Abstract
Sézary syndrome (SzS) is the leukaemic variant of cutaneous T cell lymphoma (CTCL), whose malignant T cells are of the Th2 type in most cases. In this study we investigated the tumouricidal activity of cytotoxic T lymphocytes (CTL) present in peripheral blood of a patient with Th2-type SzS, focusing on the effect of IL-2, IFN-γ and IL-12 on their cytotoxic activity, and the relationship between their lytic capacity and the patient's clinical course. At four different time points during a 2-month clinical period, CD4+ CD7− Sézary cells and CD8+ cells were separated from the patient's circulating cells. CD8+ cells were cultured with chemically attenuated, purified Sézary cells in the presence of IL-2 to develop specific cytotoxicity. The CD8+ cells thus cultured exhibited lytic activity against autologous Sézary cells. Concomitant addition of IFN-γ or IL-12 exerted a synergistic cytolytic effect with IL-2 on the tumour cells. Cytotoxicity inhibition studies using MoAbs revealed that the cytotoxicity operated in MHC class I-, CD8- and αβ T cell receptor-dependent manners. Furthermore, eight CD8+ T cell clones generated from cultured CD8+ cells exhibited a strong cytotoxicity against Sézary cells in an MHC class I-restricted fashion. During the clinical course, the activity of generated CTL and the number of CD8+ cells were inversely correlated with disease activity as assessed by the serum level of lactate dehydrogenase. These findings suggest that CTL down-regulate the growth of malignant T cells in this long-standing disease. Since Th2 cytokines such as IL-4 down-modulate CTL activity, CTL are assumed to be usually suppressed in SzS, whose malignant T cells are of Th2 type. It is likely that the administration of IFN-γ normalizes this Th2-skewing state, activates CTL, and thus exerts the therapeutic effectiveness in the treatment of CTCL.
Keywords: Sézary syndrome, cutaneous T cell lymphoma, cytotoxic T lymphocyte, interferon-gamma, IL-12
INTRODUCTION
Cytotoxic T cells (CTL) bearing CD8 molecules as well as natural killer (NK) cells play a central role as effectors against several kinds of malignant cells [1,2]. CTL lyse tumour cells following recognition by T cell receptor (TCR) of limited endogenous tumour-antigen peptides in the context of MHC class I molecules [3,4]. On the other hand, NK cells kill tumour cells by a tumour antigen peptide-non-specific manner [5]. Tumour vaccination intended to generate tumouricidal memory CTL by immunizing with relevant peptides is a current topic of tumour immunity [6,7]. CD4+ T helper (Th) cells can be divided into two distinct subsets: Th1 cells, producing IL-2 and IFN-γ, and Th2 cells, secreting IL-4, IL-5 and IL-10 [8]. These two subsets, and the cytokines derived from them, regulate each other as counterparts in several biologic events [8]. Many observations have shown that Th1 but not Th2 cytokines are the most potent cofactor in proliferation and activation of tumour-specific cytotoxic cells [9–11].
Cutaneous T cell lymphoma (CTCL) is a clonal T cell malignancy in skin, which often leads to haematogenous dissemination of malignant T cells [12]. Sézary syndrome (SzS) is the leukaemic variant of CTCL and its malignant cells have the surface phenotype and function of mature Th cells [13]. Malignant cells in SzS mostly belong to the Th2 type [14–16], although exceptional Th1 cases exist [17], In patients with CTCL, a typical set of immunological abnormalities is usually observed. This includes decreased T cell responses to mitogens, decreased NK cell activity, eosinophilia, and increased levels of IgE [18]. These abnormalities can be attributed to the malignant clone that expresses a Th2 cytokine secretion pattern in peripheral blood [14,19]. Increased production of IL-4 and IL-5 is known to underlie immunological alterations of these kinds. Thus, the administration of Th1 cytokines is expected to improve SzS, and IFN-γ has been approved in Japan by the Ministry of Health and Welfare for the treatment of CTCL. Recently, Berger et al. [20] reported that patients with advanced CTCL have CD8+ cells that specifically kill autologous tumour cells in an MHC class I-restricted fashion. The purposes of this study were to investigate (i) whether CTL are present in the peripheral blood of a Th2-type Sézary patient, (ii) whether IFN-γ exerts its tumouricidal activity by directly inhibiting Th2 malignant cells [21] or by restoring Th1 cell [22] and cytotoxic cell activities, and (iii) whether CTL are clinically relevant in the disease activity. We demonstrate that circulating CTL against malignant T cells do exist in a patient with SzS. The clinical relevance of CTL was evidenced by fluctuations of the cytotoxic activity in association with the serum level of lactate dehydrogenase (LDH). The cytotoxic activity of these cells was enhanced in vitro by cultivation with IFN-γ or IL-12 in combination with IL-2, while IFN-γ did not directly target malignant Th2 cells.
PATIENTS AND METHODS
Patient
A 59-year-old Japanese man had been diagnosed as suffering from SzS on the basis of reported criteria [23–25] and followed for up for 4 years by our Department since 1993. Histological and immunohistochemical studies of erythrodermic skin showed infiltration of CD3+, CD4+, CD8−, CD45RO+, CD45RA− lymphocytes with a convoluted nucleus. He had a marked peripheral blood lymphocytosis (300–400 × 109/l) with a high CD4/CD8 ratio (9–370; the percentage of CD8+ cells, 0.2–10.4%). Flow cytometric analyses revealed that circulating CD3+ CD4+ Sezary cells were positive for CD45RO but negative for CD7 and CD45RA. A clonally rearranged band for a TCR β-chain (Cβ1) [26] was found by Southern blot analysis in DNA extracted from crude peripheral blood mononuclear cells (PBMC), CD4+ CD7− PBMC purified as described below, and biopsy specimens of skin and lymph node. A serum specimen was negative for antibody against human T cell lymphotropic virus-1. He was treated with chemotherapy in 1993 and with total electron beam irradiation in 1995. In 1996 the patient had received i.v. injections of recombinant (r)IFN-γ (Maruho Phermaceutical Co., Osaka, Japan, and Suntory Ltd, Osaka, Japan), 2 × 106 Japan Reference Units (JRU; 1 JRU roughly corresponds to 4 NIH units) per day for 28 days, which resulted in partial and transient improvement of skin conditions and a decrease in the number of circulating CD4+ cells by ≈ 40%. The patient had not received any treatment including rIFN-γ, systemic corticosteroids, chemotherapy, or psoralen and ultraviolet A therapy for at least 2 weeks before and during this study. Oral antibiotics and topical corticosteroids were administered transiently.
Monoclonal antibodies and recombinant cytokines
The fluorescein-conjugated MoAbs used in this study included anti-CD3, -CD4, -CD7, -CD8, -CD45RA, and -CD45RO (Becton Dickinson, Mountain View, CA); anti-αβTCR and anti-pan HLA class I antigen (BMA 031 and B9.12.1, respectively; Immunotech, Marseille, France). PE-conjugated anti-CD4 MoAb was purchased from Becton Dickinson. The purified forms of anti-CD4, -CD8, -αβTCR, and -pan HLA class I antigen MoAbs were used for inhibition assay of cytotoxicity. BMA 031 and B9.12.1 recognize a monomorphic epitope expressed on all αβTCR molecules and all class I antigens, respectively. Recombinant IL-2 (6.9 × 106 U/mg; Genzyme, Cambridge, MA), rIL-12 (107 U/mg; PharMingen, San Diego, CA), and rIFN-γ (Maruho Co and Suntory Ltd) were used in in vitro studies.
Separation of Sézary cells and CD8+ cells
PBMC were isolated from heparinized venous blood by density centrifugation on Ficoll–Hypaque (Pharmacia AB, Uppsala, Sweden). PBMC were washed twice in PBS pH 7.4 and resuspended at appropriate concentrations in medium. Malignant T cells and CD8+ cells were purified from the patient's PBMC by positive and negative selections with immunomagnetic beads. PBMC at 2 × 107 cells/ml were incubated for 60 min at 4°C with anti-CD4 MoAb-conjugated magnetic beads (Dynal Inc., Great Neck, NY) at a ratio of three beads per cell. CD4+ cells bound to the magnetic beads were collected with a magnet and cultured in a CO2 incubator overnight to separate the cells from the beads. Remaining CD4− PBMC were subjected to CD8+ cell preparation. Purified CD4+ cells were further incubated for 30 min at 4°C with anti-CD7 MoAb. After washing three times, cells were suspended with anti-mouse IgG antibody-conjugated magnetic beads (Dynal Inc.) at a ratio of 20 beads per cell. Following incubation on ice for 1 h, cell-bound magnetic beads were removed with a magnet. Remaining CD4+ CD7− tumour cells were used as stimulator or target cells for CD8+ cells and CTL clones. CD8+ cells were purified by incubating CD4− PBMC with anti-CD8 MoAb-conjugated magnetic beads at 84% purity by flow cytometric analysis. Viability of purified CD4+ CD7− Sézary cells and CD8+ cells was > 96% by trypan blue dye exclusion test.
Immunofluorescence staining and flow cytometric analysis
Cells [106] were suspended in Hanks' balanced salt solution (HBSS) containing 0.1% sodium azide and 1% heat-inactivated fetal calf serum (FCS; Filtron, Karlstein, Germany) and incubated for 30 min at 4°C with FITC- or PE-conjugated MoAbs. After three washes, 104 labelled cells were analysed on a FACScan (Becton Dickinson).
Reverse transcriptase polymerase chain reaction analysis of cytokine mRNA expression
Total RNA was extracted from the freshly separated CD4+ CD7− tumour cell as described previously [27]. First stand cDNA was reverse transcribed and was amplified by polymerase chain reaction (PCR) as described previously [17]. The primers used were as follows: IL-2, 5′ primer ATGTACAGGATGCAACTCCTGTCTT, 3′ primer GTCAGTGTTGAGATGATGCTTTGAC; IL-4, 5′ primer ATGGGTCTCACCTCCCAACTGCT, 3′ primer CGAACACTTTGAATATTTCTCTCTCAT; IL-5, 5′ primer GCTTCTG-CATTTGAGTTTGCTAGCT, 3′ primer TGGCCGTCAATGTATTTCTTTATTAAG; IL-10, 5′ primer ATCAGCTGGACAACTTGTTG, 3′ primer GTCCTAGAGTCTATAGAGTC; IFN-γ, 5′ pri-mer ATGAAATATACAAGTTATATCTTGGCTTT, 3′ primer GATGCTCTTCGACCTCGAAACAGCAT; and β-actin, 5′ primer TGACGGGGTCACCCACACTGTGCCCATCTA, 3′ primer CTAGAAGCATTGCGGTGGACGATGGAGGG. The PCR products and DNA molecular weight marker VI (Boehringer, Mannheim, Germany) were separated in 2% agarose gels. The gel was stained with ethidium bromide (1 mg/ml) and visualized with an ultraviolet transilluminator.
Generation of cytotoxic CD8+ cells and CD8+ T cell clones
RPMI 1640 (Gibco Labs, Grand Island, NY) medium supplemented with 25 mmol/l HEPES, 2 mmol/ll-glutamine, 1 mmol/l non-essential amino acids, 5 × 10−5 mol/l 2-mercaptoethanol (2-ME), 1 mmol/l sodium pyruvate (all from Gibco), 100 μg/ml gentamycin sulphate (Schering-Plough, Osaka Japan) and 10% heat-inactivated FCS was used for all cultures. Purified CD8+ cells were cultured at a density of 5 × 105 cells/ml in medium with rIL-2 (50 U/ml), rIFN-γ (500, 1000 or 5000 U/ml), and/or rIL-12 (1 or 10 ng/ml) and stimulated with mitomycin-C-treated (Sigma Chemical Co., St Louis, MO; 100 mg/ml, 30 min, 37°C) CD4+ CD7− tumour cells (2 × 105 cells/ml). One week after stimulation, their cytotoxic activity was determined as below. By limiting dilution (cell density 0.5 cells/well in 96-well culture plate) following restimulation of the expanded CD8+ cells with mitomycin C-treated patient's PBMC and 50 U/ml, eight clones exhibiting high cytolysis against autologous Sézary tumour cells were established.
Cytotoxicity assay and cytotoxicity inhibition assay
To determine the cytotoxic response against tumour cells, various numbers of cultured CD8+ cells or CD8+ cell clones were assayed by incubating 1 × 10451Cr-labelled CD4+ CD7− tumour cells for 6 h at 37°C. Target tumour cells were radio-labelled by suspension at a concentration of 1 × 107 cells/ml in medium containing 200 μCi/ml Na[51CR] (Du Pont NEN, Boston, MA) for 60 min at 37°C, and were washed three times. After incubation, the radio activity in the medium and cells was counted in a gamma counter. The percentage specific lysis was calculated as described previously [28]. Inhibition of cytotoxicity was tested by the addition of anti-CD4, -CD8, -αβTCR or -pan class I antigen MoAb at various concentrations in cultures of cytotoxic assay.
Proliferation assay
Cells (2 × 105/ml) of freshly isolated CD4+ CD7− PBMC were cultured in triplicate in a final volume of 100 μl of culture medium in 96-well flat-bottomed microtitre plates (Corning Glass Works, Corning, NY) with or without concanavalin A (Con A; 1 or 5 μg/ml) for 6 h at 37°C in 5% CO2 in air and pulsed with methyl 3H-thymidine (3H-TdR; 1 μCi/well) 8 h before harvest. The cells were collected on glassfibre filters using a cell harvester (Cambridge Technologies, Watertown, MA).
RESULTS
Phenotype and cytokine profile of isolated Sézary cells
A major tumour cell population isolated from PBMC of the SzS patient was phenotyped with MoAbs to dissect contamination of other cells as well as to characterize CTCL malignant cells. Flow cytometric analyses showed that purified tumour cells, corresponding to 71% of PBMC, were positive for CD3, CD4, CD45RO and HLA class I antigen and negative for CD7, CD8 and CD45RA (Fig. 1a). Both the presence of CD45RO [29] and the absence of CD7 [30,31] are classical features of Sézary cells. The percentage of contaminating cells as assessed by CD7 positivity was < 0.1%. It was implied that the high expression of HLA class I antigen (mean fluorescence intensity (MFI) 188), compared with its level in CD4+ cells of PBMC from a normal subject (MFI 135) (Fig. 1a), potentially renders the tumour cells feasible as targets for CTL. Sézary cells thus isolated were used in the following studies.
Fig. 1.
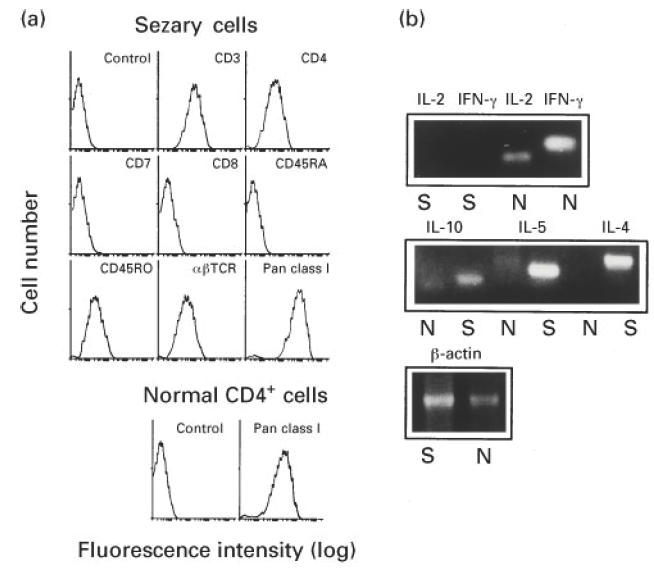
Surface phenotype and cytokine mRNA expression pattern of Sézary cells purified from peripheral blood mononuclear cells (PBMC) of the Sézary syndrome (SzS) patient by CD4+ and CD7−selections. (a) The tumour cells and CD4+ cells from PBMC of a normal subject were stained with the indicated FITC-conjugated MoAbs and analysed by flow cytometry. Fluorescein-conjugated mouse IgG MoAb was used as a control antibody. (b) Total RNA was extracted from Sézary cells (S) and PBMC from a normal subject (N). Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed by using mRNA-specific oligodeoxynucleotide primers for IFN-γ, IL-2, IL-4, IL-5 and IL-10 cytokines. Following agarose gel electrophoresis, RT-PCR products were visualized by ethidium bromide.
To examine cytokine expression in these tumour cells, reverse transcriptase (RT)-PCR was performed. The freshly isolated cells transcribed IL-4, IL-5 and IL-10 mRNA, whereas message for IL-2 or IFN-γ was not detected (Fig. 1b). Since PBMC from five normal individuals expressed mRNA for IL-2 and IFN-γ but not IL-4 or IL-5 mRNA, these results indicated that this Sézary cell population belonged to the Th2 type, as has been reported previously [14].
No inhibitory effect of IFN-γ on proliferation of Sézary cells
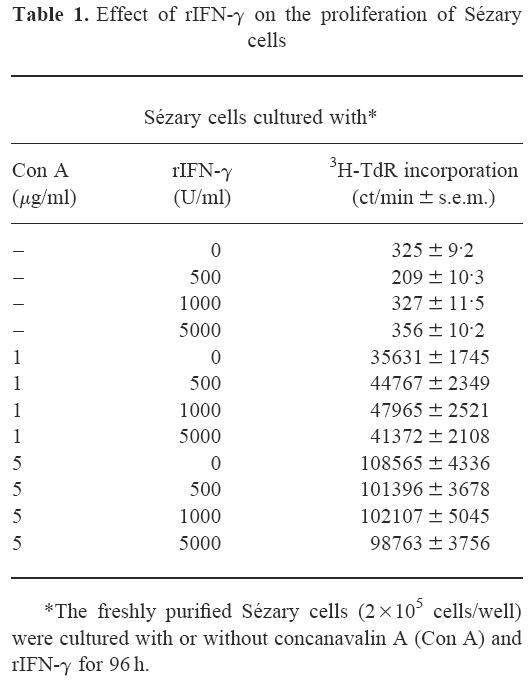
The patient showed partial and transient clinical improvement after systemic administration of rIFN-γ. To test direct attenuation of Th2-type Sézary cells by IFN-γ, we first investigated the effect of IFN-γ on the proliferation of purified Sézary cells. As shown in Table 1, neither unstimulated nor Con A (1 or 5 μg/ml)-induced Sézary cell growth was affected by rIFN-γ at any concentration (500, 1000 or 5000 U/ml), suggesting no direct action of IFN-γ against Sézary cells.
Table 1.
Effect of rIFN-γ on the proliferation of Sézary cells
Presence of Sézary cell-killing CTL whose induction is augmented by IFN-γ and IL-2 and fluctuations in their cytotoxic activity during the patient's clinical course
We examined cytotoxicity of CD8+ cells specific for autologous Sézary cells at four different time points (A–D) at intervals of 10–14 days during a 2-month patient's clinical period as indicated in Fig. 2a,b. CD8+ cells at each time point were cultured with mitomycin-C-treated purified Sézary cells for 2 weeks in the presence of rIL-2 and/or rIFN-γ to develop specific cytotoxicity, and cultured cells were assayed for their tumour-specific cytotoxicity. As observed at time point A (Fig. 2aA), CD8+ cells cultured with rIL-2 significantly lysed tumour targets. Of note is that simultaneous addition of rIFN-γ with rIL-2 enhanced the cytotoxicity in a concentration-dependent fashion. This augmentation of cytotoxicity by rIFN-γ was due to a synergistic effect with rIL-2, because cytotoxicity was not induced by rIFN-γ (1000 or 5000 U/ml) alone (data not shown). However, CD8+ cells at time points B and C exerted modest cytolytic effects only when cells were cultured with rIL-2 combined with a high concentration of rIFN-γ (5000 U/ml) (Fig. 2aBC). Again, rIFN-γ concentration-dependent induction of cytotoxicity was markedly found in CD8+ cells at time point D (Fig. 2aD).
Fig. 2.
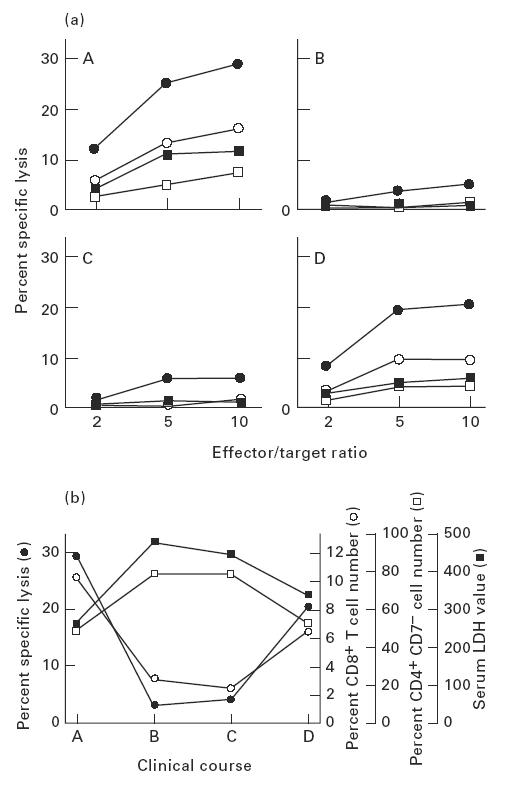
Relationships of cytotoxic activity of cultured CD8+ cells, circulating CD8+ cell percentage, and serum lactate dehydrogenase (LDH) level in the patient's clinical course. (a) CD8+ cells were obtained from the patient at four different time points (A–D) at intervals of 10–14 days during a 2-month clinical period. CD8+ cells separated at each time point were cultured in the presence of rIL-2 (50 U/ml) (□), or rIL-2 (50 U/ml) combined with rIFN-γ (500 U/ml (▪), 1000 U/ml (○), or 5000 U/ml (•)) with mitomycin-C-treated purified Sézary cells. Cytotoxicity of the cultured CD8+ cells (effector cells) was assayed by 51Cr-release of labelled Sézary tumour cells (target cells) at the indicated effector/target ratios. (b) CD8+ cells separated at each time point were cultured in medium supplemented with rIL-2 (50 U/ml) and rIFN-γ (5000 U/ml). Sézary cell-specific cytotoxicity was measured by cytolytic assay at an effector/target ratio of 10 (•). PBMC were stained by fluorescein-conjugated anti-CD8 MoAb and subjected to flow cytometric analysis (○). PBMC were double-stained by fluorescein-conjugated anti-CD7 MoAb and PE-conjugated anti-CD4 MoAb, and percentage of CD4+ CD7− cells was calculated by flow cytometry (□). LDH levels in sera were measured at each time point (▪).
A cytotoxicity inhibition assay was performed to phenotype cytotoxic effector cells. As shown in Fig. 3, cytotoxicity of the cultured CD8+ cells at time point A was blocked concentration-dependently by anti-class I, -αβTCR, and -CD8 MoAbs, but not anti-CD4 or control mouse IgG MoAbs. Thus, CTL were suggested to be a main cytotoxic effector cell in cultured CD8+ cells and to recognize Sézary tumour peptides in the context of MHC class I by specific binding of αβTCR and CD8 accessory molecules.
Fig. 3.
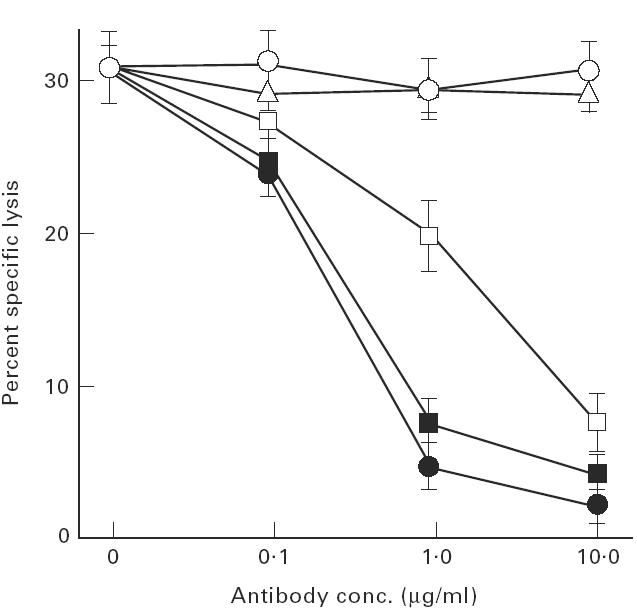
CD8-, αβTCR-, MHC class I-dependent cytolysis of Sézary cells by the cultured CD8+ cells. Inhibition assays of cytotoxicity of cultured CD8+ cells using anti-αβTCR, -pan HLA class I, -CD4 and -CD8 MoAbs were performed. Cytotoxicity was assayed by 51Cr-release of labelled Sézary tumour cells at an effector/target ratio of 10. Anti-αβTCR (▪), -pan HLA class I (•), -CD4 (○) and -CD8 (□) MoAbs were added at the start of the cytotoxic assay at the indicated concentrations. Mouse IgG MoAb (▵) was used as a control antibody. Data are expressed as mean ± s.e.m. of the results of duplicate experiments.
Changes in the cytotoxic activity of cultured CD8+ cells were monitored in relation to the patient's disease activity and the percentage of circulating CD8+ cells. Since it is widely known that increment in serum LDH level in CTCL patients is highly indicative of exacerbation or progression of the disease [32], LDH served as a function of disease activity. The cytotoxicity was represented by the lytic activity of CD8+ cells cultured with 50 U/ml rIL-2 and 5000 U/ml rIFN-γ. As summarized in Fig. 2b, during the 2-month clinical period, Sézary cell-specific CTL activity was inversely correlated with the value of serum LDH as well as the percentage of circulating CD4+ CD7− cells. On the other hand, there was a positive correlation between CTL activity and the percentage of circulating CD8+ cells at four time points. These findings suggested that elevation of tumour-specific cytotoxicity in patients' PBMC was closely associated with alleviation of the disease in its clinical course.
Comparison of rIL-12 with rIFN-γ in the augmentative effects on Sézary cell-specific cytotoxicity
In addition to rIFN-γ, IL-12, which was recently found to be a cytotoxic cell potentiator [33,34], may also be an enhancer of the cytotoxicity of CD8+ cells. Therefore, CD8+ cells separated at time point C were cultured with mitomycin-C-treated tumour cells in medium supplemented with rIL-12 and/or rIL-2. No enhancement efficacy of cytotoxicity was exhibited when CD8+ cells were cultured with rIL-12 (1 or 10 ng/ml) alone, whereas cytotoxicity was elevated in a rIL-12 concentration-dependent manner in combination with rIL-2 (50 U/ml) (Fig. 4). This augmentative effect of rIL-12 was much greater than that of rIFN-γ. Considering that rIL-12 produced a strong cytotoxicity even at time point C when CTL appeared to be functionally depressed, it was suggested that IL-12 has more potency than IFN-γ as an enhancer during the induction event of Sézary cell-specific CD8+ cells.
Fig. 4.
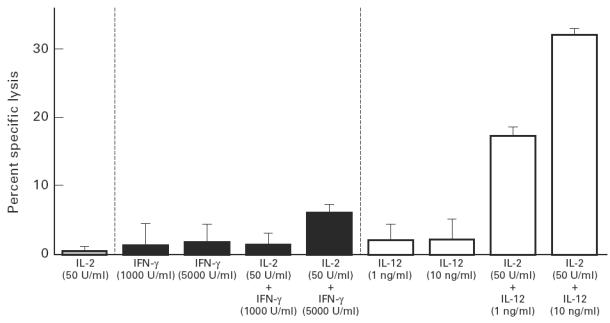
Enhancement of cytotoxicity of CD8+ cells by rIL-12 and/or rIL-2. CD8+ cells of the Sézary syndrome (SzS) patient at time point C were cultured in the presence of rIL-2 (50 U/ml), rIL-12 (1 or 10 ng/ml), rIL-12 (1 or 10 ng/ml) combined with rIL-2 (50 U/ml), rIFN-γ (1000 or 5000 U/ml), and rIFN-γ (1000 or 5000 U/ml) combined with rIL-2 (50 U/ml) with mitomycin-C- treated purified Sézary cells. Cytotoxicity of the cultured CD8+ cells (effector cells) was assayed by 51Cr-release of labelled Sézary cell (target cells) at an effector/target ratio of 10. Data are expressed as mean ± s.e.m. of the results of duplicate experiments.
Generation of CTL clones specific for Sézary cells
Eight CD8+ T cell clones were generated by limiting dilution of the cultured CD8+ cells at time point A. All of these clones exhibited an αβTCR+ CD4 CD8+ phenotype (Fig. 5a). When compared with short-term-cultured CD8+ cells (see Fig. 2a), these CTL clones exerted strong cytotoxicity even at low effector/target ratios (Fig. 5b). The cytotoxic activity was almost fully blocked by anti-MHC class I, -αβTCR, or -CD8 MoAb (Fig. 5c), as observed in crude CD8+ cells (see Fig. 3).
Fig. 5.
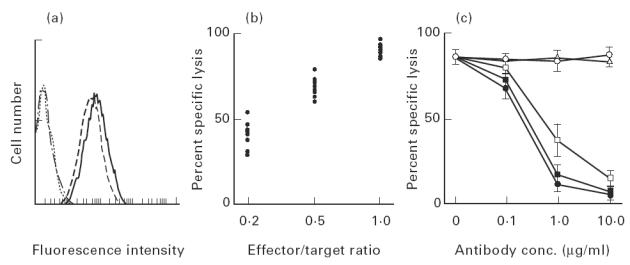
Classical cytotoxic T lymphocytes (CTL) bearing CD8 and αβTCR are the main cytotoxic cells against Sézary cells in the Sézary syndrome (SzS) patient. (a) The representative CTL clone generated from cultured CD8+ cells at a time point A was phenotyped by flow cytometric analysis using fluorescein-conjugated anti-αβTCR (- - - -), -CD4 (… …), -CD8 (– - − - –), and control mouse IgG MoAbs (–. −. –). (b) Cytotoxicities of eight CTL clones were assayed by 51Cr-release of labelled Sézary cells at the indicated effector/target ratio. Data are dot-plotted as average of the results of duplicated experiments. (c) Cytotoxicity inhibition assays of representative CTL clone using anti-αβTCR, -pan HLA class I, -CD4 and -CD8 MoAbs were performed. Cytotoxicity was assayed by 51Cr-release of labelled Sézary cells at an effector/target ratio of 1. Anti-αβTCR (▪), -pan HLA class I (•), -CD4 (○) and -CD8 (□) MoAbs were added at the start of the cytotoxic assay at the indicated concentrations. Mouse IgG MoAb (Δ) was used as a control antibody. Data are expressed as mean ± s.e.m. of the results of duplicate experiments.
DISCUSSION
This study clearly demonstrates that Sézary cell-specific CTL or their precursors do exist in advanced SzS. Of particular interest was the observation that the cytotoxic activity of these cells as well as the percentage of circulating CD8+ cells fluctuated in association with disease activity as assessed by the serum level of LDH. Thus, disease activity seems to be influenced by not only the number of CD8+ cytotoxic cells but also their functional activity on a per cell basis. However, caution is necessary in drawing definite conclusions, because we analysed only one patient in this study. Since this patient had a high percentage of Sézary cells in PBMC, it appears to evaluate the percentage of CD8+ CTL and their cytolytic activity against Sézary cells. We found that in another Sézary patient, who had ≈ 20% CD4+ CD7− cells, CTL activity was less marked than in the present case.
IL-4, IL-5 and IL-10 from Th2 cells cause IgE hyper-production, eosinophil activation, and inhibition of antigen-presenting cell capacities, respectively [35]. In vitro immunological abnormalities in SzS patients are similar to consequences of these known biological effects of Th2 cytokines [14], and malignant T cells in most cases of SzS may be of Th2 type, as observed in this patient. IL-4 inhibits the activation or proliferation of cytotoxic cells such as NK cells, lymphokine-activated killer (LAK) cells, and CTL [36,37], and down-regulates the production of IL-2 and IFN-γ by Th1 cells. In addition, IL-4 reduces the mRNA transcription of cytotoxic mediator granules such as perforin or granzyme B in cytotoxic cells activated by IL-2 [38]. Thus, IL-4 produced by Th2 Sézary cells seems to down-modulate killer cells in several possible ways in patients with SzS. In fact, the production of IL-2 and IFN-γ is inhibited and the activity of cytotoxic cells is decreased in SzS [14,19]. IL-2 and IFN-γ produced by Th1 cells promote the generation, differentiation, and activation of cytotoxic cells, including CTL, NK cells, LAK cells and tumour-infiltrating lymphocytes [6,9–11]. Deterioration of Th1 activities that is triggered by a large amount of Th2 cytokines produced by Sézary cells may lead to reduction in the percentage and cytotoxic activity of CTL.
It was demonstrated that in vitro cultivation with rIFN-γ combined with rIL-2 enhanced CTL activity. IFN-γ antagonizes the biological activity of IL-4 [39] and can even inhibit the growth of Th2 cells [40]. In our study, the addition of rIFN-γ at a concentration of 5000 U/ml to cultures did not inhibit the proliferative response of purified Sézary cells to Con A. This suggests that the therapeutic efficacy of rIFN-γ administration does not stem simply from the direct inhibitory effect of IFN-γ on malignant Th2 cells. Rather, it is likely that rIFN-γ exerts its therapeutic effect by stimulating CTL-mediated immunity. In addition, IFN-γ may promote the expression of MHC class I or cell adhesion molecules on the surface of tumour cells, which enhances tumour antigen-specific cytolysis by CTL [41]. Moreover, it is possible that the administration of IFN-γ normalizes the Th2-skewing circumstance, resulting in activation of CTL.
IL-12, when combined with IL-2, has a synergistic effect on the capacity of tumouricidal CTL [34]. Our study clearly demonstrates that IL-12 combined with IL-2 more powerfully enhances the capacity of CTL against Sézary cells than IFN-γ plus IL-2. IL-12 exhibits potent Th1 cytokine-inducing effects during the responses to microorganism antigen [42,43]. In addition, IL-12 augments NK cell activity and CTL proliferation and activation [33,34]. Therefore, IL-12 is featured as a Th2 cell inhibitor [22]. The production of IL-12 is reportedly inhibited in PBMC of SzS patients [44], resulting in the impaired cytotoxic cell function in SzS patients.
Both assistance of Th1 cytokines and stimulation with specific antigen peptide–MHC class I complex are needed for successful maturation of CTL. CTL recognition of the tumour antigen peptides in the context of MHC class I molecule is a central topic of today for tumour-specific recognition [6,7]. Whereas it is suggested by the reported [20] and current studies that the Sézary tumour antigen peptide is recognized by CD8+ CTL in an MHC class I-restricted manner [20], the antigen peptide in CTCL cells remains unknown. Further studies of purification and characterization of Sézary tumour antigen peptide that stimulate specifically CTL may provide a therapeutic strategy for killing tumour cells as well as vaccination in SzS.
Acknowledgments
We thank Ms Keiko Sugaya for technical assistance and Ms Fumiyo Ohmori for preparation of the manuscript.
References
- 1.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boon T, Cerottini JC, Van Der Eynde B, Van Der Brugenn P, Van Pel A. Tumor antigen recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–65. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 3.Rotzschke O, Falk K. Naturally occurring peptide antigens derived from the MHC class I restricted processing pathway. Immunol Today. 1991;12:447–55. doi: 10.1016/0167-5699(91)90018-O. [DOI] [PubMed] [Google Scholar]
- 4.Parham P. Pictures of MHC restriction. Nature. 1996;384:109–10. doi: 10.1038/384109a0. [DOI] [PubMed] [Google Scholar]
- 5.Malnati MS, Peruzzi M, Parker KC, Biddison WE, Ciccone E, Moretta A, Long EO. Peptide specificity in the recognition of MHC class I by natural killer cell clones. Science. 1995;267:1016–8. doi: 10.1126/science.7863326. [DOI] [PubMed] [Google Scholar]
- 6.Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nature Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 7.Young JW, Inaba K. Dendritic cells as adjuvants for class I major histocompatibility complex-restricted antitumor immunity. J Exp Med. 1996;183:7–11. doi: 10.1084/jem.183.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma: a preliminary report. N Engl J Med. 1988;319:1676–80. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 10.Siegel JP. Effects on interferon-γ on the activation of human T lymphocytes. Cell Immunol. 1988;111:461–72. doi: 10.1016/0008-8749(88)90109-8. [DOI] [PubMed] [Google Scholar]
- 11.Seo N, Egawa K. Utilization of leucine methyl ester for generation of hybridomas producing monoclonal antibodies specific to tumor-associated antigens. Cancer Immunol Immunother. 1994;38:277–80. doi: 10.1007/BF01533520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edelson R. Cutaneous T cell lymphoma (mycosis fungoides, Sézary syndrome and other variants) J Am Acad Dermatol. 1980;2:89–106. doi: 10.1016/s0190-9622(80)80385-9. [DOI] [PubMed] [Google Scholar]
- 13.Heald P, Edelson R. The immunobiology of cutaneous T-cell lymphoma. J Natl Cancer Inst. 1991;83:400–4. doi: 10.1093/jnci/83.6.400. [DOI] [PubMed] [Google Scholar]
- 14.Vowels BR, Cassin M, Vonderheid EC, Rook AH. Aberrant cytokine production by Sézary syndrome patients: cytokine secretion pattern resembles murine Th2 cells. J Invest Dermatol. 1992;99:90–94. doi: 10.1111/1523-1747.ep12611877. [DOI] [PubMed] [Google Scholar]
- 15.Saed G, Fivenson DP, Naidu Y, Nickoloff J. Mycosis fungoides exhibits a Th1-type cell-mediated cytokine profile whereas Sézary syndrome expresses a Th2-type profile. J Invest Dermatol. 1994;103:29–33. doi: 10.1111/1523-1747.ep12388985. [DOI] [PubMed] [Google Scholar]
- 16.Tendler CL, Burton JD, Danielpour D, Charley M, Mccoy JP, Pittelkow MR, Waldmann TA. Abnormal cytokine expression in Sézary and adult T-cell leukemia cells correlates with the functional diversity between these T-cell malignancies. Cancer Res. 1994;54:4430–5. [PubMed] [Google Scholar]
- 17.Yagi H, Tokura Y, Furukawa F, Takigawa M. CD7-positive Sézary syndrome with a Th1 cytokine profile. J Am Acad Dermatol. 1996;34:368–74. doi: 10.1016/s0190-9622(07)80011-9. [DOI] [PubMed] [Google Scholar]
- 18.Heald PW, Edelson RL. Lymphoma, pseudolymphoma and related conditions. In: Fitzpatrick TB, editor. Dermatology in general medicine. 4. New York: McGraw-Hill; 1993. pp. 1285–312. [Google Scholar]
- 19.Rook AH, Vowels BR, Jaworsky C, Singh A, Lessin SR. The immunopathogenesis of cutaneous T cell lymphoma: abnormal cytokine production by Sézary T cells. Arch Dermatol. 1993;129:486–9. [PubMed] [Google Scholar]
- 20.Berger CL, Wang N, Christensen I, Longley J, Heald P, Edelson RL. The immune response to class I-associated tumor specific cutaneous T-cell lymphoma antigens. J Invest Dermatol. 1996;107:392–7. doi: 10.1111/1523-1747.ep12363378. [DOI] [PubMed] [Google Scholar]
- 21.Yagi H, Tokura Y, Furukawa F, Takigawa M. Th2 cytokine mRNA expression in primary cutaneous CD30-positive lymphoproliferative disorders: successful treatment with recombinant interferon-γ. J Invest Dermatol. 1996;107:827–32. doi: 10.1111/1523-1747.ep12330845. [DOI] [PubMed] [Google Scholar]
- 22.Vowels BR, Lessin SR, Cassin M, Benoit BM, Rook AH. Normalization of cytokine secretion patterns and immune function following disappearance of malignant clone from the peripheral blood of a Sézary syndrome patient. J Invest Dermatol. 1993;100:556–62. [Google Scholar]
- 23.Lutzner MA, Jordan HW. The ultrastructure of an abnormal cell in Sézary's syndrome. Blood. 1968;31:719–26. [PubMed] [Google Scholar]
- 24.Edelson RL. Pathogenesis of T-cell lymphoma of skin. J Am Acad Dermatol. 1983;9:957–60. doi: 10.1016/s0190-9622(83)80083-8. [DOI] [PubMed] [Google Scholar]
- 25.Willemze R, Van Vloten WA, Hermans J, Damsteeg MJ, Meijer CJ. Diagnostic criteria in Sézary syndrome: a multiparameter study of peripheral blood lymphocytes in 32 patients with erythroderma. J Invest Dermatol. 1983;81:392–7. doi: 10.1111/1523-1747.ep12521991. [DOI] [PubMed] [Google Scholar]
- 26.Ikuta K, Ogura T, Shimizu A, Honjo T. Low frequency of somatic mutation in β-chain variable region genes of human T-cell receptors. Proc Natl Acad Sci USA. 1985;82:7701–5. doi: 10.1073/pnas.82.22.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 28.Seo N, Egawa K. Suppression of cytotoxic T lymphocyte activity by γ/δ T cells in tumor bearing mice. Cancer Immunol Immunother. 1995;40:358–66. doi: 10.1007/BF01525386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vonderheid EC, Bigler RD, Greenberg AS, Neukum SJ, Micaily B. Extracorporeal photopheresis and recombinant interferon alfa 2b in Sézary syndrome. Use of dual marker labeling to monitor therapeutic response. Am J Clin Oncol. 1994;17:255–63. doi: 10.1097/00000421-199406000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Labaatide WB, Rana MT, Barker CR. A new monoclonal antibody (CH-F42) recognizes a CD7 subset of normal T-lymphocytes and circulating malignant cells in adult T-cell lymphoma-leukemia and Sézary syndrome. Blood. 1990;76:1361–8. [PubMed] [Google Scholar]
- 31.Wood GS, Hong SR, Sasaki DT, Abel EA, Hoppe RT, Warnke RA, Norhenn VB. Leu-8/CD7 antigen expression by CD3+ T cells: comparative analysis of skin and blood in mycosis fungoides/ Sézary syndrome relative to normal blood values. J Am Acad Dermatol. 1990;22:602–7. doi: 10.1016/0190-9622(90)70080-2. [DOI] [PubMed] [Google Scholar]
- 32.Marti RM, Eatrach T, Reverter JC, Mascaro JM. Prognostic clinicopathologic factors in cutaneous T-cell lymphoma. Arch Dermatol. 1991;127:1511–6. doi: 10.1001/archderm.1991.01680090075007. [DOI] [PubMed] [Google Scholar]
- 33.Manetti R, Parronchi P, Giudizi MG, Piccinni M-P, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (IL-12) induces Th1-type specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi M, Fitz L, Hewick RM, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–45. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powrie F, Coffman RL. Cytokine regulation of T-cell function; potential for therapeutic intervention. Immunol Today. 1993;14:270–4. doi: 10.1016/0167-5699(93)90044-L. [DOI] [PubMed] [Google Scholar]
- 36.Nagler A, Lanier LL, Phillips JH. The effects of IL-4 on human natural killer cells. A potent regulator of IL-2 activation and proliferation. J Immunol. 1988;141:2349–51. [PubMed] [Google Scholar]
- 37.Kawakami Y, Custer MC, Rosenberg SA, Lotze MT. IL-4 regulates IL-2 induction of lymphokine-activated killer activity from human lymphocytes. J Immunol. 1989;142:3452–61. [PubMed] [Google Scholar]
- 38.Salvucci O, Chouaib FM, Moreau JL, Theze J, Chehimi J, Chouaib S. Differential regulation of interleukin-12- and interleukin-15-induced natural killer cell activation by interleukin-4. Eur J Immunol. 1996;26:2736–41. doi: 10.1002/eji.1830261128. [DOI] [PubMed] [Google Scholar]
- 39.Peleman R, Wu J, Rargeas C, Delespsse G. Recombinant interleukin 4 suppresses the production of interferon gamma by human mononuclear cells. J Exp Med. 1989;170:1751–6. doi: 10.1084/jem.170.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gajewski TF, Fitch FW. Anti-proliferative effect of IFNγ in immune regulation. I. IFNγ inhibits the proliferation of TH2 but not TH1 murine HTL clones. J Immunol. 1988;140:4245–52. [PubMed] [Google Scholar]
- 41.Imai K, Ng A-K, Glassy MC, Ferrone S. Differential effect of interferon on the expression of tumor-associated antigens and histocompatibility antigens on human melanoma cells: relationship to susceptibility to immune lysis mediated by monoclonal antibodies. J Immunol. 1981;127:505–8. [PubMed] [Google Scholar]
- 42.Chan SH, Perussia B, Gupta JW, et al. Induction of interferon-γ production by natural killer cell stimulatory factor: characterization of the responding cells and synergy with other inducers. J Exp Med. 1991;173:869–79. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'garra A, Murphy KM. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 44.Rook AH, Kubin M, Cassin M, et al. IL-12 reverses cytokine and immune abnormalities in Sézary syndrome. J Immunol. 1995;154:1491–8. [PubMed] [Google Scholar]


