Abstract
In the present study we evaluated in vitro immunoglobulin production from IgAD individuals and healthy controls. Peripheral blood mononuclear cells (PBMC) from IgAD and controls were cultured with anti-CD40 MoAb presented on a CDw32-transfected fibroblast cell line (CD40 system) in the presence of IL-10, IL-2, IL-4, transforming growth factor-beta (TGF-β) alone as well as of IL-10 in combination with each of the other three cytokines. Only IL-10 added alone induced significant changes in baseline immunoglobulin production; marked increases in median supernatant levels of all three isotypes were observed in both groups. The most striking finding of this study was the synergizing effect of IL-4 on IgA production in the IgAD group when added with IL-10; median IgA supernatant level increased to a value superimposable on that found in the normal controls which remained about the same as when stimulated with IL-10 alone. The synergic effect of IL-4 and IL-10 was specific to the IgA isotype.
Keywords: IgA deficiency, immunoglobulin production, interleukins, CD40 ligand
INTRODUCTION
Selective IgAD is the most common primary immunodeficiency in man [1–3]. The underlying immunological defect is unknown. A variety of pathologic immunoregulatory mechanisms has been postulated: IgA-specific T suppressor cells, inadequate T helper cells and intrinsic B cell defects [4–10]. Genetic studies indicate that susceptibility genes located in the MHC class II or III regions may predispose to this immunodeficiency [4,5], but the mechanism by which these genes interfere with IgA B cell differentiation remains obscure. A defect of normal differentiation of IgM B cells into IgA-secreting plasma cells is thought to be the cause of IgA deficiency, but the precise point at which the differentiation arrest occurs is a matter of controversy [9,11]. There is evidence that B cell differentiation is regulated by cytokines and that defective cytokine production may contribute to the pathogenesis of IgA deficiency [12,13].
Recent reports of the activation and proliferation of B cell cultures induced by presentation of the MoAb anti-CD40 by a CDw32-transfected fibroblast cell line (CD40 system) [14–17] have greatly facilitated study of the effects of various cytokines on immunoglobulin synthesis by B cells [18–21]. Using this system Brière et al. [22] demonstrated IgA production by B cell cultures from IgAD patients, although in concentrations lower than those from healthy controls. Friman et al. [23] obtained similar results and also showed more severely impaired IgA production in IgAD patients with recurrent infections than in healthy IgAD subjects.
In the present study we evaluate in vitro immunoglobulin production by anti-CD40-activated peripheral blood mononuclear cells (PBMC) from IgAD patients and healthy controls in baseline conditions and after addition of IL-10, IL-2, IL-4 and transforming growth factor-beta (TGF-β) alone as well as of IL-10 in combination with each of the other three cytokines, and show that IL-4 synergizes with IL-10 to normalize in vitro IgA production in the IgAD group.
SUBJECTS AND METHODS
Subjects
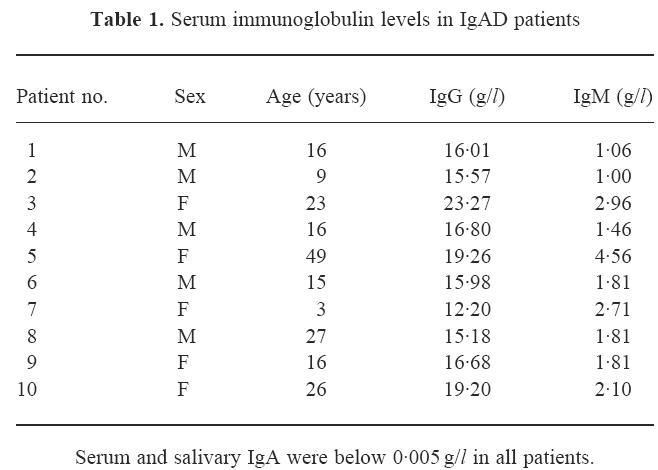
Ten healthy individuals (five males and five females) with selective IgAD ranging from 3 to 49 years of age (mean age 16 years) (Table 1) and 12 age- and sex-matched healthy controls with normal levels of immunoglobulin were included in the study. All patients had serum and secretory IgA levels < 0.005 g/l on multiple samples obtained over a period of years, while IgG and IgM were well above the lower limit of age-normal values. None of the patients had relatives with common variable immunodeficiency (CVID); however, two were mother (patient 5) and daughter (patient 3), and the mother of patient 6 had asymptomatic partial IgAD. None of the patients had a recent history of recurrent infections. Informed consent was obtained from all subjects or one of their parents before drawing blood samples. The institutional ethics committee approved the study.
Table 1.
Serum immunoglobulin levels in IgAD patients
Quantification of serum immunoglobulins
Serum concentrations of IgM, IgA, IgG and IgG subclasses were determined by single radial immunodiffusion [24,25]. Serum and salivary IgA were re-evaluated in the IgAD subjects by electroimmunodiffusion [26], a method with greater sensitivity (0.005 g/l).
Isolation of PBMC, lymphocyte phenotyping and culture conditions
PBMC were isolated using the standard Ficoll–Hypaque gradient method. Expression of surface markers on PBMC was studied by FACS analysis using fluorescein-conjugated MoAbs against CD3, CD4, CD8 and CD20 (Becton Dickinson). The PBMC (105/well) were cultured in the CD40 system alone and after addition of IL-10, IL-2, IL-4 and TGF-β alone, as well as after addition of IL-10 plus each of the other three cytokines. All cultures were set up in Iscove's medium, supplemented with 50 μg/ml human transferrin, 5 μg/ml bovine insulin, 0.5% bovine serum albumin (BSA), 5 × 10−5 m 2β-mercaptoethanol (2-βME) (all from Sigma Chemical Co.) and 5% heat-inactivated fetal bovine serum (FBS; Gibco). The CD40 system was composed of 2 × 103/well irradiated (70 Gy) CDw32 L cells and 0.5 μg/ml of anti-CD40 mAb89 (kindly provided by Professor J. Banchereau). Purified rhIL-2, rhIL-4, rhIL-10 and hTGF-β1 (Genzyme Diagnostics) were used, respectively, in concentrations of 20 U/ml, 50 U/ml, 200 ng/ml and 0.3 ng/ml. All conditions were run in triplicate to a final volume of 200 μl/well. The supernatants were harvested after 10 days of incubation at 37°C in humidified 5% CO2.
Quantification of supernatant levels of IgG, IgA, IgM
IgA, IgG and IgM concentrations in the supernatants were determined by an ELISA technique as previously described [27]. Briefly, microtitre plates were coated with rabbit anti-hIgA or anti-hIgG or anti-hIgM (Dako Immunoglobulins), by incubating for 3 h at 37°C and overnight at 4°C. The supernatants diluted 1:5 or 1:20 were incubated for 2 h at 37°C and then rabbit anti-hIgA or anti-hIgG or anti-hIgM conjugated to horseradish peroxidase (HRP) added. The concentration of each isotype was extrapolated from the standard curve included in each plate and expressed in ng/ml.
Statistical analysis
Comparisons of the IgA, IgG and IgM supernatant levels in baseline conditions and after incubation with each cytokine and combination of cytokines were performed within each group using the Wilcoxon non-parametric test; comparisons between non-paired groups were performed by means of the Mann–Whitney U-test. Parametric tests were unsuitable because parameters did not follow a normal distribution pattern; P < 0.05 was defined as statistically significant and all tests were two-sided. All calculations were performed using the package STATISTICA for Windows Release 5.0 (StatSoft Inc.).
RESULTS
Serum immunoglobulin levels and lymphocyte subsets
All patients fulfilled the generally accepted diagnostic criteria for selective IgAD. Serum and salivary IgA were below 0.005 g/l and serum IgG and IgM were normal for age (Table 1). Cell-mediated immunity, as evaluated by lymphocyte subsets and lymphoproliferative responses to mitogens (phytohaemagglutinin, concanavalin A) and antigens (tetanus toxoid, Candida albicans), was also intact (data not shown). In addition, serum IgG subclass levels were normal for age (data not shown).
IgA production
IgA production in the two groups at baseline and after stimulation with IL-10 alone and in combination with the other three cytokines is illustrated in Fig. 1. Activation of the PBMC by the CD40 system induced spontaneous IgA synthesis in the controls but not in the IgAD patients (P < 0.001). The addition of IL-10, but not of IL-2, IL-4 or TGF-β alone, resulted in significantly greater IgA production than in baseline in both groups (P = 0.004 in IgAD; P = 0.002 in controls) as well as in significantly greater (P = 0.04) IgA production in the controls than in the IgAD patients (Fig. 1).
Fig. 1.
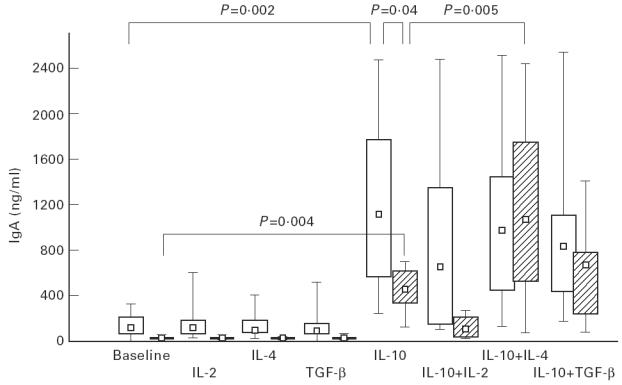
In vitro IgA production by peripheral blood mononuclear cells (PBMC) from IgAD patients (hatched bars) and controls (open bars) at baseline and after stimulation with interleukins. Box plots indicate range (whiskers), 25% and 75% interval (box) and median value (small square). For statistical analyses see text.
After stimulation with IL-10 plus each of the other cytokines, IgA production was again significantly enhanced compared with baseline, but to different extents within each group. When IL-10 was added in combination with IL-4, in the IgAD patients median IgA supernatant concentration was significantly greater than that observed with IL-10 alone (P = 0.005) and increased to a level similar to that of the controls (Fig. 1); in contrast, IgA production in the control cultures remained about the same as that observed after addition of IL-10 alone. Within both groups the combination of IL-10 plus IL-2 resulted in less IgA production than with IL-10 alone, but significantly less only in the IgAD group (P = 0.006). With this combination the difference between groups reached significance, with lower IgA production in the IgAD group (P = 0.02; Fig 1). The addition of TGF-β to IL-10 resulted in significant changes in IgA supernatant levels compared with IL-10 alone in the controls only (P = 0.03).
IgG production
IgG production by the cultures of both patients and controls in baseline conditions was similar and remained so in both groups after stimulation with IL-2, IL-4 or TGF-β alone (Fig. 2). However, significantly more IgG, compared with baseline, was produced within both groups after addition of IL-10 alone (P = 0.004 in IgAD; P = 0.007 in controls) and in combination with IL-2 (P = 0.006 in IgAD; P = 0.008 in controls), with IL-4 (P = 0.006 in IgAD; P = 0.002 in controls) and with TGF-β (P = 0.006 in IgAD; P = 0.003 in controls). When IL-2, IL-4 or TGF-β were added together with IL-10, IgG supernatant levels were similar to those observed with IL-10 alone in both patients and controls. Significantly greater IgG production was observed in patients than controls after addition of IL-10 alone (P = 0.04) or in combination with IL-2 (P = 0.02) or with TGF-β (P = 0.03), but stimulation with IL-10 plus IL-4 did not result in statistically different IgG levels between groups.
Fig. 2.
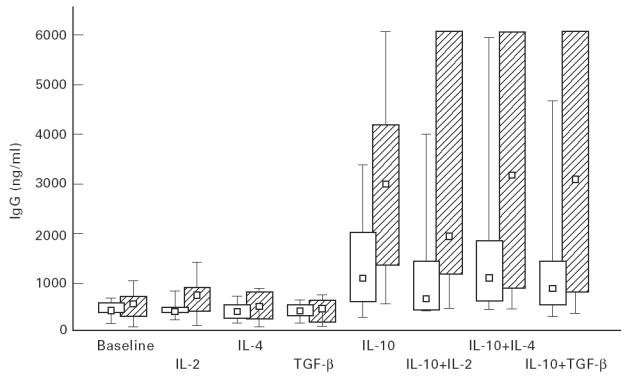
In vitro IgG production by peripheral blood mononuclear cells (PBMC) from IgAD patients (hatched bars) and controls (open bars) at baseline and after stimulation with interleukins. Box plots indicate range (whiskers), 25% and 75% interval (box) and median value (small square). For statistical analyses see text.
IgM production
In both groups IgM supernatant levels were low at baseline and increased significantly and to about the same extent with IL-10 alone (P = 0.005 in IgAD; P = 0.002 in controls) and in combination with the other three cytokines (Fig. 3), but only slightly with IL-2 alone (P = 0.005 in IgAD; P = 0.004 in controls). When IL-2, IL-4 or TGF-β were added together with IL-10, IgM production was similar to that observed with IL-10 alone within each group and did not differ between the two groups.
Fig. 3.
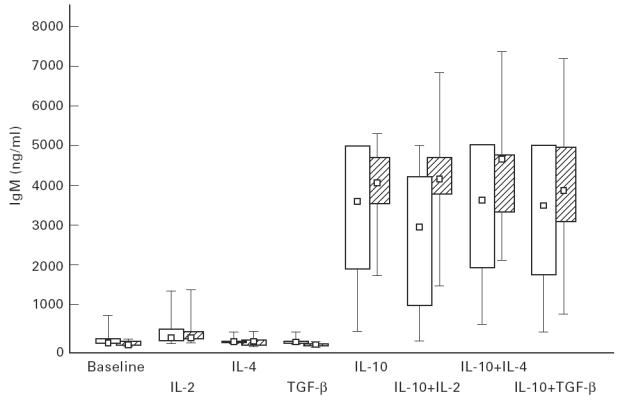
In vitro IgM production by peripheral blood mononuclear cells (PBMC) from IgAD patients (hatched bars) and controls (open bars) at baseline and after stimulation with interleukins. Box plots indicate range (whiskers), 25% and 75% interval (box) and median value (small square). For statistical analyses see text.
DISCUSSION
To our knowledge the only two studies which up to now have assessed the effects of cytokines on B cell differentiation in IgAD patients both showed that addition of IL-10 to PBMC cultures triggered by the CD40 system was essential to induce B cells from IgAD patients to differentiate into IgA-secreting plasma cells. In the first [22], IgA production, which was absent in the IgAD patients but present in the controls at baseline, was induced in the former by addition of IL-10 to the CD40-activated cultures, although in lesser concentrations than in the controls. The second study [23] likewise showed increased IgA production after addition of IL-10 to CD40-activated PBMC from controls and two groups of IgAD patients (one healthy and the other infection-prone), as well as different effects on IgA production in the two IgAD groups. IgA production was significantly greater in the healthy than the infection-prone IgAD group, but lower in the former than in controls. Moreover, when immunoglobulin production was studied after addition of IL-10 to purified B cells, the B lymphocytes from the healthy IgAD patients produced significantly less IgA than those from the normal controls, suggesting a defective overall response in vitro to IL-10. However, in neither of the above studies did IgA production in the IgAD group reach levels similar to those observed in the PBMC from the normal controls.
In the present study we evaluated the effects of IL-10, IL-2, IL-4, and TGF-β alone and of each of the latter three combined with IL-10 on immunoglobulin production by CD40-activated PBMC from IgAD patients and normal controls. As previously reported [22,23], spontaneous IgA synthesis was observed at baseline only in the controls, while that of IgG and IgM was similar in the two groups. Of the four cytokines assessed, other than IL-2 which increased IgM production in both groups (Fig. 3), only IL-10 added alone induced significant changes in baseline immunoglobulin production; marked increases in median supernatant levels of all three isotypes were observed in both groups (Figs 1–3). These data confirm the findings described in a T-independent system [21], demonstrating a powerful effect of IL-10, but not of IL-2 or IL-4, on IgG, IgA and IgM synthesis by CD40-activated B cells. Furthermore, IL-10 stimulation not only increased IgA synthesis by the controls, but also induced IgA secretion by the IgAD group, although the median IgA supernatant level remained significantly lower than that of the controls (Fig. 1). In contrast, significantly greater synthesis of IgG after IL-10 stimulation was observed in the IgAD patients than in the controls; a finding which could be due to IL-10-induced differentiation of greater numbers of IgG-committed B cells present because of the relative paucity of IgA-positive B cells in such subjects.
TGF-β alone did not alter baseline immunoglobulin production, but in combination with IL-10 less IgA was synthesized by the control cultures, and more, but not significantly more, by the IgAD PBMC than with IL-10 alone. Taken together these data are difficult to explain, as TGF-β has been reported to favour the switch from IgM to IgA production [28,29]. Furthermore, these changes in opposite directions of IgA supernatant levels in the two groups annulled the between-group difference in IgA synthesis observed with IL-10 alone. In the IgAD cultures the combination IL-2 plus IL-10 decreased IgA production (P = 0.006) compared with IL-10 alone.
The most striking finding of this study was the synergizing effect of IL-4 on IgA production in the IgAD group when added with IL-10; the median IgA supernatant level increased to a value superimposable on that found in normal controls (P = 0.8), which remained about the same as when stimulated with IL-10 alone. The synergic effect of IL-4 and IL-10 was specific for the IgA isotype. Indeed, on between-group comparison IgG production was greater with IL-10 alone and with the combination of IL-10 and both IL-2 and TGF-β in the IgAD group, but not with IL-4 plus IL-10, and no differences between groups were evident in IgM production. These results strongly suggest that the residual impairment of the capacity to differentiate into IgA-secreting cells in response to IL-10 alone can be restored by addition of IL-4 in IgAD. Indeed, in CVID, a disease considered to belong to the same spectrum of B cell defects as IgAD, but at the opposite extreme [30], defective immunoglobulin production in primary B cell cultures was observed after stimulation with IL-10 alone and the response to IL-10 was partially restored by addition of both IL-4 and IL-10 to the cultures; these data led the authors to postulate that IL-4 permitted overcoming the previous anergy to IL-10 of the CVID B cells [31]. A similar mechanism could perhaps explain the normalization of IgA production by IL-4 plus IL-10 observed in the cultures of our IgAD group.
Acknowledgments
The authors thank Professor L Å. Hanson for reviewing the manuscript and Mr Attilio Ascione for his technical assistance.
References
- 1.Ammann AJ, Hong R. Selective IgA deficiency: presentation of 30 cases and a review of the literature. Med. 1971;50:223–36. [PubMed] [Google Scholar]
- 2.Hanson LÅ, Bjorkander J, Oxelius V. Selective IgA. deficiency. In: Chandra RK, editor. Primary and secondary immunodeficiency disorders. Edinburgh: Churchill Livingstone; 1983. pp. 62–84. [Google Scholar]
- 3.Plebani A, Ugazio AG, Monafo V, Burgio GR. Clinical heterogeneity and reversibility of selective immunoglobulin A deficiency in 80 children. Lancet. 1986;i:829–31. doi: 10.1016/s0140-6736(86)90940-2. [DOI] [PubMed] [Google Scholar]
- 4.Schaffer FM, Monteiro RC, Volanakis JE, Cooper MD. IgA deficiency. Immunodefic Rev. 1991;3:15–44. [PubMed] [Google Scholar]
- 5.Burrows PD, Cooper MD. IgA deficiency. Adv Immunol. 1997;65:245–76. [PubMed] [Google Scholar]
- 6.Cassidy JT, Oldham G, Platts-Mills TA. Functional assessment of a B cell defect in patients with selective IgA deficiency. Clin Exp Immunol. 1979;35:296–305. [PMC free article] [PubMed] [Google Scholar]
- 7.Waldmann TA, Broder S, Krakauer R, Durm M, Meade B, Goldman C. Defect in IgA secretion and in IgA specific suppressor cells in patients with selective IgA deficiency. Trans Assoc Am Physicians. 1976;89:215–24. [PubMed] [Google Scholar]
- 8.Islam KB, Baskin B, Christensson B, Hammarstrom L, Smith CI. In vivo expression of human immunoglobulin germ-line mRNA in normal and in immunodeficient individuals. Clin Exp Immunol. 1994;95:3–9. doi: 10.1111/j.1365-2249.1994.tb06006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Islam KB, Baskin B, Nilsson L, Hammarstrom L, Sideras P, Smith CI. Molecular analysis of IgA deficiency. Evidence for impaired switching to IgA. J Immunol. 1994;152:1442–52. [PubMed] [Google Scholar]
- 10.King MA, Wells JV, Nelson DS. IgA synthesis by peripheral blood mononuclear cells from normal and selectively IgA deficient subjects. Clin Exp Immunol. 1979;38:306–15. [PMC free article] [PubMed] [Google Scholar]
- 11.Conley ME, Cooper MD. Immature IgA B cells in IgA-deficient patients. N Eng J Med. 1981;305:495–7. doi: 10.1056/NEJM198108273050905. [DOI] [PubMed] [Google Scholar]
- 12.Murray PD, McKenzie DT, Swain SL, Kagnoff MF. Interleukin 5 and interleukin 4 produced by Peyer's patch T cells selectively enhance immunoglobulin A expression. J Immunol. 1987;139:2669–74. [PubMed] [Google Scholar]
- 13.Banchereau J, Rousset F. Human B lymphocytes: phenotype, proliferation, and differentiation. Adv Immunol. 1992;52:125–262. doi: 10.1016/s0065-2776(08)60876-7. [DOI] [PubMed] [Google Scholar]
- 14.Clark LB, Foy TM, Noelle RJ. CD40 and its ligand. Adv Immunol. 1994;63:43–78. doi: 10.1016/s0065-2776(08)60854-8. [DOI] [PubMed] [Google Scholar]
- 15.Banchereau J, Bazan F, Blanchard D, et al. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 16.Banchereau J, De Paoli P, Valle A, Garcia E, Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 1991;251:70–72. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- 17.Splawski JB, Fu MS, Lipsky PE. Immunoregulatory role of CD40 in human B cell differentiation. J Immunol. 1993;150:1276–85. [PubMed] [Google Scholar]
- 18.Rousset F, Garcia E, Banchereau J. Cytokine-induced proliferation and immunoglobulin production of human B lymphocytes triggered through their CD40 antigen. J Exp Med. 1991;173:705–10. doi: 10.1084/jem.173.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodgkin PD, Castle BE, Kehry MR. B cell differentiation induced by helper T cell membranes: evidence for sequential isotype switching and a requirement for lymphokines during proliferation. Eur J Immunol. 1994;24:239–46. doi: 10.1002/eji.1830240138. [DOI] [PubMed] [Google Scholar]
- 20.Armitage RJ, Macduff BM, Spriggs MK, Fanslow WC. Human B cell proliferation and Ig secretion induced by recombinant CD40 ligand are modulated by soluble cytokines. J Immunol. 1993;150:3671–80. [PubMed] [Google Scholar]
- 21.Rousset F, Garcia E, Defrance T, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–3. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brière F, Bridon JM, Chevet D, Souillet G, Bienvenu F, Guret C, Martinez-Valdez H, Banchereau J. Interleukin 10 induces B lymphocytes from IgA-deficient patients to secrete IgA. J Clin Invest. 1994;94:97–104. doi: 10.1172/JCI117354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friman V, Hanson LÅ, Bridon JM, Tarkowski A, Banchereau J, Briere F. IL-10-driven immunoglobulin production by B lymphocytes from IgA-deficient individuals correlates to infection proneness. Clin Exp Immunol. 1996;104:432–8. doi: 10.1046/j.1365-2249.1996.38746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancini G, Carbonara AO, Heremans JF. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochem. 1965;2:235–54. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- 25.Plebani A, Ugazio AG, Avanzini MA, Massimi P, Zonta L, Monafo V, Burgio GR. Serum IgG subclass concentrations in healthy subjects at different age: age normal percentile charts. Eur J Pediatr. 1989;149:164–7. doi: 10.1007/BF01958271. [DOI] [PubMed] [Google Scholar]
- 26.Burgio GR, Lanzavecchia A, Plebani A, Jayakar S, Ugazio AG. Ontogeny of secretory immunity: levels of secretory IgA and natural antibodies in saliva. Pediatr Res. 1980;14:1111–4. doi: 10.1203/00006450-198010000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Plebani A, Mira E, Monafo V, Notarangelo LD, Avanzini MA, Ugazio AG. IgM and IgD concentrations in the serum and secretions of children with selective IgA deficiency. Clin Exp Immunol. 1983;53:689–96. [PMC free article] [PubMed] [Google Scholar]
- 28.Defrance T, Vanbervliet B, Brière F, Durand I, Rousset F, Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992;175:671–82. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Vlasselaer P, Punnonen J, de Vries JE. Transforming growth factor-beta directs IgA switching in human B cells. J Immunol. 1992;148:2062–7. [PubMed] [Google Scholar]
- 30.Eisenstein EM, Chua K, Strober W. B cell differentiation defects in common variable immunodeficiency are ameliorated after stimulation with anti-CD40 antibody and IL-10. J Immunol. 1994;152:5957–68. [PubMed] [Google Scholar]
- 31.Punnonen J, Kainulainen L, Ruuskanen O, Nikoskelainen J, Arvilommi H. IL-4 synergizes with IL-10 and anti-CD40 MoAbs to induce B-cell differentiation in patients with common variable immunodeficiency. Scand J Immunol. 1997;45:203–12. doi: 10.1046/j.1365-3083.1997.d01-381.x. [DOI] [PubMed] [Google Scholar]


