Abstract
Thyroid-associated ophthalmopathy (TAO) has a major effect on the two compartments of the retro-orbital (RO) space, leading to enlargement of the extraocular muscles and other RO tissues. T lymphocyte infiltration of RO tissue is a characteristic feature of TAO and there is current interest in whether these T cells are specifically and selectively reactive to RO tissue itself. We recently established 18 T cell lines (TCL) from RO adipose/connective tissue of six patients with severe TAO by using IL-2, anti-CD3 antibodies and irradiated autologous peripheral blood mononuclear cells (PBMC) to maintain the growth of T cells reactive to autologous RO tissue protein fractions. Here we report on the phenotype characteristics and cytokine gene expression profiles of these orbital TCL and on their immunoreactivity to the organ-specific thyroid antigens thyrotropin receptor (TSH-R), thyroidal peroxidase (TPO) and thyroglobulin (TG). Flow cytometry revealed that 10 TCL were predominantly of CD4+ phenotype, three being mostly CD8+ and five neither CD4+ nor CD8+. Analysis with reverse transcriptase-polymerase chain reaction (RT-PCR) of cytokine gene expression revealed both Th1- and Th2-like products in all TCL: IL-2 product (in 17 TCL), interferon-gamma (IFN-γ) (n = 10), tumour necrosis factor-beta (TNF-β) (n = 15), IL-4 (n = 12), IL-5 (n = 17), IL-6 (n = 13), TNF-α (n = 12) and IL-10 (n = 4). Reactivity to thyroid antigens was observed only in two TCL, the other 16 being uniformly unreactive. Although 10 out of 18 RO tissue-reactive TCL were predominantly CD4+ there were no significant relationships between TCL phenotype, cytokine gene profile, magnitude of reactivity to RO tissue protein or the (rare) occurrence of thyroid reactivity. The findings of both Th1- and Th2-like cytokine gene expression in all RO tissue-reactive TCL support the concept that TAO is a tissue-specific autoimmune disease, distinct immunologically from the thyroid, and involving both T cell and B cell autoimmune mechanisms in disease pathogenesis.
Keywords: thyroid-associated ophthalmopathy, orbital T cell lines, cytokine profiles, thyroid antigens
INTRODUCTION
Enlargement of the two compartments of the retro-orbital (RO) space, namely the extraocular muscles and the RO adipose/connective tissue, leads to an increase of total orbital volume. Thus, proptosis and lid retraction are prominent features in patients with ophthalmopathy associated with autoimmune thyroid disease [1,2]. Histological examination of RO tissue samples demonstrates mononuclear cell infiltration comprising mainly activated T cells, with a few B cells, macrophages and mast cells in patients with thyroid-associated ophthalmopathy (TAO) [3]. Cytokines released by activated immunocompetent cells stimulate RO fibroblasts to proliferate and to secrete glycosaminoglycans, leading to oedema and finally fibrosis [4–7]. Expression of intercellular adhesion molecule-1 (ICAM-1), HLA-DR and heat shock proteins (hsp) by orbital fibroblasts may also be induced by T cell-derived cytokines [8,9]. The phenotypes of T cells in the RO tissue of patients with TAO has been studied using mainly markers for the CD4+ and CD8+ antigens, with inconsistent results [3,10,11]. Both cell-mediated and humoral mechanisms have been postulated to be involved in the immunopathogenesis of TAO [8,12]. The few studies concerning cytokine profiles of T lymphocytes from RO tissue of patients with TAO produced various findings [11,13–15].
Factors that initiate T cell activation and an orbital immune reaction are unknown. Many putative orbital antigens have been already described and may be an immunological target in TAO [16–18]. On the other hand, known thyroidal antigens may be an antigenic link between TAO and the associated thyroidal disorder [19]. Analysis of circulating populations of peripheral blood mononuclear cells (PBMC) poorly reflect the principal pathogenic events within the orbit. Therefore, we established orbital adipose connective tissue-derived T cell lines (TCL) from patients with TAO which react to autologous RO tissue protein fractions, shown in previous investigations [16,17]. In the present study, we were interested in the phenotype characteristics and cytokine gene expression profiles of these orbital TCL and in their immunoreactivity to organ-specific thyroid antigens.
PATIENTS AND METHODS
Subjects
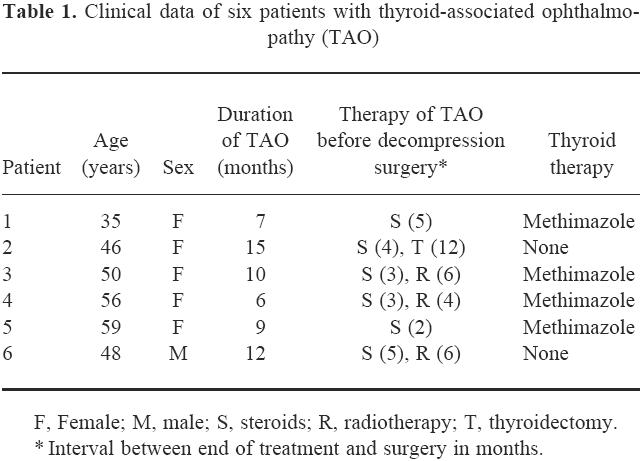
Pieces of orbital adipose/connective tissue were obtained under sterile conditions from six euthyroid patients with severe TAO undergoing orbital decompression surgery (Table 1). All patients received steroids, and three were additionally irradiated. Immunosuppressive therapy was terminated at least 2 months before surgery. Written consent was obtained from all patients and the study was approved by the local Ethics Committee.
Table 1.
Clinical data of six patients with thyroid-associated ophthalmopathy (TAO)
Antigen preparation and protein electroelution
Preparation of RO protein fractions was performed as previously described [16,17]. RO adipose/connective tissues were snap-frozen immediately in liquid nitrogen. Tissue was thawed in 10 vol of lysis buffer containing a mixture of protease inhibitors (aprotinin, phenylmethylsulfonylfluoride, and ɛ-aminocapronic acid) and non-ionic detergent (2% Nonidet P-40; INC Pharmaceuticals, Costa Mesa, CA), homogenized in a Polytron mechanical blender (Brinkmann Instruments, Westerbury, NY), and incubated on ice for 30 min. Homogenates were centrifuged at 15 000 g for 15 min to remove cell debris. The supernatant from this step was retained for use in SDS–PAGE, which was performed using a linear 5–20% polyacrylamide gradient separating gel (11 × 11 × 0.15 cm) and a 4% stacking gel in a vertical gel apparatus [20]. SDS-treated protein samples were added at a concentration of 300 μg protein in 50-μl aliquots/lane. The gel was run at 35 mA for 3 h under reducing conditions with mercaptoethanol.
To recover the separated proteins in soluble form, a Blotelutor (Biometra, Göttingen, Germany) was used [21]. They were electroeluted for 1 h under constant current (0.8 mA/cm2) to a masterplate containing 22 × 24 wells filled with 5 mmol/l Tris buffer pH 10.4. Each row of the 22 different molecular mass protein fractions was pooled, lyophilized, resuspended in 200 μl PBS, and sterilized with ultraviolet light (312 nm) for 5 min.
Preparation of T cell lines
PBMC were separated from heparinized venous blood by Ficoll gradient centrifugation. As previously described [17], in vivo activated, orbital tissue-reactive T lymphocytes were selected by cultivating small pieces of RO adipose/connective tissue in medium (RPMI 1640; 10% AB+, Rh+ human serum) containing IL-2 (20 ng/ml) for 10 days, followed by growth stimulation with anti-CD3 antibodies (30 ng/ml; CD3-UCHT1-IOT-3b; Dianova, Hamburg, Germany), thereby retaining antigen specificity of the T cells [22]. Every 10 days anti-CD3 antibodies and irradiated autologous PBMC (5 × 104; 40 Gy) were added to the cell lines as feeders. Every 3 days IL-2 medium was changed. In order to generate sufficient numbers of T cells for various investigations, six to eight culture passages were needed.
Phenotype analysis
Phenotypes of the TCL were determined by immunofluorescence staining with specific MoAbs. After transfer of the lymphocytes into round-bottomed tubes (105 cells/tube) and washing with PBS containing 0.1% bovine serum albumin (BSA), the cells were incubated with FITC- and PE-conjugated MoAb against CD3, CD4 and CD8 (each 2.5 μg/ml; Immunotech, Marseille, France) for 30 min at 4°C in the dark. Lymphocytes were washed twice with PBS containing 0.1% BSA and 0.1% NaN3, diluted in fixation buffer containing 0.5% formaldehyde, and analysed on a fluorescence-activated cell sorter (FACS analysis; Becton Dickinson Immunocytometry Systems, Mountain View, CA). Ten-thousand scatter-gated cells were analysed in each sample. The frequency and fluorescence profile of the cells were determined with logarithmic signal amplifiers.
mRNA preparation and cDNA synthesis
After restimulation of orbital T cells (105) with anti-CD3 MoAb, IL-2 medium and feeders for 5 days to induce cytokine expression, total mRNA was prepared from cultivated TCL using a modified guanidinium thiocyanate/phenol/chloroform extraction method (RotiQuick kit; Roth, Germany) according to the manufacturer's instructions. In order to analyse IL-2 expression, TCL were stimulated for 6 h with anti-CD3 MoAb without IL-2 or feeders, in microtitre plates coated with anti-CD28 MoAb (1 μg/well, overnight, 4°C; Pharmingen, Germany) and washed twice in PBS before isolation of total mRNA. Samples were used immediately to synthesize cDNA or stored at −70°C. One microgram of total RNA obtained from each TCL was used to generate cDNA by reverse transcription (RT) with oligo-desoxythymidine primer (cDNA synthesis kit; Boehringer, Mannheim, Germany).
Polymerase chain reaction amplification of cytokine cDNA
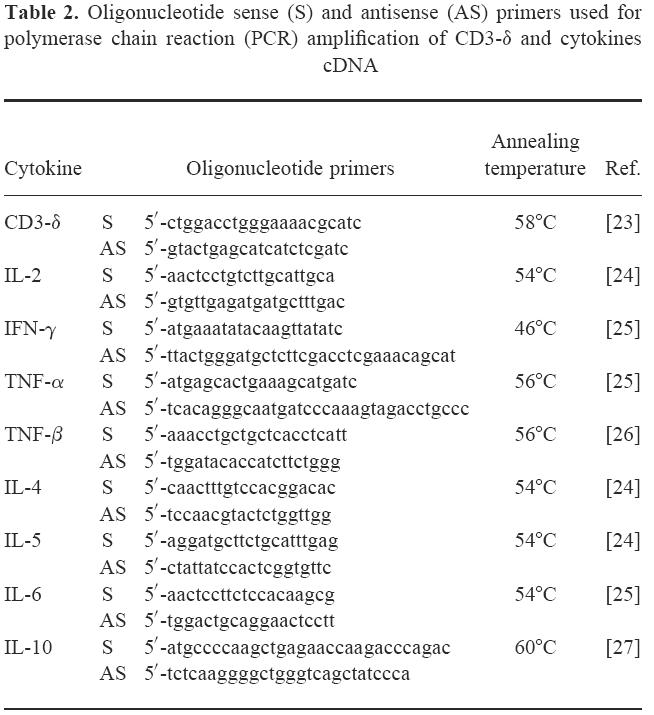
PCR was performed in 50 μl amplification reaction containing 5 μl of 10 × buffer with 20 mm Tris–HCl pH 8.55, 16 mm (NH4)2SO4, 1.5 mm MgCl2, 2.0 U of Taq DNA polymerase, 0.2 mm each dNTP (all from AGS, Heidelberg, Germany), 25 pmol of the corresponding primer pairs, 1 μl of cDNA and autoclaved deionized water. Primers were chosen according to references [23–27] (Table 2). Amplifications were carried out using cycles of 94°C for 30 s, primer corresponding annealing temperature for 30 s, and 72 °C for 1 min. Thirty-five cycles were performed to amplify T cell-specific CD3-δ and eight different cytokine products. As positive control, PBMC from a normal donor were cultivated in RPMI 1640, 10% human AB+ serum, and stimulated with 2 μl/ml phytohaemagglutinin (PHA; Biochrom, Germany) for 72 h. Cells were separated by centrifugation and used to prepare total RNA and cDNA, as described above. Control reaction without cDNA and with total RNA, in which RT was omitted in order to exclude a possible amplification of contaminating genomic DNA, were carried out in parallel, and were consistently negative (data not shown). Polymerase chain reaction (PCR) products were separated by agarose gel electrophoresis, visualized by staining with ethidium bromide, and their size was verified by comparison with a 100-bp ladder. Gels were permanently recorded using an automatic video system (Eagle Eye; Stratagene, La Jolla, CA).
Table 2.
Oligonucleotide sense (S) and antisense (AS) primers used for polymerase chain reaction (PCR) amplification of CD3-δ and cytokines cDNA
T lymphocyte proliferation assay
TCL or PBMC were cultivated at a cell density of 1 × 104 or 1.5 × 105 cells per well, respectively, in 96-well round-bottomed plates containing 200 μl culture medium (RPMI 1640; 10% AB+, Rh+ human serum and antibiotics) at 37°C in a humid atmosphere comprising 95% air and 5% CO2, together with irradiated autologous feeders (4 × 104; 40 Gy) and autologous protein fractions of different molecular masses obtained from SDS–PAGE or thyroidal proteins in different concentrations as antigen [16,17]. The following thyroidal antigens were used: recombinant human thyrotropin receptor (TSH-R), recombinant human thyroidal peroxidase (TPO) in soluble form [28], and native human thyroglobulin (TG; Elias, Freiburg, Germany). Triplicate cultures were set up, and incubation was for 5 days. Eighteen to 24 h before termination, 3H-thymidine (1 μCi, 925 GBq/mmol; Amersham Buchler, Braunschweig, Germany) was added to each well, cultures were harvested and thymidine incorporation was measured in a beta counter. Control cultures without orbital or thyroidal antigens, with tuberculin (Tb; 66 U/well; Behring, Marburg, Germany) as negative control, and with PHA (0.3 μg/well) as positive control, were included in all assays. Results were expressed as stimulation index (SI) calculated as (ct/min (lymphocytes + antigens)/ct/min (controls)) and Δct/min (ct/min (lymphocytes + antigens) − ct/min (controls)). An SI > 3.0 was taken as positive response. Proliferative responses were compared using Student's t-test.
RESULTS
Phenotype analysis of orbital T cell lines
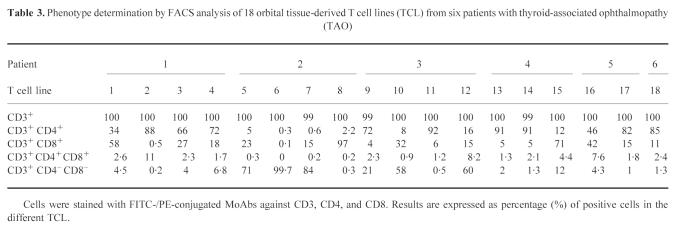
In all 18 orbital TCL derived from six patients with TAO, the T cell marker CD3+ could be detected in almost 100% of cases. TCL from all patients except patient 2 consisted predominantly of CD4+ cells (Table 3). Ten out of 18 TCL were mostly CD4+. The percentage of these CD4+ helper/inducer cells of each total TCL population ranged between 46% and 92% with a mean of 78.5%. Three TCL, nos 1 (patient 1), 8 (patient 2), and 15 (patient 4), were predominantly CD8+. Low numbers of CD4+CD8+ cells were detected in all samples. Three TCL, derived from patient 2, and two TCL (patient 3) were mainly CD4−CD8−. All remaining TCL contained a low proportion of CD4−CD8− cells.
Table 3.
Phenotype determination by FACS analysis of 18 orbital tissue-derived T cell lines (TCL) from six patients with thyroid-associated ophthalmopathy (TAO)
Cytokine profile of orbital T cell lines
We analysed the cytokine gene expression using RT-PCR. Each cDNA transcript of the predicted size could be detected in the positive control (X), prepared from stimulated PBMC from a normal subject (Fig. 1a,b). Consistent amplification of CD3-δ cDNA, which was used as an internal control for studies on T cell transcripts, could also be detected in all TCL samples from six patients with TAO, demonstrating an intact mRNA preparation and the integrity of T lymphocytes as a specific T cell marker. The generation of cytokine DNA products was variable, although the RT-PCR technique provides qualitative information only. Transcripts of IL-2, interferon-gamma (IFN-γ) and tumour necrosis factor-beta (TNF-β) typically produced by Th1 lymphocytes were amplified in all 18 TCL. IL-2 cDNA could be detected in all samples except TCL no. 11, whereas IFN-γ was detectable in 10 TCL. All six patients were positive for IFN-γ cDNA transcript products. TNF-β was found in all TCL except nos 8, 10 and 15. Amplification of TNF-α, produced by Th1 but also by Th2 lymphocytes, was generated from cDNA of 12 TCL. All analysed TCL were also positive for at least one of the Th2 cytokines. cDNA transcripts for IL-4, IL-5 and IL-6 were each demonstrated in 10 TCL. IL-4 was detectable in 12 TCL, IL-5 in all TCL except no. 15, and IL-6 in 13 TCL. IL-10 cDNA could be amplified in four samples. In TCL nos 8 and 15 with high predominance of CD8+ cells (97% and 71%, respectively; Table 3) cDNA transcripts for IFN-γ, TNF-β, IL-4 and IL-10 could not be amplified, and cDNA for IL-5 (TCL no. 8, patient 2) and IL-6 (TCL no. 15, patient 4) gave only very weak signals. No cytokine transcripts were amplified from orbital TCL mRNA without performing cDNA synthesis (data not shown), thereby excluding the presence of contaminating genomic DNA as a source of template.
Fig. 1.
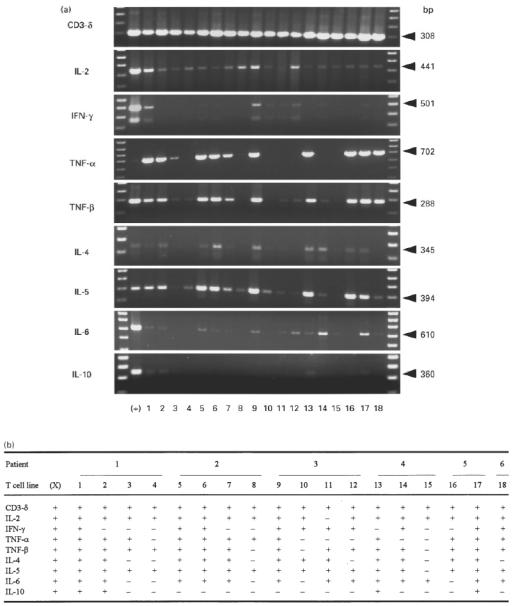
Cytokine gene expression of 18 T cell lines (TCL) derived from orbital tissue of six patients with thyroid-associated ophthalmopathy (TAO) using reverse transcription-polymerase chain reaction (RT-PCR). Following cDNA synthesis, all TCL (lanes 1–18) and as positive control cDNA prepared from stimulated peripheral blood mononuclear cells (PBMC) of a normal donor (X), were amplified by PCR simultaneously for each cytokine-specific oligonucleotide primer pair. Orbital TCL were generated from six patients with TAO (patient 1, lanes 1–4; patient 2, lanes 5–8; patient 3, lanes 9–12; patient 4, lanes 13–15; patient 5, lanes 16 and 17; patient 6, lane 18). Lanes right and left sideways represent the DNA molecular weight markers. Only the expected range of base pairs (bp) size with visualized transcripts after electrophoretic separation are shown (a). Additionally, positive signals are demonstrated by + and negative by − (b).
Immunoreactivity to thyroidal antigens
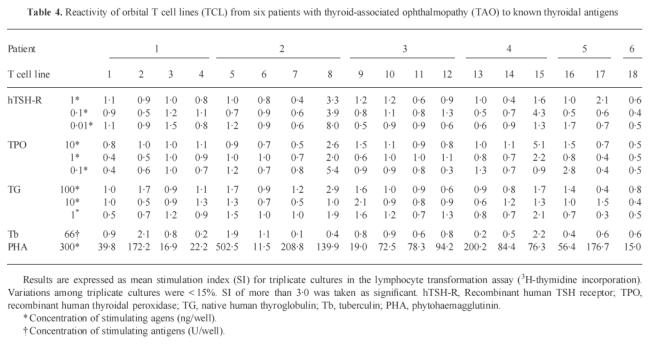
Immunological responses of 18 orbital TCL to known thyroidal antigens are summarized in Table 4. In no case was specific proliferation demonstrated. None of the TCL showed significant proliferation when incubated with TG (SI < 3.0 in all cases). TPO stimulated two TCL from two different patients, with SI values of 5.4 (TCL no. 8, patient 2) and 5.1 (TCL no. 15, patient 4), respectively. The same two TCL responded to TSH-R (SI 8.0 and 4.3, respectively). Reaction of TCL was not dependent on the concentrations of the antigens used. None of the other orbital TCL was stimulated by the thyroidal antigens used (SI < 3.0). Orbital TCL did not proliferate when incubated with Tb, but showed an increased proliferation with PHA as positive control.
Table 4.
Reactivity of orbital T cell lines (TCL) from six patients with thyroid-associated ophthalmopathy (TAO) to known thyroidal antigens
Correlations between various characteristics of orbital T cell lines
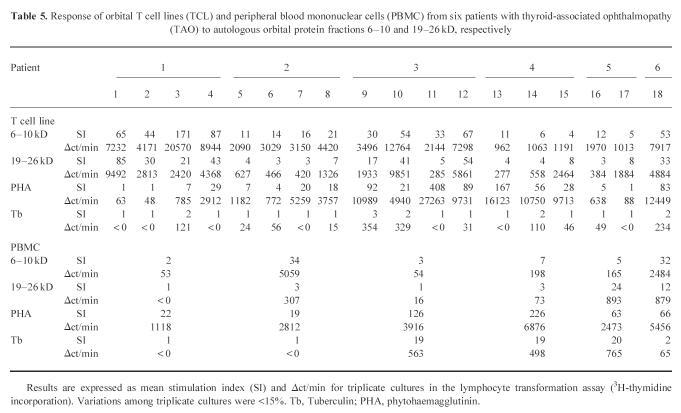
Previous investigations showed that incubation of orbital-derived TCL from six TAO patients with autologous protein fractions obtained from RO adipose/connective tissue resulted in markedly elevated T cell proliferation with proteins of 6–10 and 19–26 kD. The results are shown in Table 5 expressed in SI and Δct/min. Incubation of the two RO adipose/connective protein fractions with autologous PBMC from the investigated TAO patients induced a significant proliferation of lymphocytes from patient 6. PBMC from patients 2 and 4 responded to the protein fraction 6–10 kD and from patient 5 to the protein fraction 19–26 kD. Comparison between the magnitude of reactivity to RO tissue proteins, TCL phenotype, cytokine gene profile or the rare occurrence of thyroid reactivity showed no significant relationships. Furthermore, no correlation of the TCL characteristics was found with previous treatments of the TAO patients, e.g. orbital irradiation or steroid therapy.
Table 5.
Response of orbital T cell lines (TCL) and peripheral blood mononuclear cells (PBMC) from six patients with thyroid-associated ophthalmopathy (TAO) to autologous orbital protein fractions 6–10 and 19–26 kD, respectively
DISCUSSION
In previous investigations, we were able to establish orbital adipose/connective tissue-derived TCL from six patients with severe TAO that react to autologous protein fractions of RO tissue, as well as of cultured autologous orbital fibroblasts [16,17]. In the present study, we analysed these 18 orbital TCL in order to study their phenotype, functional and immunological properties. Since the majority of T cells infiltrating RO space are presumed to be activated [3], T lymphocytes were selected from surgical material and cultivated in IL-2-conditioned medium. With this procedure, in vivo activated T cells that already express IL-2 receptor were stimulated. After cell amplification with anti-CD3 antibodies, TCL showed specific responses to orbital antigens [16,17], thus maintaining their antigen specificity.
Immunohistological analysis in TAO patients showed a predominance of CD4+ phenotype T cells in extraocular muscle biopsies [3] and RO adipose/connective tissue [10]. In comparison, mainly CD8+ phenotype profiles were reported on RO adipose/connective tissue-derived TCL [11], and RO T cell clones displayed almost equal proportions of CD4+ and CD8+ cells [13]. Recently, TCL derived from extraocular muscles were demonstrated to be predominantly CD4+[15]. In our laboratory, previous phenotype screening of 62 orbital T cell clones revealed a CD4/CD8 ratio of 8.2, compared with a normal ratio of 2.1 in PBMC [29]. In the present study, phenotype analysis of the 18 RO adipose/connective tissue-derived TCL showed that 10 of the TCL were predominantly CD4+ lymphocytes, while only three were mainly CD8+. Five TCL were CD3+CD4−CD8−. The phenotype of RO T cells might depend more on the stage of disease than on the origin of the biopsy material like extraocular muscles or adipose/connective tissue. Although our TCL prepared from the same individual had different phenotypes, indicating that both CD4+ and CD8+ lymphocytes were present in the RO space at the same time, the predominance of CD4+ cells in the investigated TCL favours a predominantly T helper cell-mediated immune process. Since the number of TCL studied was low, it is not possible to provide definitive information about the nature of the tissue infiltrates.
T helper cells are characterized by their profile of cytokine production. In patients with TAO, but not in controls, the presence of IL-1, TNF-α and IFN-γ has been observed in orbital tissue [30]. Furthermore, in investigation of orbital fat/connective tissue by RT-PCR the presence of IL-2, IL-4 and IL-10 but not of IFN-γ in two out of five patients with TAO was reported, supporting a humoral autoimmunity [14]. Recently, analysis of extraocular muscle biopsies showed a predominance of IL-4 gene expression, and cultured TCL derived from the same tissue released a mixture of IL-2, IL-4, IFN-γ, IL-10 and TNF-β [15]. In another study, orbital fat/connective tissue-derived T cell clones of four TAO patients were established, and secretion of cytokines was measured in vitro[13]. In contrast, only a small proportion of clones secreted IL-4 and IL-5, whereas the majority secreted IL-2 and IFN-γ, suggesting a predominance of Th2-mediated cytolytic T cells in the orbit [13]. Investigated by RT-PCR, our TCL showed cytokine gene expression of Th1 cells, IL-2, IFN-γ and TNF-β. In each of the 18 TCL at least one of these cytokines were demonstrable, and most were positive for two or all three cytokines. Strong evidence for a Th2 response was also found in our patients: Th2 cytokines were detectable in all 18 orbital TCL, and most TCL expressed IL-4, IL-5 and IL-6 simultaneously. IL-6, positive in 13 TCL, is a known B and T cell activator and its over-expression has been associated with the production of antibodies [31]. IL-10, a potent immunosuppressant of macrophage function and cell-mediated immunity, with a wide array of immunostimulatory actions on B cells and autoantibody production [32], does not discriminate between a Th1 or Th2 profile. In humans, this cytokine is produced by both Th1 and Th2 cells as well as by B cells and stimulated macrophages [33]. Gene expression of TNF-α, that is predominantly of macrophage origin under in vivo circumstances, and is produced by Th1 and Th2 cells as well [23], was found in 12 out of 18 TCL. As recently shown for thyroid-derived lymphocytes [34], orbital T cells from patients with TAO are also producers of TNF-α.
In this study, gene expression of cytokines in 18 orbital TCL derived from adipose/connective tissue of TAO patients has only been demonstrated qualitatively. Although a low contamination of PBMC in analysis of RO tissue cannot be excluded and the two major types of T cell subsets are negatively interactive [35], we found gene expression of both Th1- and Th2-mediated cell activity without a preference for one or the other autoimmune process. As recently shown for the neonatal immune system [36–38], T helper cell reaction in an autoimmune disorder changes during the course of the disease and is never purely antibody- or T cell-driven. The cytokine profile and the type of autoimmune activity in the RO space may depend on the activity of TAO and may change during the course of the eye disease, thus explaining the apparent differences in the investigations. In addition, TCL cytokine gene expression could depend on the origin of the lymphocytes, either from the extraocular muscles or the orbital adipose/connective tissue.
The relationship between TAO and thyroid autoimmunity is unclear, and thyroidal antigens may also play a role in T cell immunity of the RO space [39]. Known thyroidal antigens such as TSH-R, TG and TPO, all well studied as immunological targets in Graves' disease and Hashimoto's thyroiditis, did not lead to disease-specific proliferation of our 18 TCL. Only two TCL, that consisted predominantly of CD8+ cells with a Th1 cytokine profile, were stimulated by TSH-R and TPO. In comparison with non-specific stimulation with PHA, responses were very low and not antigen dose-dependent, suggesting that only small proportions of the cell lines were responsive. It seems that T lymphocytes directed against these antigens are not predominant in the RO space. Although our T lymphocytes showed no guiding reaction pattern against known thyroidal antigens, cross reactivity with a thyroid antigen cannot be excluded.
Comparisons of the response to RO protein fraction, phenotype or cytokine profile of our investigated TCL were without significance. Previous treatment, e.g. orbital irradiation or steroid therapy of the TAO patients, from whom the TCL were derived, showed no significant influence on the characteristics of these TCL. Investigation of organ-specific T cells from an early stage of disease without previous immunosuppressive treatment would be useful to show the initial situation in TAO.
In conclusion, while results derived from the study of 18 TCL from six patients with TAO may not reflect the usual inflammatory reaction in the RO space, they suggest that infiltrating immunological cells in TAO are T lymphocytes, comprising mainly cells of the helper phenotype. Both immunological immune mechanisms, involving antibodies and cytolytic T lymphocytes, respectively, were demonstrated by cytokine gene expression of orbital derived TCL. These findings of RO tissue-reactive TCL support the concept that TAO is a tissue-specific autoimmune disease, immunologically distinct from the thyroid.
Acknowledgments
This study contains parts of the doctoral thesis of E.O.
References
- 1.Burch HB, Wartofsky L. Graves' ophthalmopathy. Current concepts regarding pathogenesis and management. Endocr Rev. 1993;14:747–93. doi: 10.1210/edrv-14-6-747. [DOI] [PubMed] [Google Scholar]
- 2.Weetman AP. Thyroid-associated eye disease: pathophysiology. Lancet. 1991;338:25–28. doi: 10.1016/0140-6736(91)90013-f. [DOI] [PubMed] [Google Scholar]
- 3.Weetman AP, Cohen S, Gatter KC, Fells P, Shine B. Immunohistochemical analysis of retrobulbar tissues in Graves' ophthalmopathy. Clin Exp Immunol. 1989;75:222–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Smith TJ, Bahn RS, Gorman CA, Cheavens M. Stimulation of glycosaminoglycan accumulation by interferon gamma in cultured human retroocular fibroblasts. J Clin Endocrinol Metab. 1991;72:1169–71. doi: 10.1210/jcem-72-5-1169. [DOI] [PubMed] [Google Scholar]
- 5.Kahaly G, Stover C, Otto E, Beyer J, Schuler M. Glycosaminoglycans in thyroid-associated ophthalmopathy. Autoimmunity. 1992;13:81–88. doi: 10.3109/08916939209014639. [DOI] [PubMed] [Google Scholar]
- 6.Hansen C, Otto E, Kuhlemann K, Förster G, Kahaly G. Glycosaminoglycans in autoimmunity. Clin Exp Rheumatol. 1996;14:59–67. [PubMed] [Google Scholar]
- 7.Tan GH, Dutton CM, Bahn RS. Interleukin-1 (IL-1) receptor antagonist and soluble IL-1 receptor inhibit IL-1 induced glycosaminoglycan production in cultured human orbital fibroblasts from patients with Graves' ophthalmopathy. J Clin Endocrinol Metab. 1996;81:449–52. doi: 10.1210/jcem.81.2.8636247. [DOI] [PubMed] [Google Scholar]
- 8.Bahn RS, Heufelder AE. Pathogenesis of Graves' ophthalmopathy. N Engl J Med. 1993;329:1468–75. doi: 10.1056/NEJM199311113292007. [DOI] [PubMed] [Google Scholar]
- 9.Hiromatsu Y, Tanaka K, Ishisaka N, et al. Human histocompatibility leucocyte antigen-DR and heat shock protein-70 expression in eye muscle tissue in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 1995;80:685–91. doi: 10.1210/jcem.80.2.7531718. [DOI] [PubMed] [Google Scholar]
- 10.Kahaly G, Hansen C, Felke B, Dienes HP. Immunohistochemical staining of retrobulbar adipose tissue in Graves' ophthalmopathy. Clin Immunol Immunopathol. 1994;73:53–62. doi: 10.1006/clin.1994.1169. [DOI] [PubMed] [Google Scholar]
- 11.Grubeck-Loebenstein B, Trieb K, Sztankay A, Holter W, Anderl H, Wick G. Retrobulbar T cells from patients with Graves' ophthalmopathy are CD8+ and specifically recognize autologous fibroblasts. J Clin Invest. 1994;93:2738–43. doi: 10.1172/JCI117289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weetman AP. The role of T lymphocytes in thyroid-associated ophthalmopathy. Autoimmunity. 1992;13:69–73. doi: 10.3109/08916939209014637. [DOI] [PubMed] [Google Scholar]
- 13.De Carli M, D'Elios MM, Mariotti S, et al. Cytolytic T cells with Th1-like cytokine profile predominate in retroorbital lymphocytic infiltrates of Graves' ophthalmopathy. J Clin Endocrinol Metab. 1993;77:1120–4. doi: 10.1210/jcem.77.5.8077301. [DOI] [PubMed] [Google Scholar]
- 14.McLachlan SM, Prummel MF, Rapoport B. Cell-mediated or humoral immunity in Graves' ophthalmopathy? Profiles of T-cell cytokines amplified by polymerase chain reaction from orbital tissue. J Clin Endocrinol Metab. 1994;78:1070–4. doi: 10.1210/jcem.78.5.8175962. [DOI] [PubMed] [Google Scholar]
- 15.Pappa A, Calder V, Ajjan R, Fells P, Ludgate M, Weetman AP, Lightman S. Analysis of extraocular muscle-infiltrating T cells in thyroid-associated ophthalmopathy (TAO) Clin Exp Immunol. 1997;109:362–9. doi: 10.1046/j.1365-2249.1997.4491347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otto E, Ochs K, Leyendecker E, et al. Autoimmune endocrine ophthalmopathy and retrobulbar antigens. Horm Metab Res. 1995;27:533–8. doi: 10.1055/s-2007-980020. [DOI] [PubMed] [Google Scholar]
- 17.Otto E, Ochs K, Hansen C, Wall J, Kahaly G. Orbital tissue-derived T lymphocytes from patients with Graves' ophthalmopathy recognize autologous orbital antigens. J Clin Endocrinol Metab. 1996;81:3045–50. doi: 10.1210/jcem.81.8.8768872. [DOI] [PubMed] [Google Scholar]
- 18.Arnold K, Tandon N, McIntosh RS, Elisei R, Ludgate M, Weetman AP. T cell responses to orbital antigens in thyroid-associated ophthalmopathy. Clin Exp Immunol. 1994;96:329–34. doi: 10.1111/j.1365-2249.1994.tb06562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perros P, Kendall-Taylor P. Demonstration of thyrotropin binding sites in orbital connective tissue: possible role in the pathogenesis of thyroid-associated ophthalmopathy. J Endocrinol Invest. 1994;17:163–70. doi: 10.1007/BF03347708. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli EK. Cleavage structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Gulle H, Schoel B, Kaufmann SHE. Direct blotting with viable cells of protein mixtures separated by two-dimensional gel electrophoresis. J Immunol Methods. 1990;133:253–61. doi: 10.1016/0022-1759(90)90366-4. [DOI] [PubMed] [Google Scholar]
- 22.Londei M, Grubeck-Loebenstein B, de Berardinis P, Greenall C, Feldmann M. Efficient propagation and cloning of human T cells in the absence of antigen by means of OKT3, interleukin-2, and antigen-presenting cells. Scand J Immunol. 1988;27:35–46. doi: 10.1111/j.1365-3083.1988.tb02321.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamamura M, Uyemura K, Deans RJ, et al. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–9. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 24.Sorg R, Enczmann J, Sorg U, Heermeier K, Schneider EM, Wernet P. Rapid and sensitive mRNA phenotyping for interleukins (IL-1 to IL-6) and colony-stimulating factors (G-CSF, M-CSF, and GM-CSF) by reverse transcription and subsequent polymerase chain reaction. Exp Hematol. 1991;19:882–7. [PubMed] [Google Scholar]
- 25.Brenner CA, Tam AW, Nelson PA, et al. Message amplification phenotyping (MAPPing): a technique to simultaneously measure multiple mRNAs from small numbers of cells. Bio Techniques. 1989;7:1096–103. [PubMed] [Google Scholar]
- 26.Zhou LJ, Tedder TF. A distinct pattern of cytokine gene expression by human CD83+ blood dendritic cells. Blood. 1995;86:3295–301. [PubMed] [Google Scholar]
- 27.Corry DB, Ampel NM, Christian L, Locksley RM, Galgiani JN. Cytokine production by peripheral blood mononuclear cells in human coccidioidomycosis. J Infect Dis. 1996;174:440–3. doi: 10.1093/infdis/174.2.440. [DOI] [PubMed] [Google Scholar]
- 28.Haubruck H, Mauch L, Cook NJ, et al. Expression of recombinant human thyroid peroxidase by the baculovirus system and its use in ELISA screening for diagnosis of autoimmune thyroid disease. Autoimmunity. 1993;15:275–84. doi: 10.3109/08916939309115749. [DOI] [PubMed] [Google Scholar]
- 29.Stover C, Otto E, Beyer J, Kahaly G. Cellular immunity and retrobulbar fibroblasts in Graves' ophthalmopathy. Thyroid. 1994;4:161–5. doi: 10.1089/thy.1994.4.161. [DOI] [PubMed] [Google Scholar]
- 30.Heufelder AE, Bahn RS. Detection and localization of cytokine immunoreactivity in retro-ocular connective tissue in Graves' ophthalmopathy. Eur J Clin Invest. 1993;23:10–17. doi: 10.1111/j.1365-2362.1993.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 31.Hirano T. The biology of IL-6. Chem Immunol. 1992;51:153–80. [PubMed] [Google Scholar]
- 32.Moore KW, O'Garra A, de Waal Malefyt R, Viera P, Mosmann TR. Interleukin-10. Ann Rev Immunol. 1993;11:165–90. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 33.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1), type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–60. [PubMed] [Google Scholar]
- 34.Aust G, Heuer M, Laue S, et al. Expression of tumor necrosis factor-alpha (TNF-α) mRNA and protein in pathological thyroid tissue and carcinoma cell lines. Clin Exp Immunol. 1996;105:148–54. doi: 10.1046/j.1365-2249.1996.d01-726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwarz EM, Salgame P, Bloom BR. Molecular regulation of human interleukin 2 and T-cell function by interleukin 4. Proc Natl Acad Sci USA. 1993;90:7734–8. doi: 10.1073/pnas.90.16.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–6. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 37.Sarzotti DS, Robbins PM, Hoffman I. Induction of protective CTL responses in newborn mice by murine retrovirus. Science. 1996;271:1726–8. doi: 10.1126/science.271.5256.1726. [DOI] [PubMed] [Google Scholar]
- 38.Forsthuber HC, Yip PV, Lehmann H. Induction of Th1 and Th2 immunity in neonatal mice. Science. 1996;271:1728–30. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 39.Arnold K, Weetman AP. Cell-mediated immunity in thyroid-associated ophthalmopathy. Orbit. 1996;15:159–64. [Google Scholar]