Abstract
Antibodies against food antigens are usually produced in healthy people. This humoral response can be detected both in serum and secretions. The characterization of this response can be useful for a better understanding of food-related immunological alterations. In this study, IgA and IgG antibodies specific to ovalbumin, β-lactoglobulin or gliadin were measured in serum, saliva, colostrum and milk from 40 healthy breast-feeding women. Specific IgA and IgG to the three antigens were measured by indirect ELISA. Specific IgG levels were highest in serum and very low in the other biological fluids. No correlation between the IgG specific to the different antigens was found. Specific IgA reactivity was found in all the samples analysed. Levels observed were higher in colostrum and milk than in serum and saliva. In spite of being three different unrelated food antigens, a correlation between the levels of specific IgA was found in saliva, colostrum and milk samples of all subjects studied. The specificity of IgA anti-gliadin antibodies from serum, saliva and colostrum was analysed by immunoblotting of SDS–PAGE-separated wheat proteins. Each sample presented a unique pattern of recognition. No common pattern of recognition was found either among the same biological fluids of the different subjects tested, or among the different samples—either serum, colostrum or saliva—of the same individual. Different degrees of specificity to wheat proteins among IgA from colostrum, saliva or serum were observed, suggesting that the local IgA-producing populations are functionally different in the different tissues of the organism.
Keywords: IgA, IgG, gliadin, ovalbumin, β-lactoglobulin
INTRODUCTION
Food antigens, although generally harmless, behave as ‘non-self’ agents, triggering an immune response. The study and characterization of the humoral response against food antigens has become of interest due to the potential importance of anti-food antigen antibodies in clinical tests performed to detect food-related immunological illnesses [1]. This characterization is also useful for a better understanding of the role of secretory antibodies in the modulation of the immune response against food antigens [2].
The presence of specific IgG to different food antigens in serum has been reported in recent years [3,4]. The presence of IgA specific to different food antigens in saliva and breast milk was also described [5–7]. There is a consensus in that food antigen-specific B cell clones are expanded in the gut and migrate to other distant mucosal sites, where they secrete IgA [8,9].
The presence of IgA and IgG specific to three common different food antigens, not structurally related, such as hen ovalbumin (OVA), bovine β-lactoglobulin (β-LG) and gliadins, in serum, saliva, colostrum and milk of healthy individuals, is reported in this study. A study of the specificity of the anti-gliadin IgA in the different biological fluids is described. Our results suggest that local influences may have a greater importance than previously thought with regard to the specificity and the levels in which secretory IgA is produced.
SUBJECTS AND METHODS
Subjects studied
Samples were taken from healthy breast-feeding mothers seen at the Department of Obstetrics of San Roque's Hospital of La Plata and the Department of Neonatology of the Sor Maria Ludovica Children's Hospital of La Plata, Argentina. All of them were following an unrestricted diet that included bovine milk and dairy products, eggs and wheat-derived products.
Ethical considerations
The protocol carried out was approved by both Ethics and Research committees of San Roque's and Sor Maria Ludovica's Hospitals. All mothers were informed of the purpose of the study and were enrolled with their consent.
Samples
An aliquot of 1 ml of serum was separated from the samples obtained 48–72 h after delivery to perform routine analysis at the Hospital laboratory. The same day a 2-ml sample of colostrum was obtained either by manual extraction or by using a breast pump. At the same time a saliva sample was collected as described by Fitzsimmons et al. [10]. Breast milk sample was obtained either by manual extraction or using a breast pump 5–16 days after delivery. Colostrum and milk samples were centrifuged at 8°C 12 000 g, 10 min. The aqueous phase between the cellular pellet and the overlying fat phase was separated and employed to perform antibody studies. Saliva samples were centrifuged under the same conditions, the pellet containing cells and debris was discarded and overlying transparent phase was separated for study. Immediately after processing, all samples were frozen at − 80°C until analysis.
Total IgA levels
The content of IgA in all samples was measured employing a Kallestadt (USA) radial immunodiffusion kit, following the specifications of the manufacturer.
Specific antibody measurement
The presence of specific antibodies to gliadins, hen OVA and bovine β-LG was tested by indirect ELISA.
Antigen preparation
Gliadin from Sigma (St Louis, MO) was dissolved in 70% aqueous ethanol and quantified by Kjeldahl's method. Hen OVA and bovine β-LG (Sigma) were dissolved in PBS (140 mm NaCl, 2.7 mm KCl, 1.5 mm KPO4H2, 8.1 mm Na2PO4H; pH 7.4).
Coating
Polystyrene strips (Maxisorp; Nunc, Roskilde, Denmark) were incubated with either OVA, β-LG or gliadin, dissolved in PBS at 1 μg/ml, 100 μl/well, 16 h at 4°C.
Blocking
Non-specific binding sites were blocked by incubating with 200 μl/well of 2% (w/v) bovine serum albumin (BSA; Sigma) in PBS for 2 h at 37°C.
Incubation
One hundred microlitres per well of each sample diluted 1:5 (v/v) in PBS containing 0.5% (w/v) BSA (diluent solution) were incubated at 37°C during 1 h (for the measurement of specific IgG, serum samples were diluted 1:50).
Conjugate
Either goat anti-human IgA horseradish peroxidase (HRP) conjugate (Sigma) or goat anti-human IgG HRP conjugate (Dako, Glostrup, Denmark), both diluted 1:1500 (v/v) in diluent solution, were incubated (100 μl/well) for 1 h at 37°C.
Colour development
The colour reaction was developed by adding a solution containing o-phenylenediamine (1 mg/ml; Merck, Darmstadt, Germany) and 30% H2O2 (1 μl/ml) in 0.1 m citrate-phosphate buffer pH 5.0. The enzymatic reaction was stopped after 20 min with 40 μl/well of 2 m SO4H2. Optical density (OD) was determined at 490 nm.
After each incubation step, wells were washed three times with PBS containing 0.05% (v/v) Tween 20 (PBS–T) during 10 min. Each sample was analysed in duplicate. A set of five control samples was analysed in every experiment and its values were used to normalize the results. All chemical reagents were reagent grade, from Sigma unless otherwise stated.
Immunoblotting
Wheat flour proteins were extracted with sample buffer (125 mm Tris–HCl, 0.1% (w/v) SDS, 40% (v/v) glycerol, 5% (v/v) 2-mercaptoethanol (2-ME), 0.5 mg% bromophenol blue, pH 6.8) at a flour/buffer rate of 100 mg/ml. This protein extract was separated by SDS–PAGE according to the method described by Laemmli [11], employing a polyacrylamide gradient of 10–15%. After that, they were transferred to nitrocellulose using a trans-blot cube (BioRad, USA) as described by Towbin [12]. Membranes were blocked by incubating overnight at 4°C in 1% (w/v) skimmed milk Tris–HCl buffer (TBS; 0.05 m Tris–HCl, 0.15 m NaCl, pH 7.4). Membranes were then incubated for 3 h at 37°C with the sample under study (either serum, saliva, colostrum or milk). After that, they were incubated with an affinity-purified peroxidase-conjugated anti-human IgA serum (Sigma) at 1:400 in 0.5% (w/v) skimmed milk–TBS during 1 h at 37°C. After each incubation step, membranes were washed three times during 5 min with TBS. Immunoblotting was performed employing 4-chloronaphtol 0.5 mg/ml in a 12% methanol–TBS buffer containing 0.1% H2O2 (30% v/v). Reaction was stopped after 10 min incubation by immersion in distilled water. As a control, a dilution 1:1000 of a rabbit anti-gliadin serum in TBS supplemented with 0.5% w/v skimmed milk was employed. The production and characterization of the rabbit anti-gliadin serum has been previously described [13].
RESULTS
IgA specific to different food antigens
Specific IgA to gliadin, OVA and β-LG were determined. Results are summarized in Table 1. The three antigens studied showed similar behaviour: colostrum and milk presented the highest values, whereas levels of saliva and serum were the lowest. For each subject the highest level of specific IgA to either gliadin, OVA or β-LG was found in colostrum samples; lower levels were observed in the other biological fluids. A great dispersion in results can be observed, with overlapping values for the different biological fluids studied. High individual variability of colostrum- and milk-specific IgA against pathogens [14] and food antigens [15] has been described. High variability in the specific IgA levels throughout breast feeding as a consequence of immunological, hormonal and psychological influences has also been reported [16–18].
Table 1.
Specific IgA determination
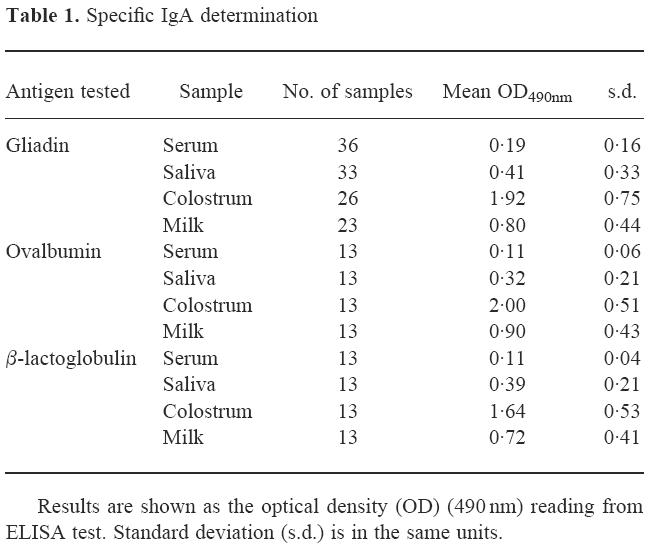
Total IgA and specific IgA to these food antigens were measured. Serum samples showed a mean IgA level of 135 mg/dl (s.d. = 30 mg/dl). Levels found in saliva were 45 mg/dl (s.d. = 25 mg/dl), while colostrum and milk levels were 300 mg/dl (s.d. = 120 mg/dl) and 100 mg/dl (s.d. = 30 mg/dl), respectively. No correlation was found between total and specific IgA levels in any of the four different biological fluids studied (not shown). Samples presenting the highest levels of specific IgA did not necessarily show high levels of total IgA. Samples with low levels of specific IgA presented in some cases high values of total IgA.
The analysis of the levels of specific IgA to the different antigens studied in every subject showed a striking similarity. Among the different samples tested (saliva, colostrum or milk) the same behaviour was always observed: every time that a sample presented high levels of specific IgA against one of the antigens tested, high levels against the other two antigens were also observed. The correlation plot between the levels of OVA-specific IgA versus gliadin-specific IgA in the different biological fluids is shown in Fig. 1a. Most of the colostrum samples can be seen in the upper right part of the figure, since they present high values of both IgA-specific antibodies. Milk samples are located mainly in the central part of the figure and most saliva samples are grouped in the lower left side of the plot. In Fig. 1b the plot of anti-β-LG IgA versus anti-gliadin IgA can be observed. The distribution of the colostrum, saliva and milk samples is similar to that observed in Fig. 1a. There is a statistically significant correlation between the analysed levels of specific IgA in both cases.
Fig. 1.

Correlation between the levels of IgA specific to the different antigens studied. The different types of samples are represented by the symbols: □, colostrum sample; ○, milk sample; Δ, saliva sample. (a) Anti-ovalbumin (OVA) IgA versus anti-gliadin IgA. The correlation coefficient is 0.928. (b) Anti-β-lactoglobulin (β-LG) IgA versus anti-gliadin IgA. The correlation coefficient is 0.836. In both cases there is a statistically significant correlation (P < 0.001).
IgG specific to different food antigens
Results of specific IgG measurement are summarized in Table 2. The same behaviour was observed in the three antigens tested. Antibody levels in samples of saliva, milk and colostrum were very low. On the other hand, serum levels were much higher, in agreement with the results reported in literature [3,6,19]. A great dispersion of serum-specific IgG was observed for anti-gliadin, anti-OVA or anti-β-LG antibodies. As in the case of IgA, an analysis of correlation of the levels of the specific IgG observed in each individual for the different antigens was performed. Results are shown in Fig. 2, where the serum levels of anti-OVA IgG (Fig 2a) or anti-β-LG IgG (Fig 2b) were plotted against anti-gliadin IgG levels. Subjects that showed high specific IgG for one antigen do not always have high specific IgG for the other antigens. No statistically significant correlation was found in any case.
Table 2.
Specific IgG determination
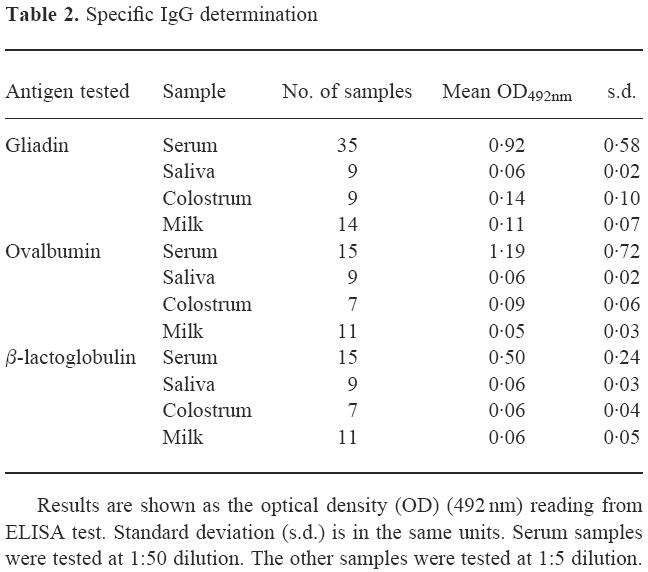
Fig. 2.
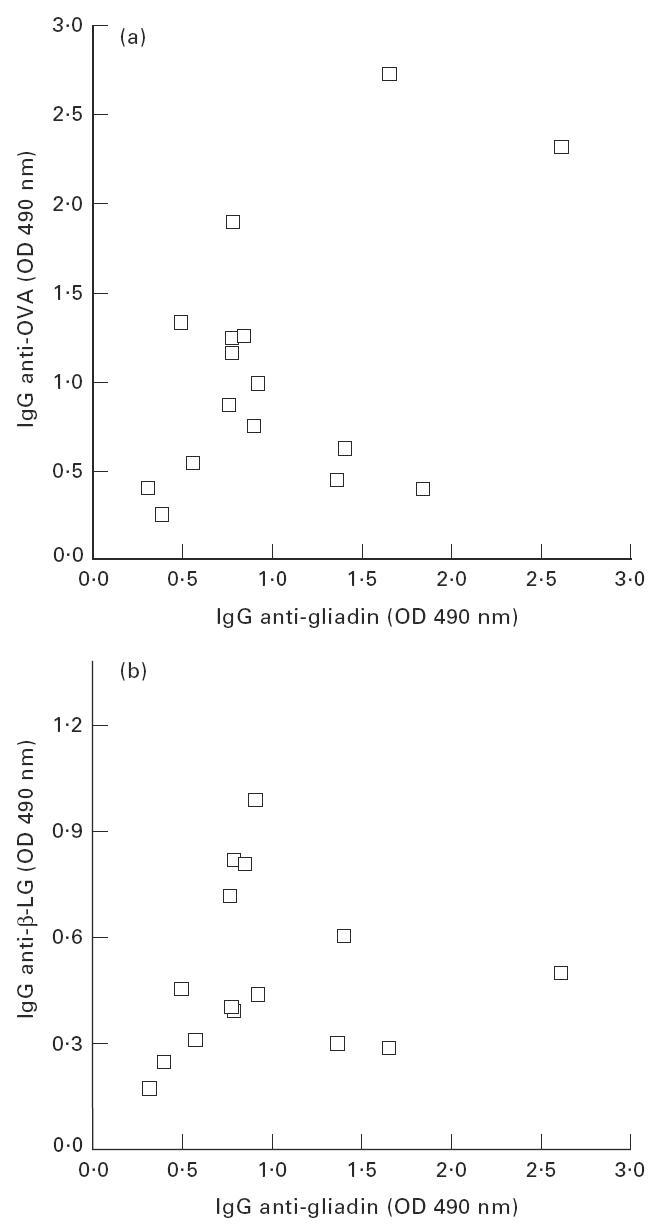
Correlation between the levels of serum IgG specific to the different antigens studied. (a) Anti-ovalbumin (OVA) IgG versus anti-gliadin IgG. Correlation coefficient 0.508. (b) Anti-β-lactoglobulin (β-LG) IgG versus anti-gliadin IgG. The correlation coefficient was 0.076. In both cases there is not a statistically significant correlation (P < 0.01).
Immunoblotting
Saliva, colostrum and serum from seven different subjects were studied. Three of them were selected as an example of the general behaviour observed. Figure 3 shows the blots of the specific IgA of serum, saliva and colostrum samples against wheat grain proteins separated by SDS–PAGE. Lanes 1–3 show the molecular weight markers, wheat protein extract and the blotting of the extract performed with an anti-gliadin rabbit serum, respectively. Lanes 4–12 show the IgA reactivity of serum, saliva and colostrum of three different subjects against wheat proteins. It can be observed that each sample presents a particular pattern of recognition. Proteins from the high molecular weight glutenin (HMW) fraction, the gliadin fraction and components from albumin and globulin fractions are recognized by different samples. In control experiments with no transferred gliadins non-specific bands were not detected (not shown).
Fig. 3.
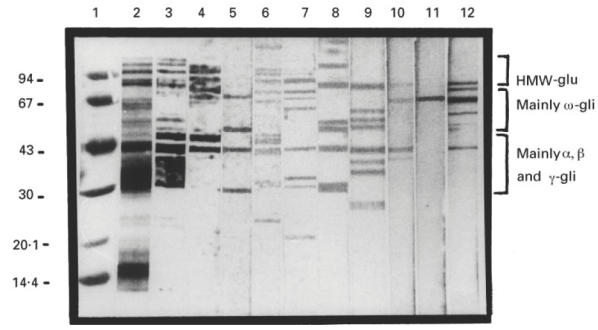
Immunoblotting of IgA antibodies of the different samples analysed that reacted against the SDS–PAGE-separated wheat proteins. Lane 1, molecular weight markers; lane 2, electrophoretic separation of total wheat proteins; lanes 3–12, immunoblots of: lane3, rabbit anti-gliadin serum; lanes 4–6, IgA from samples of subject I: lane 4, serum; lane 5, saliva; lane 6, colostrum; lanes 7–9, IgA from samples of subject II: lane 7, serum; lane 8, saliva; lane 9, colostrum; lanes 10–12, IgA from samples of subject III: lane 10, serum; lane 11, saliva; lane 12, colostrum.
The comparison of the sera-specific IgA fluid from the three different subjects (lanes 4, 7 and 10) shows that there is not a common pattern of recognition. Some samples present a broad recognition pattern (e.g. lane 4 or 7), whereas others show a restricted one, recognizing some components that are not recognized by the others (e.g. lane 10). A similar situation is observed when the blots of the different saliva samples (lanes 5, 8 and 11) or colostrum samples (lanes 6, 9 and 12) are analysed.
The comparison of the patterns of recognition of samples obtained from the same subject also show differences (e.g. lanes 4, 5 and 6, revealed with serum, saliva and colostrum, respectively). Each sample reacted against several components of the gliadin fraction. Some components were recognized by the three samples, but each sample recognized components that were not recognized by the others. The same happened with the other subjects, as can be seen in lanes 7–9 and 10–12 (serum, saliva and colostrum, respectively).
DISCUSSION
The presence of specific IgG and IgA against three different food antigens, usually present in a normal diet, such as OVA, β-LG and gliadins, is analysed here. The three antigens have been extensively studied and their primary structure has been reported [20–22]. No homology among them could be found. They showed no common immunological reactivity, either. Moreover, these three antigens come from independent, completely unrelated, food sources. For these reasons the three antigens should trigger specific, independent immune responses.
In spite of the high variability of the levels of specific IgA in the kinds of samples analysed, the lack of structural similarity and independence of the response against the antigens studied, a correlation in the specific IgA levels against them was found, as shown in Fig. 1. Samples showing high IgA levels to a specific antigen also showed high IgA levels to the other two antigens studied. This correlation was observed for saliva-, colostrum- and milk-specific IgA. To our knowledge this observation has not yet been reported, and it may be part of a general behaviour that involves the humoral mucosal immune response to other food antigens. No correlation could be found between specific IgG against the different antigens studied (Fig. 2).
Wheat proteins present multiple homologous components with similar structures and the consequent cross-reactivity among them [23,24]. The use of such a complex antigenic mixture in the Western blot, instead of a single food protein, made possible the detection of differences in the specificity of the anti-gliadin antibodies. These differences were detected as a different recognition pattern. If a single protein had been used, the blot would have detected only the presence or absence of antibodies against it. As can be seen in Fig. 3, there is not a conserved pattern of recognition among anti-gliadin IgA of different individuals.
There are some components that were recognized by most of the samples, but each sample recognized a particular group of components that were not recognized by the others. No recurrent pattern of recognition among serum, salivary or breast milk samples of different individuals was found (Fig. 3). Moreover, IgA from different biological fluids from the same individual showed a different pattern of recognition, indicating that there are differences in the specificity of the antibodies produced in the different tissues of the organism. This fact may contribute to the different individual susceptibility to infectious diseases, because there may be individuals who can develop a high IgA-based response to certain pathogens, but with a non-protective specificity pattern or a weak response with an appropriate profile of specificities. This situation may extend to the different mucosal sites of the body.
There is general agreement that the IgA specific to food antigen is produced by differentiated lymphocytes that are sensitized by the antigen, expanded in the gut and afterwards migrate to other peripheral mucosal microenvironments and differentiate to IgA-secreting plasmatic cells [7,8]. However, a concomitant local activation of B lymphocytes in mucosal sites can not be ruled out [25]. If all the gliadin-reactive plasma cells present in the mammary gland and the oral cavity originate from expanded clones in the gut, milk and saliva, IgA antibodies would present a comparable pattern of recognition. Results described here do not support this hypothesis. On the contrary, results may be explained if there is either a differential homing or direct activation of different subpopulations in the different mucosal sites. To differentiate between these two hypotheses is beyond the scope of the present work, and deserves further study.
In conclusion, in this study we have shown that there is a correlation among the levels of IgA produced against the three different antigens studied. This indicates the importance of local influences that regulate the production of antibodies in the different mucosal sites. Besides, the differences found in the recognition patterns of IgA anti-wheat proteins of different samples coming from the same individual suggest that the local IgA-producing cell populations are functionally different in the different tissues of the organism.
Acknowledgments
The authors wish to thank the Department of Neonatology of Sor Maria Ludovica's Children's Hospital and the Department of Obstetrics of San Roque's Hospital for their collaboration in the collection of the samples. This work was financed with grants from the National Research Council of Argentina (CONICET).
References
- 1.Barnes RMR. IgG and IgA antibodies to dietary antigens in food allergy and intolerance. Clin Exp Allergy. 1995;25(Suppl. 1):7–9. doi: 10.1111/j.1365-2222.1995.tb01124.x. [DOI] [PubMed] [Google Scholar]
- 2.Lovegrove JA, Hampton SM, Morgan JB. The immunological and long term outcome of infants born to women following a milk free diet during late pregnancy and lactation. A pilot study. Br J Nutr. 1994;24:246–9. doi: 10.1079/bjn19940129. [DOI] [PubMed] [Google Scholar]
- 3.Barnes RMR, Jonhson PM, Blears J, Harvey MM, Finn R. Human serum antibodies reactive with dietary proteins. IgG subclass distribution. Int Arch Allergy Appl Immunol. 1988;87:184–8. doi: 10.1159/000234670. [DOI] [PubMed] [Google Scholar]
- 4.Barnes RMR, Jonhson PM, Blears J, Harvey MM, Finn R. Human serum antibodies reactive with dietary proteins. Antigenic specificity. Int Arch Allergy Appl Immunol. 1988;87:189–93. doi: 10.1159/000234671. [DOI] [PubMed] [Google Scholar]
- 5.Cruz JR, Garcia B, Urrutia JJ. Food antibodies in milk from Guatemalan women. J Pediatr. 1981;99:600–2. doi: 10.1016/s0022-3476(81)80269-7. [DOI] [PubMed] [Google Scholar]
- 6.Hanson LA, Ahlstedt S, Carlsson B. Secretory IgA antibodies against cow's milk protein in human milk and their possible effect in mixed feeding. Int Arch Allergy Appl Immunol. 1977;54:457–61. doi: 10.1159/000231862. [DOI] [PubMed] [Google Scholar]
- 7.Juto P, Holm S. Gliadin-specific and cow's milk protein-specific IgA in human milk. J Pediatr Gastr Nutr. 1992;15:159–62. doi: 10.1097/00005176-199208000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Czerkinsky C, Prince SJ, Michalek SM. IgA antibody-producing cells in the peripheral blood after antigen ingestion. Evidence for a common mucosal immune system in humans. Proc Natl Acad Sci USA. 1987;84:2449–53. doi: 10.1073/pnas.84.8.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandtzaeg P. Development of the intestinal immune system and its relation to coeliac disease. Scand J Nutr. 1996;40:50–57. [Google Scholar]
- 10.Fitzsimmons SP, Evans MK, Pearce CL, Sheridan JM, Wientzen R, Cole MF. Immunoglobulin A subclasses in infants' saliva and in saliva and milk from their mothers. J Pediatrics. 1994;124:566–73. doi: 10.1016/s0022-3476(05)83135-x. [DOI] [PubMed] [Google Scholar]
- 11.Laemmli UK. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Towbin H. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–6. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chirdo FG, Fossati CA, Añón MC. Optimisation of a competitive ELISA with polyclonal antibodies for quantification of prolamins in foods. Food Agricult Immunol. 1995;7:333–43. [Google Scholar]
- 14.Cruz JR, Arevalo C. Fluctuation of specific IgA antibodies in human milk. Acta Pædiatr Scand. 1985;74:897–903. doi: 10.1111/j.1651-2227.1985.tb10055.x. [DOI] [PubMed] [Google Scholar]
- 15.Savilahti E, Tainio V, Salmenperä L, Arjomaa P, Kallio M, Perheentupa J, Siimes M. Low colostral IgA associated with cow's milk allergy. Acta Pediatr Scand. 1991;80:1207–13. doi: 10.1111/j.1651-2227.1991.tb11810.x. [DOI] [PubMed] [Google Scholar]
- 16.Hennart PF, Brasseur DJ, Delogne-Desnoeck JB, Dramaix MM, Robyn CE. Lysozyme, lactoferrin and secretory immunoglobulin. A content in breast milk: influence of duration of lactation, nutrition status, prolactin status and parity of the mother. Am J Clin Nutr. 1991;53:32–39. doi: 10.1093/ajcn/53.1.32. [DOI] [PubMed] [Google Scholar]
- 17.Butte NF, Goldblum RM, Fehl LM, Loftin K, Smith EO, Garza C, Goldman AS. Daily ingestion of immunologic components in human milk during the first four months of life. Acta Pædiatr Scand. 1991;73:296–301. doi: 10.1111/j.1651-2227.1994.tb17738.x. [DOI] [PubMed] [Google Scholar]
- 18.Lovegrove JA, Morgan JB, Hampton SM. Dietary factors influencing levels of food antibodies in breast milk. Acta Pædiatrica. 1996;85:778–84. doi: 10.1111/j.1651-2227.1996.tb14151.x. [DOI] [PubMed] [Google Scholar]
- 19.Hvatum M, Scott H, Brandtzaeg P. Serum IgG subclass antibodies to a variety of food antigens in patients with coeliac disease. Gut. 1992;33:632–8. doi: 10.1136/gut.33.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nibset AD, Saundry RH, Moir AJG, Forthergill LA, Forthergill JE. Aminoacid sequence of hen ovalbumin. Eur J Biochem. 1981;115:335–9. doi: 10.1111/j.1432-1033.1981.tb05243.x. [DOI] [PubMed] [Google Scholar]
- 21.Eigel WN, Butler JE, Ernstrom CA, Farrell HM, Harwalker VR, Jennes R, Whitney L. Nomenclature of proteins of cow's milk: fifth revision. J Dairy Sci. 1984;67:1599–631. [Google Scholar]
- 22.Kasarda DD, Okita TW, Bernardin JE, Baecker PA, Nimmo CC, Lew EJ. Nucleic acid (cDNA) and aminoacid sequences of α-type gliadins from wheat (Triticum aestivum) Proc Natl Acad Sci USA. 1984;81:4712–6. doi: 10.1073/pnas.81.15.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shewry PR, Tatham AS. The prolamin storage proteins of cereal seeds: structure and evolution. Biochem J. 1990;267:1–12. doi: 10.1042/bj2670001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skerrit JH. Immunochemistry of cereal grain storage proteins. In: Pomeranz Y, editor. Advances in cereal science and technology. IX. Am Asociation Cereal Chemist Inc Minessota; 1988. pp. 263–339. Chap. 8. [Google Scholar]
- 25.Neutra MN, Pringault E, Kraehenbuhl JP. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu Rev Immunol. 1996;14:275–300. doi: 10.1146/annurev.immunol.14.1.275. [DOI] [PubMed] [Google Scholar]


