Abstract
MRL/Mp-lpr/lpr (MRL/lpr) mice spontaneously develop destructive inflammation of the salivary and lachrymal glands resembling Sjögren's syndrome (SS), representing an animal model to study this disease. We used in situ hybridization with synthetic radiolabelled oligonucleotide probes to examine expression of mRNA encoding pro- and anti-inflammatory cytokines in submandibular glands of 2, 3, 4 and 5-month-old MRL/lpr mice. Phenotypic composition of submandibular gland infiltrates was evaluated by immunohistochemistry. Cells expressing tumour necrosis factor-alpha (TNF-α), IL-1β, IL-6 and IL-12 mRNA were strongly up-regulated at about the time of onset of sialoadenitis, suggesting a role of these cytokines in development of the disease. Interferon-gamma (IFN-γ) and cytolysin mRNA-expressing cells were gradually up-regulated over the disease course up to 5 months of age, the time when sialoadenitis is at its height, favouring a role of these cytokines in progression of the disease as well. Low levels of IL-10 and transforming growth factor-beta (TGF-β) mRNA-expressing cells were observed at 2, 3 and 4 months of age, and were almost undetectable at 5 months. Maximum levels of CD4+, CD8+ and interdigitating/dendritic cells, as well as of MHC class II and MHC class I expression were seen at 3 months, with CD4+ outnumbering CD8+ cells. Maximum levels of macrophages were seen at 4 months of age. These data argue for a major role of the proinflammatory cytokines TNF-α, IL-1β, IL-6, IL-12, IFN-γ and cytolysin in initiation and perpetuation of autoimmune sialoadenitis in MRL/lpr mice, probably in conjunction with an insufficiency of the anti-inflammatory cytokines TGF-β and IL-10.
Keywords: cytokine mRNA, sialoadenitis, MRL/lpr mice
INTRODUCTION
Sjögren's syndrome (SS) is an autoimmune disease characterized by progressive lymphocytic infiltration of the salivary and lachrymal glands, leading to insufficient secretion and hence dry mouth and eye symptoms [1,2]. Malignant lymphoproliferative diseases such as lymphomas and macroglobulinaemia may develop in some patients with SS [3,4].
MRL/Mp-lpr/lpr (MRL/lpr) mice spontaneously develop lymphadenopathy, serum autoantibodies, hypergammaglobulinaemia and generalized autoimmune disorders including glomerulonephritis and arthritis [5,6]. Both T and B cells are considered necessary for the development of the generalized autoimmune disease in MRL/lpr mice [7–11]. The autoimmune disorder in MRL/lpr mice also includes development of a destructive inflammation of the salivary and lachrymal glands, resembling SS and representing a well characterized animal model to study this human disease [12,13].
A differentiated and age-related pattern of cytokine expression has been suggested to play an important role in development and progression of the autoimmune process in the salivary glands of MRL/lpr mice [14–16]. Production of interferon-gamma (IFN-γ) and IL-2 receptor within inflammatory foci in submandibular glands suggests an active role of these cytokines in local tissue destruction in these mice [14]. Tumour necrosis factor-alpha (TNF-α) gene expression and protein production, and IL-1β gene expression were detected in salivary glands of 2-month-old MRL/lpr mice, as were IL-6 gene expression and protein production in 3-month-old mice. Expression of these cytokine genes was consistently detected in mice up to 8 months old [16].
In the present study we examine the profiles of cells expressing mRNA for pro- and anti-inflammatory cytokines in submandibular glands and among spleen mononuclear cells (MNC) of 2, 3, 4 and 5-month-old female MRL/lpr mice, using in situ hybridization. Immunohistochemistry was used to define the phenotypic composition of submandibular gland infiltrates at each time point.
MATERIALS AND METHODS
Animals
Female MRL/lpr mice and BALB/c mice purchased from Charles River Co. (Sulzfeld, Germany) were housed at the animal facilities of Huddinge University Hospital with water ad libitum. Five mice from each group were killed at 2, 3 and 4 months of age, and four mice at 5 months of age. Submandibular glands were dissected, snap-frozen in liquid nitrogen and stored at −70°C.
Isolation of spleen MNC
Spleens were dissected and ground, followed by osmotic lysis of erythrocytes. MNC were washed three times in culture medium. Cell concentration was thereafter adjusted to 10 × 106 viable cells/ml as evaluated by trypan blue staining. The proportion of viable cells generally exceeded 90%. MNC (1 × 105) were dried at 55°C for 15 min onto restricted areas of electrically charged microscope slides (ProbeOn slides; Fisher Scientific, Pittsburgh, PA), fixed for 1 min in 1% formaldehyde and rinsed in PBS. Slides were then stored in sealed boxes with silica at −20°C until hybridization.
Histological evaluation
Cryostat sections (10 μm) of submandibular glands were thaw-mounted onto gelatin-coated glass slides. For evaluation of submandibular gland pathology, submandibular gland tissue sections were haematoxylin and eosin-stained, and examined using light microscopy. The noted inflammation was roughly categorized as follows: 0, no inflammatory cells present; +, few perivascular and periductal inflammatory infiltrates (< 100 cells); + +, moderate number of perivascular and periductal inflammatory infiltrates (100–500 cells); and + + +, extensive inflammation with large inflammatory foci (> 500 cells) infiltrating the glandular tissues.
Immunohistochemical evaluation
For characterization of the phenotypic composition of submandibular gland infiltrates, sections were exposed to rat MoAbs MAS 110 (MoAb against mouse L3/T4 on helper/inducer T cells); MAS 111c (MoAb against Lyt-2 on mouse suppressor/cytotoxic T cells, CD8 equivalent); MAS 740 (MoAb anti-mouse pan macrophage) and MAS 755 (MoAb anti-mouse interdigitating/dendritic cells) (Harlan, Sera-Lab, Crawley Down, UK) as well as to Ox6 (mouse anti-rat MHC class II) and Ox18 (mouse anti-rat MHC class I). Sections were stained according to the avidin-biotin technique (Vectastain Elite kit; Vector Labs, Burlingame, CA). Omission of the primary antibody served as a negative control. Specificity of the staining was also controlled on sections of peripheral lymphoid organs. The tissue areas were measured by image analysis and the numbers of stained cells were counted, using light microscopy, and calculated per 100 mm2 of tissue area.
In situ hybridization
In situ hybridization was performed as described for tissue sections [17] and modified for MNC [18]. Synthetic oligonucleotide probes (Scandinavian Gene Synthesiz AB, Köping, Sweden) were labelled using 35S-deoxyadenosine-5′-α-(thio)-triphosphate (New England Nuclear, Cambridge, MA) with terminal deoxynucleotidyl transferase (Amersham, Aylesbury, UK). The oligonucleotide sequences were obtained from GenBank and probes were designed using MacVector software (IBI, New Haven, CT) (Table 1). A mixture of four probes was used for each cytokine to increase the sensitivity of the method. A constant ratio of the guanine/cytosine content of ≈ 60% was employed. The oligonucleotide probes were ≈ 48 bases long and checked for absence of palindromes or long sequences of homology within the species against available GenBank data. After hybridization, emulsion autoradiography and developing, slides were stained with cresyl violet and mounted with Entellan (Merck, Darmstadt, Germany). Coded slides were examined by dark field microscopy at ×10 magnification. The results were expressed as numbers of mRNA-positive cells per 105 spleen MNC or 100 mm2 tissue section. Cells were judged as positive when containing > 15 silver grains per cell with star-like distribution over the cytoplasm (Fig. 1a,b). In cells judged negative, the number of grains was mostly 0–2 per cell, and the grains were randomly scattered over the cells and not in a star-like fashion. The cellular distribution of the grains was always checked under light microscopy at × 20 and/or × 40 magnification. There were no difficulties in differentiating between cytokine mRNA-positive and -negative cells. Variation between duplicates was < 10%. A sense probe with the nucleotide sequence for rat IFN-γ exon 4 was used as control for each cytokine. This control probe used in parallel with the cytokine probe on the cells from each specimen revealed no cells with grains exceeding background counts.
Table 1.
A survey of the cytokine probes used
Fig. 1.
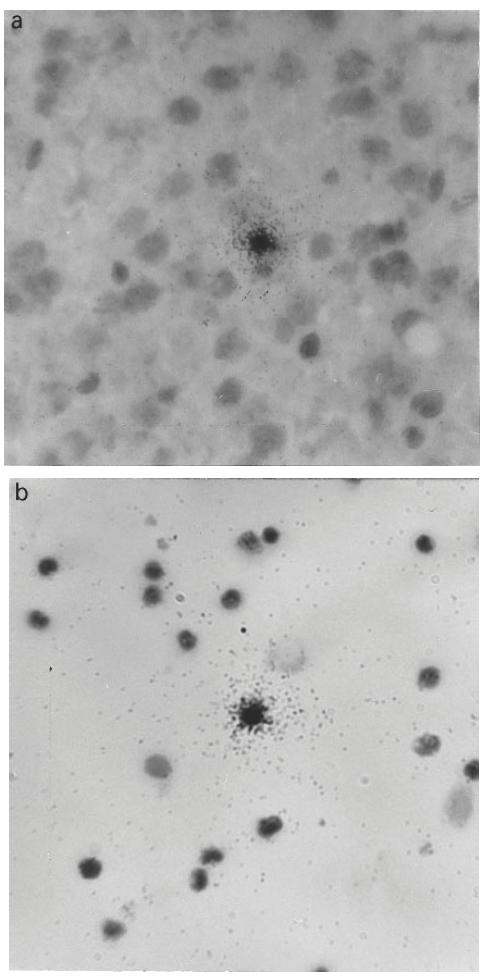
Autoradiograms of in situ hybridization. A cluster of grains over individual cells show: (a) transforming growth factor-alpha mRNA-expressing cell in the submandibular gland inflammatory infiltrate in 2-month-old MRL/lpr mouse (×400), and (b) IL-6 mRNA-expressing cell among spleen mononuclear cells in 5-month-old MRL/lpr mouse (×400).
Statistical analysis
Differences between pairs of groups were tested by unpaired Student's t-test. Differences between three or more groups were tested by one-factor analysis of variance (anova). Post hoc tests between pairs of means were performed according to Scheffé. The level of significance was set to α = 0.05. All tests were two-sided.
RESULTS
Submandibular gland pathology in MRL/lpr mice
Table 2 summarizes the histopathology observed in submandibular glands of MRL/lpr mice at 2, 3, 4 and 5 months old. A mild submandibular gland inflammation with small perivascular MNC infiltration was observed in three mice at 2 months of age (Fig. 2a). Moderate inflammation with small to large perivascular and periductal MNC infiltration was observed in all mice at 3 months of age. Most mice at 4 and 5 months of age showed severe inflammation with large perivascular and periductal MNC infiltration of the submandibular glands, with multiple focal infiltration and tissue destruction (Fig. 2b). In contrast, none of the control BALB/c mice at any age showed more than mild inflammatory cell infiltrates in submandibular glands.
Table 2.
Submandibular gland pathology in groups of MRL/lpr mice under study*
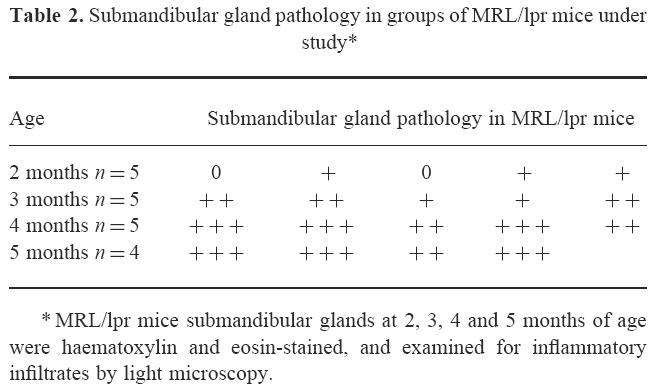
Fig. 2.
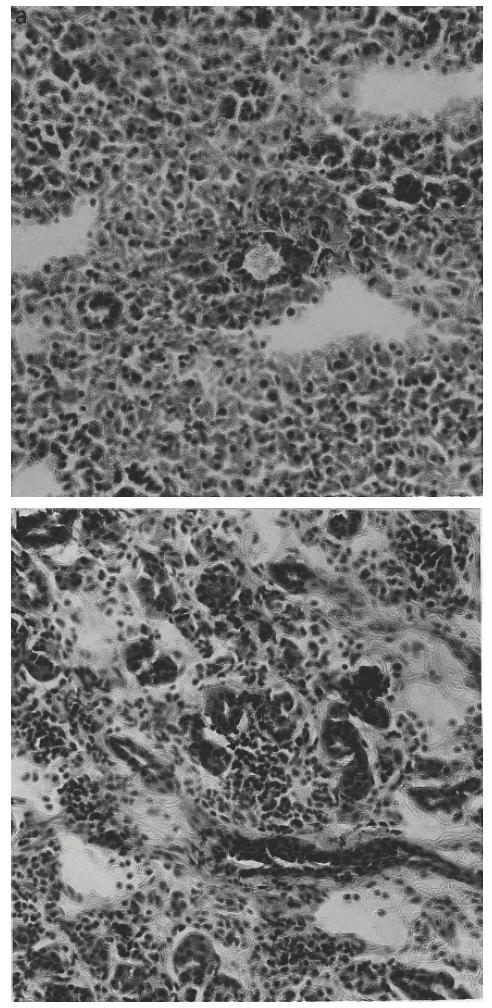
The histological appearance of the submandibular glands of MRL/lpr mice. (a) Mild mononuclear cell infiltration in 2-month-old mouse (×200), and (b) severe mononuclear cell infiltration in 5-month-old mouse (×200).
Immunohistochemical analysis of submandibular gland infiltrates
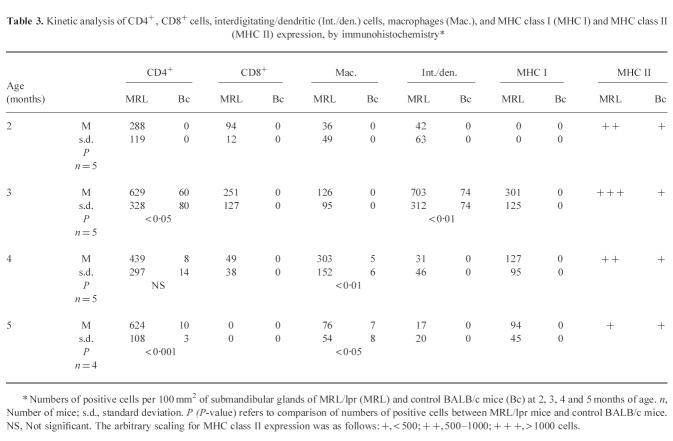
Extensive CD4+, CD8+, and interdigitating/dendritic cells and macrophage infiltrations, as well as increased MHC class I and MHC class II expression, were detected in submandibular glands of MRL/lpr mice at 2, 3, 4 and 5 months of age (Table 3). The maximum levels of CD4+, CD8+ and interdigitating/dendritic cell infiltrations in submandibular glands of MRL/lpr mice appeared at 3 month of age. The maximum levels of CD4+ cells outnumbered CD8+ cells. Five-month-old MRL/lpr mice showed extensive infiltrations of CD4+ cells and few macrophages and interdigitating/dendritic cells in submandibular glands. Increased MHC class I expression was detected in submandibular glands of 3 and 5-month-old MRL/lpr mice. In contrast, only few CD4+ cells, macrophages and interdigitating/dendritic cells, and very low levels of MHC class II expression were seen in submandibular gland sections of the BALB/c control mice of the same age.
Table 3.
Kinetic analysis of CD4+, CD8+ cells, interdigitating/dendritic (Int./den.) cells, macrophages (Mac.), and MHC class I (MHC I) and MHC class II (MHC II) expression, by immunohistochemistry*
Analysis of cytokine mRNA-expressing cells
TNF-α, IL-1β and IL-6 mRNA expression
Patterns of TNF-α, IL-1β and IL-6 mRNA-expressing cells in relation to age of the MRL/lpr mice were rather similar in the submandibular glands. The highest numbers of cells expressing mRNA were observed at about 3 months of age (Figs 3a, 4a, 5a). At 5 months of age, numbers of TNF-α (P < 0.01), IL-1β (P < 0.05) and IL-6 (P < 0.05) were lower compared with numbers detected at 3 months of age, though levels of TNF-α (P < 0.01; Fig. 3a) and IL-6 (P < 0.05; Fig. 5a) remained elevated at this time point in the MRL/lpr mice compared with BALB/c mice.
Fig. 3.
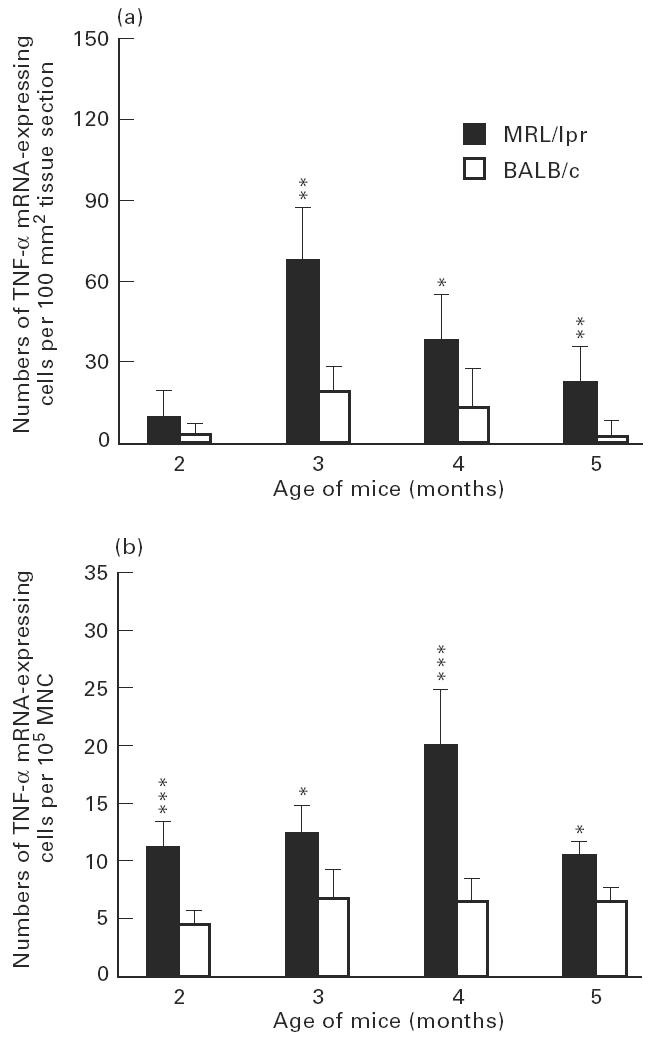
Numbers of tumour necrosis factor-alpha (TNF-α) mRNA-expressing cells, detected by in situ hybridization with 35S-labelled synthetic oligonucleotide probes, per 100 mm2 submandibular gland tissue sections (a), and per 105 spleen mononuclear cells (MNC) (b), of MRL/lpr mice and BALB/c mice at 2, 3, 4 and 5 months of age. Each column indicates mean value of five mice and bars indicate s.d. Levels of significance for differences between MRL/lpr mice and age-matched control BALB/c mice: *P < 0.05; **P < 0.01; ***P < 0.001. There were significantly higher numbers of TNF-α mRNA-expressing cells both in submandibular glands and among spleen MNC in MRL/lpr mice compared with control BALB/c mice at 3, 4 and 5 months of age. Peak numbers of submandibular gland TNF-α mRNA-expressing cells occurred at 3 months of age, while peak numbers of TNF-α mRNA-expressing spleen MNC were registered at 4 months of age.
Fig. 4.
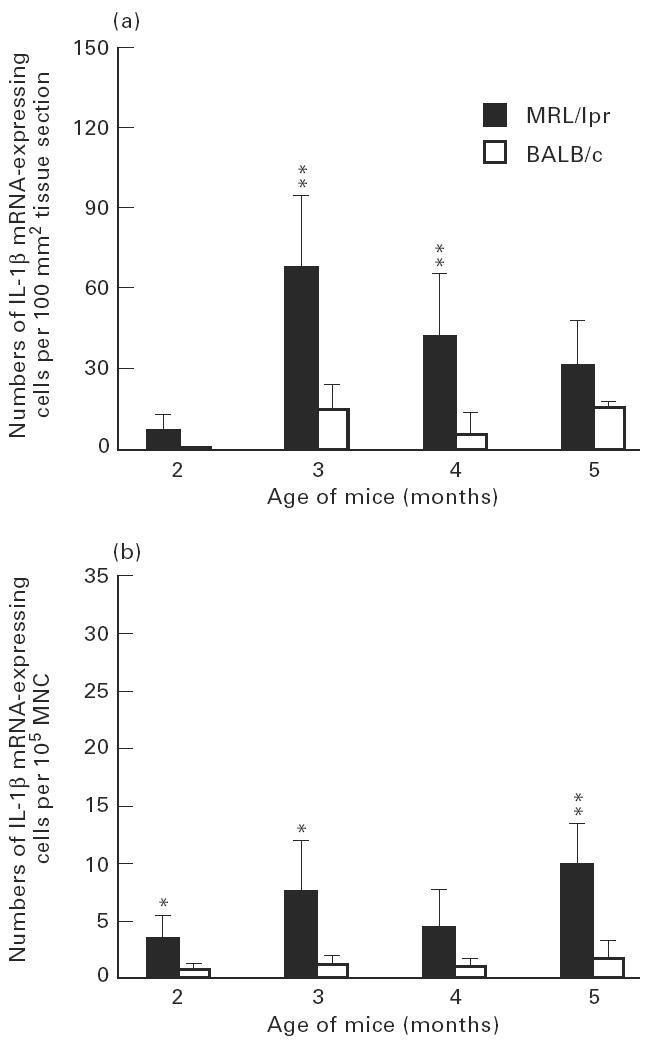
Numbers of IL-1β mRNA-expressing cells in submandibular glands (a) and among spleen mononuclear cells (MNC) (b) of MRL/lpr mice and control BALB/c mice. For explanation see legend to Fig. 3.
Fig. 5.
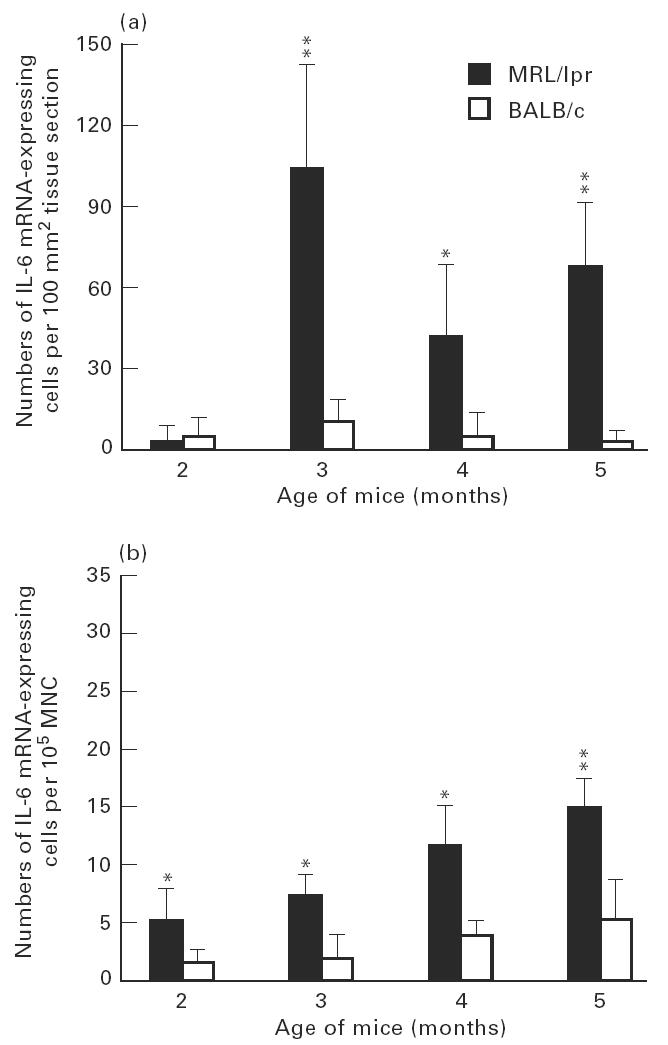
Numbers of IL-6 mRNA-expressing cells in submandibular glands (a) and among spleen mononuclear cells (MNC) (b) of MRL/lpr mice and control BALB/c mice. For explanation see legend to Fig. 3.
In spleen MNC, patterns of mRNA expression of these cytokines were different from submandibular glands. The level of TNF-α mRNA-expressing cells peaked at 4 months of age, when it was higher (P < 0.01) than at 5 months (Fig. 3b). In contrast, the highest numbers of IL-1β and IL-6 mRNA-expressing cells were observed at 5 months of age (Figs 4b and 5b).
IFN-γ and cytolysin mRNA-expressing cells
The highest numbers of IFN-γ and cytolysin mRNA-expressing cells in submandibular glands were detected at 5 months of age (Figs 6a and 7a). This level of IFN-γ mRNA-expressing cells was higher (P < 0.05) than at 2 months. Among spleen MNC, in contrast, higher levels IFN-γ and cytolysin mRNA-expressing cells were observed at 2 months of age (Figs 6b and 7b). This level of IFN-γ mRNA-expressing cells was higher (P < 0.01) than at 5 months.
Fig. 6.
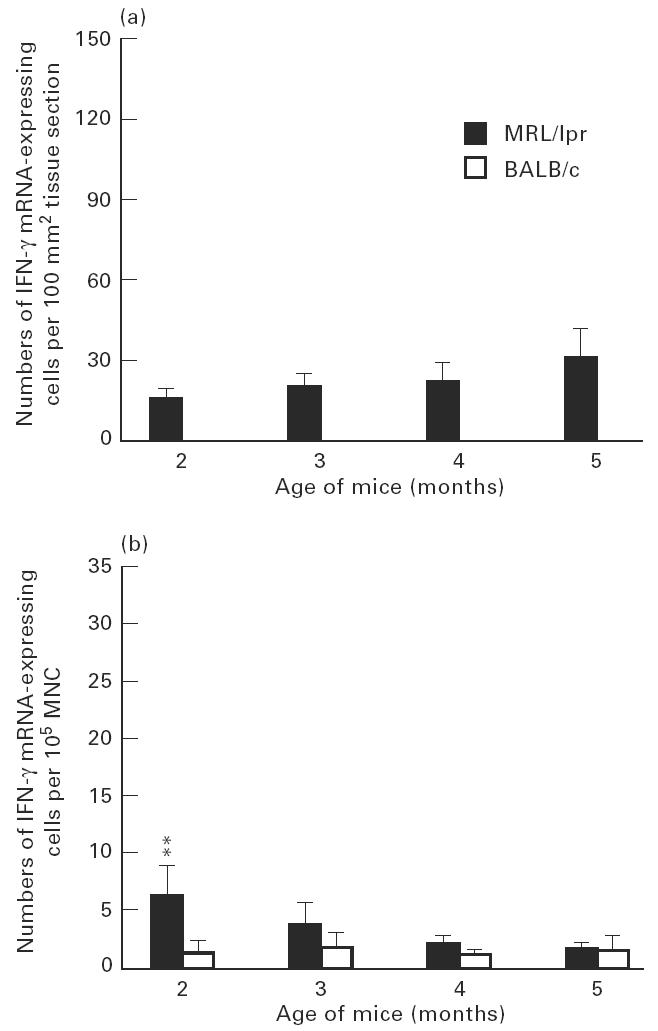
Numbers of IFN-γ mRNA-expressing cells in submandibular glands (a) and among spleen mononuclear cells (MNC) (b) of MRL/lpr mice and control BALB/c mice. For explanation see legend to Fig. 3. The numbers of IFN-γ mRNA-expressing cells of MRL/lpr mice at 2, 3, 4 and 5 months of age were significantly higher compared with age-matched BALB/c mice, where IFN-γ mRNA expression was not detectable.
Fig. 7.
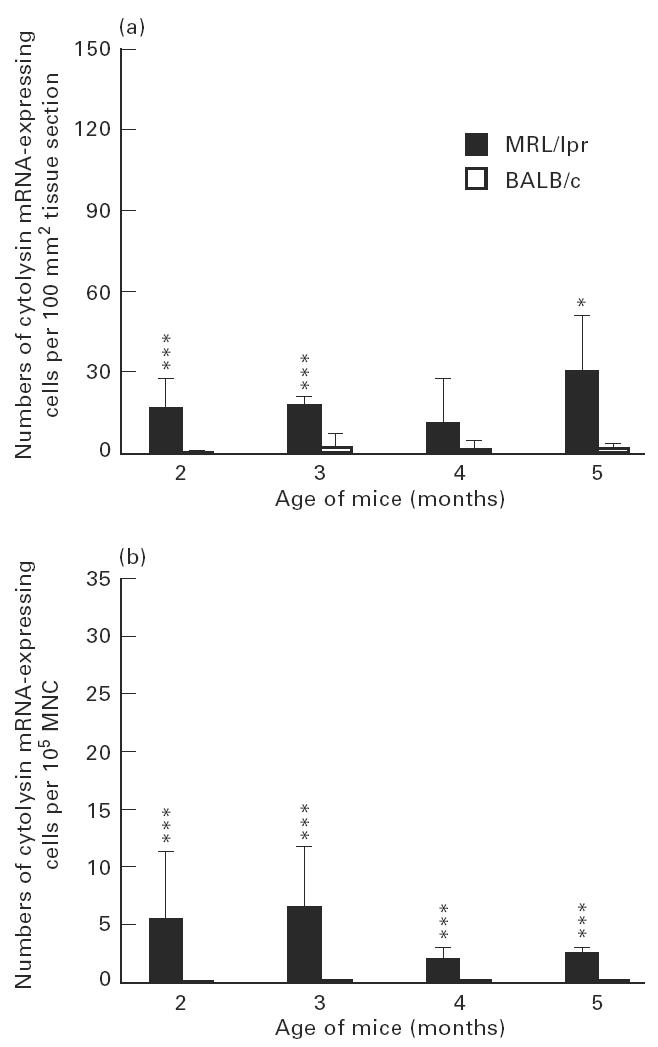
Numbers of cytolysin mRNA-expressing cells in submandibular glands (a) and among spleen mononuclear cells (MNC) (b) of MRL/lpr mice and control BALB/c mice. For explanation see legend to Fig. 3.
IL-12 and TNF-β mRNA-expressing cells
Peak levels of both IL-12 and TNF-β mRNA-expressing cells were observed in the submandibular glands at 3 months of age. This level of IL-12 mRNA-positive cells was higher (P < 0.01) than at 5 months (Fig. 8). Among spleen MNC, the peaks of IL-12 and TNF-β mRNA-expressing cells were observed at 3 and 4 months, respectively (data not shown).
Fig. 8.
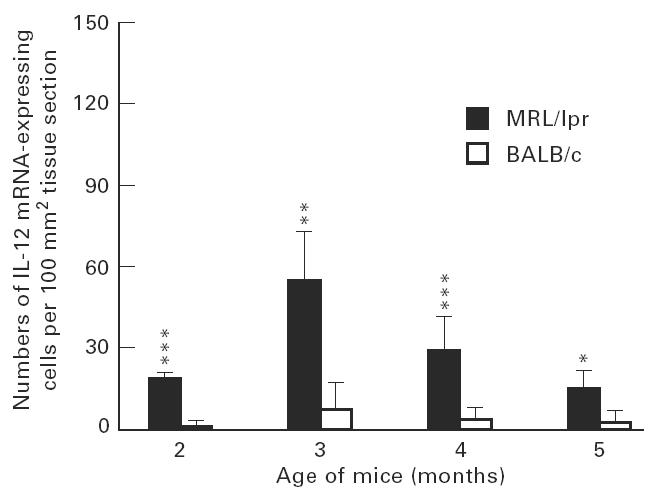
Numbers of IL-12 mRNA-expressing cells in submandibular glands of MRL/lpr mice and control BALB/c mice. For explanation see legend to Fig. 3.
Transforming growth factor-beta, IL-10, and IL-4 mRNA-expressing cells
Low numbers of IL-10, IL-4 and transforming growth factor-beta (TGF-β) mRNA-expressing cells were observed in the submandibular glands over the course of the disease. The numbers of both IL-10 and IL-4 mRNA-expressing cells were higher at 2 months than 5 months (P < 0.001) (data not shown). Similarly, TGF-β mRNA-expressing cells were low at 3 and 4 months, and almost undetectable at 5 months (data not shown). Among spleen MNC, higher numbers of both TGF-β and IL-4 mRNA-expressing cells were observed at 2 months of age (P < 0.001) compared with 3, 4 and 5 months; IL-10 mRNA-expressing cells were higher at 3 months of age (P < 0.001) compared with 5 months (data not shown).
DISCUSSION
In this study, we used in situ hybridization to examine the expression of mRNA encoding a number of cytokines in submandibular glands of autoimmune disease-prone MRL/lpr mice over the course of autoimmune sialoadenitis. We also evaluated the expression of mRNA encoding the same cytokines among spleen MNC, and the phenotypic composition of submandibular gland infiltrates at each time point. Although the recorded cellular mRNA expression may not necessarily have a direct relation to the secretion of these cytokines or their final in vivo activities, the detection of cytokines at the cellular level is a useful alternative to investigate their involvement in disease processes.
A rapid, pronounced and sustained accumulation of TNF-α, IL-1β and IL-6 mRNA-expressing cells occurred in the submandibular glands in 3-month-old MRL/lpr mice, following an early up-regulation of mRNA expression of these proinflammatory cytokines at 2 months old, and a subsequent decrease at 5 months. The local production preferentially by monocytes and macrophages of both TNF-α and IL-1β has been suggested to contribute to tissue injury in organs affected by autoimmune diseases [19–21]. TNF-α and IL-1β in synergy recruit macrophages and induce up-regulation of endothelial adhesion molecules [22,23]. Our findings are in agreement with previous reports which describe the histopathologic features of the inflammatory submandibular gland lesions in 3-month-old MRL/lpr mice as typical of developing human sialoadenitis [24], thereby suggesting an important role of TNF-α and IL-1β in the initiation of sialoadenitis. In the spleen, however, peak levels of TNF-α, IL-1β and IL-6 mRNA-expressing cells occurred later.
Numbers of IFN-γ mRNA-expressing cells increased in the submandibular glands in parallel with the ongoing autoimmune tissue destruction, with a maximum at 5 months old. This maximum production of IFN-γ thus coincides with other parameters of autoimmune disease, the number of rheumatoid factor-secreting cells and circulating immune complexes, which reached their peak values in 4–5-month-old MRL/lpr mice [25]. IFN-γ production by infiltrating MNC in MRL/lpr mice salivary glands has been reported previously [14]. IFN-γ induces MHC class II expression and up-regulates adhesion molecules during the inductive phase of immune responses, thereby promoting T cell homing to sites of inflammation [26]. Interestingly, IL-12 mRNA-expressing cells were strongly up-regulated in both submandibular glands and spleen in 3-month-old mice. IL-12, also being produced by macrophages, promotes Th1 cell development and IFN-γ production [27,28]. Thus, the observed increases in levels of IFN-γ mRNA-expressing cells in submandibular glands over the course of autoimmune sialoadenitis in MRL/lpr mice may be IL-12-dependent.
The present study extends previous findings by reporting a time-dependent up-regulation of mRNA encoding cytolysin and TNF-β in submandibular gland and spleen MNC over the course of autoimmune sialoadenitis in MRL/lpr mice. Cytolysin mRNA-expressing cells were up-regulated in the submandibular glands of MRL/lpr mice to a maximum at 5 months of age. Expression of cytolysin in lymphocyte-infiltrated tissue correlated with T cell-mediated cytotoxicity [29,30]. TNF-β mRNA-expressing cells were maximally up-regulated in the submandibular glands in 3-month-old mice, and at 4 and 5 months of age in the spleen MNC. TNF-β, primarily produced by activated T lymphocytes, enhances expression of endothelial cell receptors for various leucocyte recognition structures, thereby facilitating leucocyte and lymphocyte adhesion at sites of cell-mediated immunity [31,32]. Taken together, these data may indicate that expression of cytolysin and TNF-β mRNA in the submandibular glands of MRL/lpr mice reflects disease-promoting cytokines in autoimmune sialoadenitis in MRL/lpr mice.
The observed low numbers of cells expressing mRNA for IL-10 reported previously [33], and for IL-4 reported here for the first time, in submandibular glands of MRL/lpr mice may suggest that these two cytokines have local disease-associated homeostatic or compensatory activities in these mice. IL-10 and IL-4 are both known to down-regulate inflammation by inhibiting the production of proinflammatory cytokines, whilst up-regulating production of proinflammatory cytokine inhibitors [34]. Accumulating evidence suggests an important anti-inflammatory role for IL-10 as well as for the IL-1 receptor antagonist (IL-1Ra) and for soluble TNF-α receptors (sTNF-αR) in rheumatoid arthritis. IL-4 (but not IL-10) induces production of the IL-1 inhibitor IL-1Ra in vitro, and IL-10 (but not IL-4) induces production of the endogenous TNF inhibitor sTNF-αR [35]. An additional immunomodulating cytokine in the MRL/lpr mice submandibular glands found in the present study is probably TGF-β, showing low levels of mRNA expression in the submandibular glands at 3 and 4 months old, but high levels in the spleen at 2 months old. TGF-β has potentially immunosuppressive properties as it down-regulates both B and T cell immunity, and promotes tissue repair [36,37]. The low TGF-β mRNA expression detected and its subsequent absence in old MRL/lpr mice may be one reason for disease progression.
Our findings are consistent with evidence accumulated from animal models suggesting that the balance of cytokines produced by Th1/Th2 cell subsets plays an important role in the development of immune-mediated and inflammatory diseases, such as collagen-induced arthritis and inflammatory bowel disease [38,39]. Data available in human diseases favour prevalence of the Th1 cytokine profile in target organs of patients with organ-specific autoimmunity, such as rheumatoid arthritis and Crohn's disease [40,41]. Similarly, Th1 cytokine predominance was found in human tuberculous and sarcoid granulomas [42].
In conclusion, this study shows in general higher and persistent expression of mRNA of proinflammatory cytokines and low mRNA expression of anti-inflammatory and immunomodulatory cytokines in the submandibular glands over the course of autoimmune sialoadenitis in female MRL/lpr mice, correlating with induction and perpetuation of the disease. It could be of interest therefore to study these cytokine profiles during immunomodulatory or immunopharmacological treatments in young and old MRL/lpr mice.
References
- 1.Bloch KJ, Buchanan WW, Wohl MJ, et al. Sjögren's syndrome. A clinical, pathological and serological study of sixty-two cases. Med. 1965;44:187–231. [PubMed] [Google Scholar]
- 2.Fox RI, Robinson CA, Curd JG, et al. Sjögren's syndrome. Proposed criteria for classification. Arthritis Rheum. 1986;29:577–85. doi: 10.1002/art.1780290501. [DOI] [PubMed] [Google Scholar]
- 3.Talal N, Bunim JJ. The development of malignant lymphoma in the course of Sjögren's syndrome. Am J Med. 1964;36:529–40. doi: 10.1016/0002-9343(64)90101-9. [DOI] [PubMed] [Google Scholar]
- 4.Anderson LG, Talal N. The spectrum of benign to malignant lymphoproliferation in Sjögren's syndrome. Clin Exp Immunol. 1971;9:199–221. [PMC free article] [PubMed] [Google Scholar]
- 5.Theophilopoulous AN, Balderas RS, Shawler DL, et al. Influence of thymic genotype on the SLE-like disease and T cell proliferation of MRL/Mp-lpr/lpr mice. J Exp Med. 1981;153:1405–14. doi: 10.1084/jem.153.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen PL, Eisenberg RA. Lpr and gld: single gene model of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–69. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 7.Hang L, Theofilopoulos AN, Bladeras RS, et al. The effect of thymectomy on lupus-prone mice. J Immunol. 1984;132:1809–13. [PubMed] [Google Scholar]
- 8.Jabs DA, Burek CL, Hu Q, et al. Anti-CD4 monoclonal antibody therapy suppresses autoimmune disease in MRL/Mp-lpr/lpr mice. Cell Immunol. 1992;141:496–507. doi: 10.1016/0008-8749(92)90166-m. [DOI] [PubMed] [Google Scholar]
- 9.Sobel ES, Katagiri T, Katagiri K, et al. An intrinsic B cell defect is required for the production of autoantibodies in the lpr model of autoimmunity. J Exp Med. 1991;173:1441–9. doi: 10.1084/jem.173.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe-Fukunaga R, Brannan CI, Copeland NG, et al. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–7. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 11.Shlomchik MJ, Madaio MP, Ni D, et al. The role of B cells in lpr/lpr induced autoimmunity. J Exp Med. 1994;180:1295–306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman RW, Alspaugh MA, Waggie KS, et al. Sjögren's syndrome in MRL/1 and MRL/n mice. Arthritis Rheum. 1984;27:157–65. doi: 10.1002/art.1780270206. [DOI] [PubMed] [Google Scholar]
- 13.Jonsson R, Tarkowski A, Bäckman K, et al. Sialadenitis in the MRL/1 mouse: morphological and immunohistochemical characterization of resident and infiltrating cells. Immunol. 1987;60:611–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Jonsson R, Holmdahl R. Infiltrating mononuclear cells in salivary glands and kidneys in autoimmune MRL/lpr mice express IL-2 receptor and produce IFN-γ. J Oral Pathol. 1990;19:330–4. doi: 10.1111/j.1600-0714.1990.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 15.Scott L, Wolff A, Fox PC. A histologic assessment of the submandibular glands in autoimmune-disease-prone mice. J Oral Pathol. 1990;19:131–5. doi: 10.1111/j.1600-0714.1990.tb00811.x. [DOI] [PubMed] [Google Scholar]
- 16.Hamano H, Saito I, Haneji N, et al. Expression of cytokine genes during development of autoimmune sialadenitis in MRL/lpr mice. Eur J Immunol. 1993;23:2387–91. doi: 10.1002/eji.1830231002. [DOI] [PubMed] [Google Scholar]
- 17.Dagerlind A, Friberg K, Bean AL, et al. Sensitive DNA detection using unfixed tissue: combined radioactive and nonradioactive in situ hybridization histochemistry. Histochem. 1992;98:39–49. doi: 10.1007/BF00716936. [DOI] [PubMed] [Google Scholar]
- 18.Link J. Interferon-γ, interleukin-4 and transforming growth factor-β mRNA expression in multiple sclerosis and myasthenia gravis. Acta Neurol Scand. 1994;90(Suppl. 158):1–58. [PubMed] [Google Scholar]
- 19.Boswell JM, Yui MA, Burt DW, et al. Increased tumour necrosis factor and IL-1β gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988;141:3050–4. [PubMed] [Google Scholar]
- 20.Jacob CO, McDevitt HO. Tumor necrosis factor-α in murine autoimmune lupus nephritis. Nature. 1988;331:356–8. doi: 10.1038/331356a0. [DOI] [PubMed] [Google Scholar]
- 21.Donelly RP, Levine J, Hartwell QD, et al. Aberrant regulation of IL-1 expression in macrophages from young autoimmune-prone mice. J Immunol. 1990;145:3231–9. [PubMed] [Google Scholar]
- 22.Mizel SB. The interleukins. FASEB J. 1989;3:2379–88. doi: 10.1096/fasebj.3.12.2676681. [DOI] [PubMed] [Google Scholar]
- 23.Dinarello CA. The biological properties of interleukin-1. Eur Cytokine Netw. 1994;5:517–31. [PubMed] [Google Scholar]
- 24.Hayashi H, Haneji N, Hamano H. Pathogenesis of Sjögren's syndrome-like lesions in MRL/lpr mice. Pathol Int. 1994;44:559–68. doi: 10.1111/j.1440-1827.1994.tb01716.x. [DOI] [PubMed] [Google Scholar]
- 25.Tarkowski A, Czerkinsky C, Nilsson LA. Detection of IgG rheumatoid factor secreting cells in autoimmune MRL/lpr mice: a kinetic study. Clin Exp Immunol. 1984;58:7–12. [PMC free article] [PubMed] [Google Scholar]
- 26.Duijvestijn AM, Schreiber AB, Butcher EC. Interferon-gamma regulates an antigen specific for endothelial cells involved in lymphocyte traffic. Proc Natl Acad Sci USA. 1986;83:9114–8. doi: 10.1073/pnas.83.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manetti R, Parronchi P, Guidizi MG, et al. Natural killer cell stimulatory factor (interleukin 12 (IL-12)) induces T helper (Th1)-specific immune responses and inhibits the development of IL-4 producing Th cells. J Exp Med. 1992;177:1199–204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.German T, Gately MK, Schoenhaut DS, et al. Interleukin 12/T cell stimulating factor, a cytokine with multiple effects on T helper type 1 (Th1) but not on Th2 cells. Eur J Immunol. 1993;23:1762–70. doi: 10.1002/eji.1830230805. [DOI] [PubMed] [Google Scholar]
- 29.Young LH, Peterson LB, Wicker LS, et al. In vivo expression of perforin by CD8 lymphocytes in autoimmune disease. Studies on spontaneous and adaptively transferred diabetes in nonobese diabetic mice. J Immunol. 1989;149:3994–9. [PubMed] [Google Scholar]
- 30.Wu Z, Podack ER, McKenzie JM, et al. Perforin expression by thyroid-infiltrating T cells in autoimmune thyroid disease. Clin Exp Immunol. 1994;98:470–7. doi: 10.1111/j.1365-2249.1994.tb05515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul NL, Ruddle NH. Lymphotoxin. Annu Rev Immunol. 1988;6:407–38. doi: 10.1146/annurev.iy.06.040188.002203. [DOI] [PubMed] [Google Scholar]
- 32.Cavander DE, Edelbaum D, Ziff M. Endothelial cell activation induced by tumour necrosis factor and lymphotoxin. Am J Pathol. 1989;134:551–60. [PMC free article] [PubMed] [Google Scholar]
- 33.Yanagi K, Haneji N, Hamano H, et al. In vivo role of IL-10 and IL-12 during development of Sjögren's syndrome in MRL/lpr mice. Cell Immunol. 1996;168:243–50. doi: 10.1006/cimm.1996.0072. [DOI] [PubMed] [Google Scholar]
- 34.Vanroon JAG, Vanroy J, Gemligmeyling FHJ, et al. Prevention and reversal of cartilage degradation by interleukin 10 and interleukin-4. Arthritis Rheum. 1996;39:829–35. doi: 10.1002/art.1780390516. [DOI] [PubMed] [Google Scholar]
- 35.Brennan F, Feldman M. Cytokines in autoimmunity. Curr Opin Immunol. 1996;8:872–7. doi: 10.1016/s0952-7915(96)80018-5. [DOI] [PubMed] [Google Scholar]
- 36.Fontana A, Constam DB, Frei K, et al. Modulation of the immune response by transforming growth factor beta. Int Arch Allergy Immunol. 1992;99:1–7. doi: 10.1159/000236328. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki H, Pollard RB, Schmitt D, et al. Transforming growth factor-beta in the regulation of the immune response. Clin Immunol Immunopathol. 1992;65:1–9. doi: 10.1016/0090-1229(92)90241-f. [DOI] [PubMed] [Google Scholar]
- 38.Mauri C, Williams RO, Walmsley M, et al. Relationship between Th1/Th2 cytokine patterns and the arthritogenic response in collagen-induced arthritis. Eur J Immunol. 1996;26:1511–8. doi: 10.1002/eji.1830260716. [DOI] [PubMed] [Google Scholar]
- 39.Powrie F, Leach MW. Genetic and spontaneous inflammatory bowel disease in rodents: evidence for abnormalities in mucosal immune regulation. Theraputic Immunol. 1995;2:115–23. [PubMed] [Google Scholar]
- 40.Dolhain RJ, van der Heiden AN, ter Haar NT, et al. Shift toward T lymphocytes with a T helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961–9. doi: 10.1002/art.1780391204. [DOI] [PubMed] [Google Scholar]
- 41.Niessner M, Volk AB. Altered Th1/Th2 cytokine profile in the intestinal mucosa of patients of inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR) Clin Exp Immunol. 1995;101:428–35. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergeron A, Bonay M, Kambouchner M, et al. Cytokine patterns in tuberculous and sarcoid granuloma: correlation with histopathologic features of the granulomatous response. J Immunol. 1997;159:3034–43. [PubMed] [Google Scholar]