLR with CB. The expression of HLA-DP and DQ did not increase with cytokine pretreatment. However, the expression of HLA-DR was increased significantly by IL-4 + IFN-γ pretreatment: 14.3 ± 0.7% versus 10.3 ± 1.6% in cytokine and no cytokine pretreatment, respectively (P < 0.05) (Fig. 3).
Fig. 3.
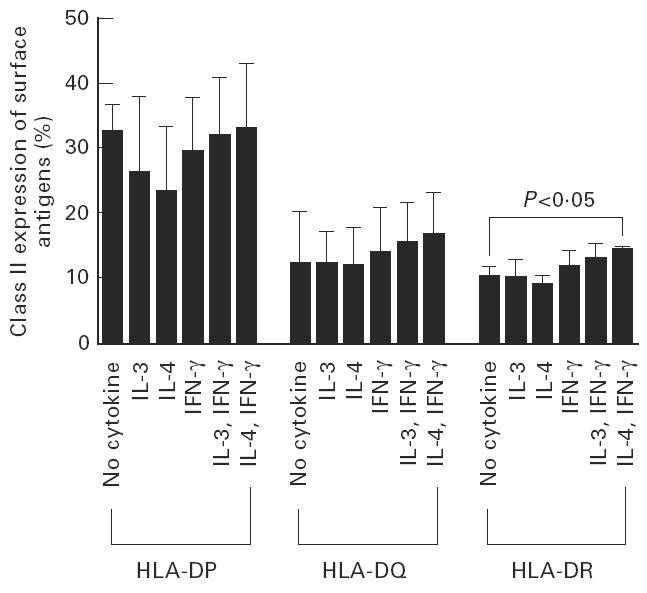
Class II MHC expression by the stimulator peripheral blood cells treated with cytokines. The expression of HLA-DR was increased significantly by IL-4 + IFN-γ pretreatment: 14.3 ± 0.7% versus 10.3 ± 1.6% in cytokine and no cytokine pretreatment, respectively (P < 0.05).
Clinical samples with pure erythrocyte aplasia case
The RRI of modified MLR between CB cells as responder samples and patient cells as stimulator samples with IL-3, IL-4 and IFN-γ were 140%, 0% and 0%, respectively. When the stimulator cells were treated with IL-3, RRI was significantly up-regulated.
DISCUSSION
The immaturity of lymphocytes in CB probably reduces the risk and severity of GVHD [11–14]. One study has shown that the cytotoxic activity in MLR of CB T cells was minimal [15]. Although HLA disparity between marrow donor and recipient is the most powerful determinant of the severity and kinetics of GVHD [16], a study of allogeneic sibling umbilical CB transplantation in children showed that grade II acute GVHD and limited chronic GVHD were seen in patients with an HLA-identical sibling donor [5]. In our clinical case, IL-3 significantly enhanced MLR even if class II antigen (HLA-DR) was genotypically identical. This suggests that cytokines stimulated proliferative responses other than HLA-A, -B, -DR, i.e. minor histocompatibility antigens. Moreover, HLA typing may not entirely predict GVHD, because CB NK cells are readily able to lyse targets found in PB cells, as we have recently reported [17]. A method to predict GVHD is needed.
Recently, one report suggested the use of cytokines to predict GVHD in BMT [8]. Another also suggested that increased cytokine mRNA expression and cytokine products in the two-way MLC enhanced by concanavalin A may predict the development of GVHD [18]. We have shown that the standard MLR with CB cells was either normal or slightly reduced compared with adult PB cells. A low positivity of standard MLR (26%; 19 out of 74) with CB cells may be due to either genetic homogeneity in Japanese [19] or immaturity of cord blood, or both. To magnify the MLR, we treated the stimulator PB cells with IFN-γ and IL-4 cytokines and added exogenous IL-2 and inflammatory cytokine TNF-α to MLR cultures to stimulate the responder CB cells. By using this powerful combination, 90% of standard MLR-negative cases in CB became positive. Consequently, CB cells could exert a strong proliferative response in vitro following these appropriate stimuli.
In vivo, several cytokines play an important role in acute GVHD and chronic GVHD [20]. Acute GVHD is observed more frequently in patients with increased IL-6, IFN-γ and TNF-α levels than in those with normal levels [21]. Up-regulated expression of IL-2 and IL-4 may also be part of the inflammatory cascade of human acute GVHD [22]. IFN-γ can induce MHC class I and II expression [23]. IL-4 can also up-regulate class II MHC antigens [24]. Our data demonstrated that the expression of HLA-DR was increased significantly by IL-4 + IFN-γ pretreatment. We showed that it was necessary to have both up-regulation of the class II MHC in the stimulator cells (e.g. pretreatment by IFN-γ and IL-4) and cytokine treatment of the responder cells (e.g. pretreatment by TNF-α and IL-2) to magnify the proliferative response of CB cells to allo-antigen. These findings suggest that CB could recognize the allo-antigen by activating the cytokine cascade, even if the standard MLR was negative.
GVHD did not develop in our single clinical case. RRI was significantly up-regulated, but it was slightly up-regulated (140%). There are no data as to whether RRI% may correlate to GVHD grade or not. As of now, we can not correlate our enhanced MLR results with outcomes of transplants, because few CB transplants have been attempted in Japan. However, we think that this study has clinical benefit in deciding a GVHD prophylactic combination (e.g. steroid, cyclosporine, methotrexate, antithymocyte globulin…) because there are various grades of GVHD in a clinical setting. Based on the results described here, the prospective study is ongoing in unrelated CB transplantation in Japan.
Acknowledgments
This study was supported by Grants-in-Aid from the Ministry of Health and Welfare, Japan, and Ministry of Education, Science, Sports and Culture, Japan (Scientific Research C).
References
- 1.Ferrara J, Deeg H. Graft-versus-host disease. N Engl J Med. 1991;324:667–74. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 2.Gluckman E, Broxmeyer HE, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–8. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 3.Wagner JE, Broxmeyer HE, Byrd RL, et al. Transplantation of umbilical cord blood after myeloablative therapy: analysis of engraftment. Blood. 1992;79:1874–81. [PubMed] [Google Scholar]
- 4.Kurtzberg J, Laughlin M, Graham ML, Smith C. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. New Engl J Med. 1996;335:157–70. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 5.Wagner JE, Kernan NA, Steinbuch M, Broxmeyer HE, Gluckman E. Allogeneic sibling umbilical-cord-blood transplantation in children with malignant and non-malignant disease. Lancet. 1995;346:214–9. doi: 10.1016/s0140-6736(95)91268-1. [DOI] [PubMed] [Google Scholar]
- 6.Goulmy E, Schipper R, Pool J, et al. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. New Engl J Med. 1996;334:281–5. doi: 10.1056/NEJM199602013340501. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan K. Graft-versus-host disease. In: Forman SBK, Thomas ED, editors. Bone marrow transplantation. Oxford: Blackwell Scientific Publications; 1994. p. 341. [Google Scholar]
- 8.Bishara A, Brautbar C, Nagler A, et al. Prediction by a modified mixed leukocyte reaction assay of graft-versus-host disease and graft rejection after allogeneic bone marrow transplantation. Transplantation. 1994;57:1474–9. [PubMed] [Google Scholar]
- 9.Sorensen SF. The genetic basis for reactivity in human mixed lymphocyte culture. 1. Studies of HL-A typed siblings. Acta Path Microbiol Scand. 1970;78:711–8. doi: 10.1111/j.1699-0463.1970.tb04361.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonno M, Azuma E, Umemoto M, et al. Successful hematopoietic reconstitution by transplantation of umbilical cord blood cells in a transfusion-dependent child with Diamond-Blackfan anemia. Bone Marrow Transplant. 1997;19:83–85. doi: 10.1038/sj.bmt.1700607. [DOI] [PubMed] [Google Scholar]
- 11.Harris DT, Schumacher MJ, Locascio J, et al. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc Natl Acad Sci USA. 1992;89:10006–10. doi: 10.1073/pnas.89.21.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Risdon G, Gaddy J, Stehman FB, Broxmeyer HE. Proliferative and cytotoxic responses of human cord blood T lymphocytes following allogeneic stimulation. Cellular Immunol. 1994;154:14–24. doi: 10.1006/cimm.1994.1053. [DOI] [PubMed] [Google Scholar]
- 13.Berthou C, Legros-Maida S, Soulie A. Cord blood T lymphocytes lack constitutive perforin expression in contrast to adult peripheral blood T lymphocytes. Blood. 1995;85:1540–6. [PubMed] [Google Scholar]
- 14.Risdon G, Gaddy J, Horie M, Broxmeyer H. Alloantigen priming induces a state of unresponsiveness in human umbilical cord blood T cells. Proc Natl Acad Sci USA. 1995;92:2413–7. doi: 10.1073/pnas.92.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keever C, Abu-Hajir M, Graf W. Characterization of the alloreactivity and anti-leukemia reactivity of cord blood mononuclear cells. Bone Marrow Transplant. 1995;15:407–19. [PubMed] [Google Scholar]
- 16.Beatty P, Hansen J, Longton G. Marrow transplantation from HLA-matched unrelated donors for treatment of hematologic malignancies. Transplantation. 1991;51:443–7. doi: 10.1097/00007890-199102000-00034. [DOI] [PubMed] [Google Scholar]
- 17.Umemoto M, Azuma E, Hirayama M, et al. Two cytotoxic pathways of natural killer cells in human cord blood: implications in cord blood transplantation. Br J Haematol. 1997;98:1037–40. doi: 10.1046/j.1365-2141.1997.3183135.x. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka J, Imamura M, Kasai M, et al. Cytokine gene expression in the mixed lymphocyte culture in allogeneic bone marrow transplants as a predictive method for transplantation-related complications. Br J Haematol. 1994;87:415–8. doi: 10.1111/j.1365-2141.1994.tb04935.x. [DOI] [PubMed] [Google Scholar]
- 19.Morishima Y, Morishita Y, Tanimoto M, et al. Low incidence of acute graft-versus-host disease by the administration of methotrexate and cyclosporine in Japanese leukemia patients after bone marrow transplantation from human leukocyte antigen compatible siblings; possible role of genetic homogeneity. Blood. 1989;74:2252–6. [PubMed] [Google Scholar]
- 20.Jadus MR, Wepsic HT. The role of cytokines in graft-versus-host reactions and disease. Bone Marrow Transplant. 1992;10:1–14. [published erratum appeared in Bone Marrow Transplant 1993 Jan; 11:89]. [Rev] [PubMed] [Google Scholar]
- 21.Smith SR, Terminelli C, Kenworthy BL, Phillips DL. A study of cytokine production in acute graft-vs-host disease. Cellular Immunol. 1991;134:336–48. doi: 10.1016/0008-8749(91)90307-w. [DOI] [PubMed] [Google Scholar]
- 22.Roy J, Blazar BR, Ochs L, Weisdorf DJ. The tissue expression of cytokines in human acute cutaneous graft-versus-host disease. Transplantation. 1995;60:343–8. doi: 10.1097/00007890-199508270-00008. [DOI] [PubMed] [Google Scholar]
- 23.Farrar M, Schreiber R. The molecular cell biology of interferon-gamma and its receptor. Ann Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 24.te Velde AA, Klomp JPG, Yard BA, et al. Modulation of phenotype and functional properties of human monocytes by IL-4. J Immunol. 1988;140:1548–54. [PubMed] [Google Scholar]


