Abstract
Pirfenidone has been shown to modify some cytokine regulatory actions and inhibit fibroblast biochemical reactions resulting in inhibition of proliferation and collagen matrix synthesis by fibroblast. We have investigated the effect of pirfenidone on the expression of cell adhesion molecules. The synovial fibroblasts were treated with IL-1α in the presence or absence of pirfenidone (range 0–1000 μm), and assayed for the expression of adhesion molecules such as ICAM-1 and endothelial-leucocyte adhesion molecule-1 (E-selectin) by cell ELISA. Pirfenidone significantly down-regulated the expression of ICAM-1 on cultured synovial fibroblasts in a dose-dependent manner. In contrast, expression of E-selectin was not affected. Furthermore, we examined whether pirfenidone affects the cellular binding between cultured lymphocytes and IL-1α-stimulated synovial fibroblasts by in vitro binding assay and found their mutual binding was significantly suppressed in a dose-dependent manner by pirfenidone. It is speculated that down-regulation of ICAM-1 might be one of the novel mechanisms of action of pirfenidone. These data indicate a novel mechanism of action for pirfenidone to reduce the activation of synovial fibroblasts.
Keywords: pirfenidone, cell adhesion molecules, ICAM-1, synovial fibroblasts
INTRODUCTION
Cell adhesion molecules that mediate interactions between lymphocytes and fibroblasts are a crucial factor in inflammatory or fibrotic reactions [1–3]. Cell adhesion molecules including ICAM-1 and E-selectin mediate a variety of cell–cell interactions by binding to leucocyte adhesion receptor molecules. ICAM-1 is a cell surface glycoprotein expressed on many cell lineages [4,5]. It functions in intercellular adhesion reactions by binding to its ligand, leucocyte function-associated antigen-1 (LFA-1) (CD11a/CD18) [4–7], a heterodimer complex that is a member of the leucocyte integrin family, which is present on all leucocytes [6–8]. E-selectin is 110-kD glycoprotein. E-selectin binds to its lignads, sialyl Lewisx and sialyl Lewisa which has been found on the surface of peripheral blood polymorphs, monocytes, T lymphocytes, and natural killer (NK) cells [9,10]. ICAM-1 and E-selectin can be up-regulated on the surface of cells in response to inflammatory cytokines such as interferon-gamma (IFN-γ) [11–13], IL-1 [13–15], and tumour necrosis factor-alpha (TNF-α) [2,5,11–13,15,16], suggesting that these adhesion molecules are important in the selective recruitment of leucocytes [17,18]. Recently, it has been reported that cell adhesion molecules, including ICAM-1, are expressed on synovial fibroblasts in rheumatoid arthritis (RA) [1,13,19–21]. These findings suggest that up-regulated expression of adhesion molecules in RA synovium plays an important role in the pathophysiology of RA.
The anti-fibrotic activities of pirfenidone (5-methyl-1-phenyl-2-(1H)-pyridone) have been demonstrated in vivo and in vitro and it has been observed that it prevents the formation of excessive fibrosis and thus maintains normal organ function. Fibrotic lesions are characterized by the formation of excessive interstitial fibrous tissue. Pirfenidone is a drug that exerts a pharmacological ability to prevent and remove the excessive tissue found in fibrosis associated with injured tissue of various body organs [22,23]. Previous studies supported the hypothesis for the mechanism of pirfenidone, anti-fibrotic activity based on in vitro cell culture studies involving alterations at cellular and molecular level in response to growth-promoting cytokines such as transforming growth factor-beta 1 (TGF-β1), fibroblast growth factor (FGF), epidermal growth factor (EGF), and platelet-derived growth factor (PDGF), and reflected in alterations to: (i) the proliferation of fibroblast cells, (ii) the rate of chemical synthesis of collagen and other cell biochemical products, (iii) formation of fibrotic matrix, (iv) accumulation of fibrotic matrix, (v) turnover rate of the collagen in the fibrotic matrix, and (vi) the remodelling or removal of previously formed fibrotic matrix by the fibroblasts [22–26]. However, its precise action remains to be elucidated. We therefore examined whether pirfenidone modulates the expression of adhesion molecules on human synovial fibroblasts in vitro. Our study clearly demonstrates that pirfenidone down-regulates expression of ICAM-1 in a dose-dependent manner. We also found that pirfenidone significantly inhibits the cellular binding between human T cell leukaemia cells and IL-1α-stimulated synovial fibroblasts.
MATERIALS AND METHODS
Cell culture
The synovia obtained by synovectomy from the knee joints of RA patients were minced, and 50 mg of the tissue were cultured in 48-well plates (Costar, Cambridge, MA) in 0.5 ml Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY) containing 10% fetal bovine serum (FBS; Gibco), 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells were grown to confluence in DMEM (Gibco) supplemented with 10% heat-inactivated FBS (Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin in a humidified atmosphere of 5% CO2 at 37°C. Because the culture cells may lose their original characteristics, no cells after more than three serial passages were used for the study. Fibroblast cultures did not contain endothelial cells and monocyte/macrophage lineage cells, as determined by negative staining for factor VIII (a marker for endothelial cells) and CD14 (a marker for monocytes/macrophages). Human umbilical vein endothelial cells and U937 cells (monocyte/macrophage lineage cells) as positive controls for factor VIII and CD14 were used. The viability of cells was always examined morphologically with an inverted microscope and by the trypan blue dye exclusion test during 4–24-h pirfenidone treatment period.
Reagents and antibodies
Pirfenidone was obtained from Dr S. B. Margolin (Marnac Inc., Dallas, TX), dissolved in acetone and kept as a stock solution. IL-1α was purchased from Otsuka Pharmaceutical Co. (Tokushima, Japan). Murine MoAbs against human adhesion molecules, including ICAM-1 (BBA3) and E-selectin (BBA1), were obtained from British Bio-Technology (Oxford, UK).
Cell ELISA
Ten thousand human synovial fibroblasts were seeded into 96-well culture plates (Costar). Confluent cultures of human synovial fibroblasts were stimulated with various concentrations of IL-1α in the presence or absence of various doses of pirfenidone. Cells were subsequently fixed with 3% paraformaldehyde/8% saccharose/PBS(−). Non-specific binding was blocked by the sequential addition of Block Ace (Yukijirushi, Sapporo, Japan)/PBS(−) and 5% goat serum/PBS(−) for 1 h. Either anti-ICAM-1 or anti-E-selectin antibody and alkaline phosphatase-conjugated goat anti-mouse IgG antibody (Tago Inc., Burlingame, CA) were used as the first and second antibodies, respectively, followed by the addition of phosphate substrate (Sigma Chemical Co., St Louis, MO). The optical density (OD) of each well was determined by a microplate reader (BioRad Labs, Richmond, CA) at 405 nm. The percentage inhibition of adhesion was calculated by the following formula: (1 − adhesion in the presence of pirfenidone (OD)/adhesion in the absence of pirfenidone (OD)) × 100. Data are expressed as mean + s.d. of three experiments.
Cell binding assay
Ten thousand fibroblasts were seeded into each well of 96-well cultured plates. Confluent cultures of synovial fibroblasts were stimulated by IL-1α and various concentrations of pirfenidone. Human T cell leukaemia cell line, Molt 4 cells, were labelled with 10 μmol/l 2′, 7′-bis(carboxyethyl)-5(6′) carboxyfluorescein (BCECF) tetraacetoxymethyl ester (Dojindo Labs, Kumamoto, Japan), as reported previously, for 1 h at 37°C in RPMI 1640 with 10% FBS and then washed three times with serum-free RPMI [27]. Twenty thousand labelled cells were added to each well and incubated with fibroblasts for 1 h. Cells that were non-specifically bound to synovial fibroblasts were removed by inverting the plates for 30 min. Wells were subsequently washed once with serum-free RPMI, and the remaining cells were lysed with 1% Nonidet P-40 (Nakarai Chemicals, Kyoto, Japan). Fluorescence intensity in cell lysates was measured with an automated microplate fluorometer (Nihon Bunko, Tokyo, Japan) at 490 nm. The percentage inhibition of cellular binding was calculated by the following formula: (1 − adhesion in the presence of pirfenidone (OD)/adhesion in the absence of pirfenidone (OD)) × 100. Data are expressed as mean + s.d. of three experiments.
Statistical analysis
The data were analysed by analysis of variance (anova) from each individual experiment.
RESULTS
IL-1α induces the expression of adhesion molecules on human synovial fibroblasts in a dose-dependent fashion
We first performed dose–response studies of IL-1α-induced adhesion molecule expression on human synovial fibroblasts using cell ELISA to obtain optimal conditions for further study. Human synovial fibroblasts were treated with various doses of IL-1α (0–100 ng/ml). A small amount of ICAM-1 was constitutively expressed on human synovial fibroblasts and there was a dose-dependent increase of ICAM-1 when stimulated with IL-1α. The stimulation with 1 ng/ml of IL-1α showed a maximum ICAM-1 expression after 24 h and slightly decreased thereafter (Fig. 1a). E-selectin expression was maximally induced by 1 ng/ml of IL-1α at 4–6 h (Fig. 1b). We therefore decided to use a suboptimal dose of IL-1α for the later experiments.
Fig. 1.

(a) A dose-dependent increase in ICAM-1 expression induced by IL-1α. Fibroblasts were cultured with various concentrations of IL-1α for 24 h, and ICAM-1 expression was assayed by cell ELISA. (b) A dose-dependent increase in E-selectin expression induced by IL-1α. Fibroblasts were cultured with various concentrations of IL-1α for 4 h, and E-selectin expression was assayed by cell ELISA. The experiment is representative of three separate experiments.
Pirfenidone significantly inhibits the expression of ICAM-1 on IL-1α-stimulated human synovial fibroblasts
We next examined the effect of pirfenidone on the expression of ICAM-1 and E-selectin on human synovial fibroblasts. Human synovial fibroblasts was treated with 10 pg/ml IL-1α for ICAM-1 expression and with 100 pg/ml IL-1α for E-selectin expression in the presence of pirfenidone (range 0–1000 μm) for 24 h for ICAM-1 expression and for 4 h for E-selectin expression, and assayed for their expression by cell ELISA. The expression of ICAM-1 on human synovial fibroblasts was significantly down-regulated by pirfenidone in a dose-dependent manner (P < 0.001) (Fig. 2a). In contrast, the expression of E-selectin suppressed only at 1000 μm (P < 0.001) (Fig. 2b). Cell viability was > 98% by the trypan blue dye exclusion test at each concentration of pirfenidone, and this inhibition could not be explained by detachment of the cells from the culture plates. However, decreased viability of synovial fibroblasts was observed when > 1000 μm of pirfenidone was used (70% viability, on average).
Fig. 2.

(a) Inhibition of ICAM-1 expression by pirfenidone. Various concentrations of pirfenidone were added to the fibroblast culture system together with IL-1α (10 pg/ml) for 24 h. ICAM-1 expression was measured by cell ELISA. Significant suppression of ICAM-1 expression was observed: *P < 0.001. (b) Inhibition of E-selectin expression by pirfenidone. Various concentrations of pirfenidone were added to the fibroblast culture system together with IL-1α (100 pg/ml) for 4 h and tested for their ability to alter E-selection expression in the system. The experiment is representative of three separate experiments.
Pirfenidone inhibits the cellular binding of cultured human lymphocytes to IL-1α-stimulated synovial fibroblasts
To assess whether pirfenidone really affects lymphocytic cell binding with activated fibroblasts, an in vitro cellular binding assay was performed between Molt 4- and IL-1α-stimulated synovial fibroblasts in the presence or absence of pirfenidone. Synovial fibroblasts were treated with pirfenidone (range 0–1000 μm) for either 4 h or 12 h in the presence of 10 pg/ml IL-1α, and the binding of Molt 4 cells to synovial fibroblasts was measured thereafter. As shown in Fig. 3, the adhesion of Molt 4 cells to IL-1α-stimulated synovial fibroblasts was significantly inhibited by 24-h and 36-h treatment with pirfenidone (P < 0.001). However, 4 h and 12 h treatment with pirfenidone did not affect their mutual binding.
Fig. 3.
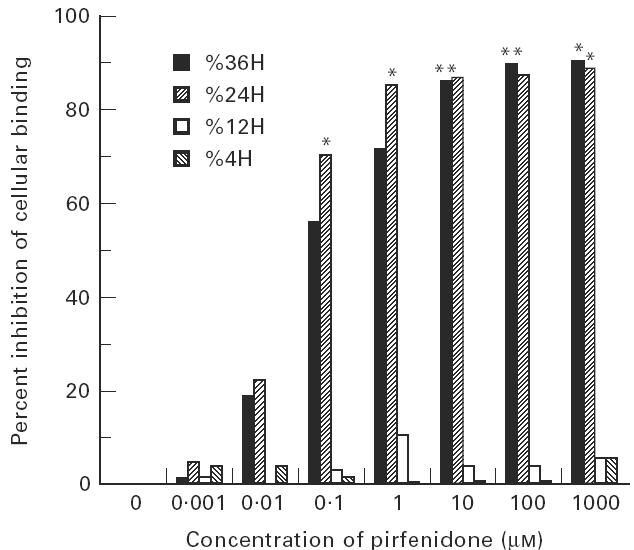
Inhibition of cellular binding between Molt 4 cells and IL-1α-stimulated fibroblasts with pirfenidone. Various concentrations of pirfenidone were added to the fibroblast culture system together with IL-1α (10 pg/ml) for 24 h. The binding between Molt 4 and IL-1α-stimulated fibroblasts was measured by the cell binding assay described in Materials and Methods. Significant inhibition was observed: *P < 0.001. The experiment is representative of three separate experiments.
DISCUSSION
Pirfenidone is established as producing anti-fibrotic and anti-inflammatory effects in a variety of animals with fibrotic lesions in vivo [22–26] and in vitro [23]. The preliminary studies indicate that pirfenidone is the first medicinal agent capable of causing a complete arrest of fibrotic lesion growth, as well as reduction or complete removal of long established scar or fibrotic lesions in animal models of fibrosis. Iyer et al. [26] demonstrated the anti-fibrotic potential of pirfenidone in the bleomycin hamster model of pulmonary fibrosis. In addition, a preventive effect of pirfenidone against experimental sclerosing peritonitis in the rat has been reported [25]. Furthermore, pirfenidone has been shown to modify some cytokine regulatory actions such as TGF-β1, PDGF, EGF, and FGF, and inhibit fibroblast biochemical reactions resulting in inhibition of proliferation and collagen matrix synthesis by fibroblasts [23]. It is therefore possible that part of the therapeutic efficacy of pirfenidone could be ascribed to down-regulation of these factors on fibroblasts. However, its precise mechanism remains to be elucidated, especially the relation between pirfenidone and adhesion molecules. We therefore examined whether pirfenidone down-regulates expression of ICAM-1 and E-selectin in a dose-dependent manner on IL-1α-stimulated synovial fibroblasts. Our study demonstrated that pirfenidone down-regulated ICAM-1 expression in a dose-dependent manner on IL-1α-stimulated synovial fibroblasts, suggesting that pirfenidone inhibits the transcription of genes such as AP-1 induced by IL-1α.
A variety of cell–cell interactions by binding of leucocyte adhesion receptor molecules with synovial fibroblasts clearly occurs and may be intimately involved. The previous studies have identified leucocyte adhesion is a crucial step in the development of the inflammatory or fibrotic processes at the synovium, including carpal tunnel syndrome (CTS) [28] and RA [1,13,20,21]. It is already known that ICAM-1 is expressed on all major components of the synovial microenvironment in RA and the inflamed synovium. That strong expression of ICAM-1 occurred within areas of active inflammation is consistent with its induction by inflammatory mediators related to these sites. Therefore, ICAM-1 could be one of the key molecules in the formation of fibrotic disease. Our findings indicate there is some possibility that part of the therapeutic efficacy of pirfenidone could be ascribed to the down-regulation of adhesion molecules on synovial fibroblasts.
It has been reported that pirfenidone modifies some cytokine regulatory actions and inhibits fibroblast proliferation and collagen synthesis, although its precise in vitro molecular action remains to be clarified [23,24]. As we examined whether pirfenidone exerts its effects upon the cytokine-induced expression of ICAM-1 and E-selectin on human synovial fibroblasts in vitro, our data indicate that pirfenidone reduced the expression of ICAM-1 on IL-1α-stimulated fibroblasts after 24 h of treatment in a dose-dependent manner, in contrast to that of E-selectin. Furthermore, pirfenidone significantly inhibited the cellular binding between Molt 4 and IL-1α-stimulated fibroblasts after 24 h and 36 h, but not 4 h or 12 h of treatment. We used synovial fibroblasts in a binding assay with Molt 4 cells, which expressed LFA-1 (the ligand of ICAM-1), to assess the functional capability of up-regulated adhesion molecules. Previous studies show that enhancement of ICAM-1 expression develops gradually and reaches a plateau at ≈ 24 h and continues at 72 h [2,13,29]. Also, E-selectin is expressed relatively early and its peak is at about 4 h and largely disappears by 24 h [1,30]. Taken together, our results indicate that pirfenidone may act directly on synovial fibroblasts to alter the expression of adhesion molecule ICAM-1. This ICAM-1/LFA-1 pathway is predominantly used in mediating the adherence of Molt 4 cells and IL-1α-stimulated synovial fibroblasts, and operates in leucocyte recruitment and retention to inflammatory sites. On the other hand, pirfenidone dose not affect the expression of E-selectin. This also suggests that ICAM-1 and E-selectin expression are differentially regulated in synovial fibroblasts and indicates that induction of ICAM-1 expression can be uncoupled from that of E-selectin.
Excessive cell proliferation occurs as a result of the presence of various cytokine growth factors such as TGF-β1, PDGF, EGF, and FGF. Because the development of fibrosis appears to be preceded by infiltration of affected tissues by mononuclear cells, inflammatory cell-derived cytokines, such as TGF-β1, account for the up-regulation of fibroblast collagen production. It is possible that pirfenidone may inhibit cytokine production from the inflammatory fibroblasts, which results in the suppression of adhesion molecule expression. These results suggest that pirfenidone might prevent the progression of disease by inhibiting induced monocyte or leucocyte adhesion to activated fibroblasts.
In conclusion, this is the first study to show that pirfenidone down-regulates ICAM-1 expression on human synovial fibroblasts in vitro. Regulation of adhesion molecule expression is now becoming one of the innovative treatments in inflammation both in vitro and in vivo. Further development of therapeutic agents to regulate the expression of adhesion molecules on fibroblasts may create a new mode of treatment for inflammatory diseases.
REFERENCES
- 1.Hale LP, Martin ME, McCollum DE, et al. Immunohistologic analysis of the distribution of cell adhesion molecules within the inflammatory synovial microenvironment. Arthritis Rheum. 1989;32:22–29. doi: 10.1002/anr.1780320105. [DOI] [PubMed] [Google Scholar]
- 2.Schwachula A, Riemann D, Kehlen A, et al. Characterization of the immunophenotype and functional properties of fibroblast-like synoviocytes in comparison to skin fibroblasts and umbilical vein endothelial cells. Immunobiol. 1994;190:67–92. doi: 10.1016/S0171-2985(11)80284-6. [DOI] [PubMed] [Google Scholar]
- 3.Marlor CW, Webb DL, Bombara MP, et al. Expression of vascular cell adhesion molecule-1 in fibroblasts like synoviocytes after stimulation with tumor necrosis factor. Am J Pathol. 1992;140:1055–60. [PMC free article] [PubMed] [Google Scholar]
- 4.Rothein R, Dustin ML, Marlin SD, et al. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986;137:1270–3. [PubMed] [Google Scholar]
- 5.Dustin ML, Rothlein AK, Bhan CA, et al. Induction by IL-1 and interferon-γ, tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–53. [PubMed] [Google Scholar]
- 6.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 7.Albelda SM, Buck CA. Integrins and other cell adhesion molecules. FASEB J. 1990;4:2868–80. [PubMed] [Google Scholar]
- 8.Sanchez-Madrid F, Nagy J, Robbins E, et al. A human leukocyte differentiation antigen family with district α-subunits and a common β-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983;158:1785–801. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walz G, Aruffo A, Kolanus W, et al. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990;250:1132–5. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- 10.Munro JM, Lo SK, Corless C, et al. Expression of sialyl-Lex, an E-selectin (ELAM-1) ligand, in inflammation, immune processes, and lymphoid tissues. Am J Pathol. 1992;141:1397–408. [PMC free article] [PubMed] [Google Scholar]
- 11.Piela TH, Korn JH. ICAM-1-dependent fibroblast–lymphocyte adhesion: discordance between surface expression and function of ICAM-1. Cell Immunol. 1990;129:125–37. doi: 10.1016/0008-8749(90)90192-t. [DOI] [PubMed] [Google Scholar]
- 12.Piela TH, Korn JH. Lymphocyte-fibroblast adhesion induced by interferon-γ. Cell Immunol. 1988;114:149–60. doi: 10.1016/0008-8749(88)90262-6. [DOI] [PubMed] [Google Scholar]
- 13.Chin JE, Winterrowd GE, Krzesicki RF, et al. Role of cytokines in inflammatory synovitis: the coordinate regulation of intercellular adhesion molecule 1 and HLA class I and class II antigens in rheumatoid synovial fibroblasts. Arthritis Rheum. 1990;33:1776–86. doi: 10.1002/art.1780331204. [DOI] [PubMed] [Google Scholar]
- 14.Miyasaka N, Sato K, Goto M, et al. Augmented interleukin-1 production and HLA-DR expression in the synovium of rheumatoid arthritis patients. Arthritis Rheum. 1988;31:480–6. doi: 10.1002/art.1780310404. [DOI] [PubMed] [Google Scholar]
- 15.Rothlein R, Czajkowski M, O'Neill MM, et al. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines: regulation by pharmacologic agents and neutralizing antibodies. J Immunol. 1988;141:1665–9. [PubMed] [Google Scholar]
- 16.Birdsall HH, Lane C, Ramser MN, et al. Induction of VCAM-1 and ICAM-1 on human neural cells and mechanisms of mononuclear leukocyte adherence. J Immunol. 1992;148:2717–23. [PubMed] [Google Scholar]
- 17.Cavender D, Haskard D, Yu CL, et al. Pathways to chronic inflammation in rheumatoid synovitis. Fed Proc. 1987;46:113–7. [PubMed] [Google Scholar]
- 18.Selvaraj P, Pluckett ML, Dustin M, et al. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. Nature. 1987;326:400–3. doi: 10.1038/326400a0. [DOI] [PubMed] [Google Scholar]
- 19.Needleman BW. Increased expression of intercellular adhesion molecule 1 on the fibroblasts of scleroderma patients. Arthritis Rheum. 1990;33:1847–6. doi: 10.1002/art.1780331214. [DOI] [PubMed] [Google Scholar]
- 20.Piela-Smith TH, Broketa G, Hand A, et al. Regulation of ICAM-1 expression and function in human dermal fibroblasts by IL-4. J Immunol. 1992;148:1375–81. [PubMed] [Google Scholar]
- 21.Iigo Y, Takashi T, Tamatani T, et al. ICAM-1 dependent pathway is critically involved in the pathogenesis of adjuvant arthritis in rats. J Immunol. 1991;147:4167–71. [PubMed] [Google Scholar]
- 22.Shetla MR, Schiedt CL. Effect of antifibrosis drug on the survivel of keloid transplants in athymic mice. FASEB J. 1995;9:A967. [Google Scholar]
- 23.Margolin SB, Lefkowitz S. Pirfenidone: a novel pharmacologic agent for prevention and resolution (removal) of lung fibrosis. FASEB J. 1994;8(4):A117. [Google Scholar]
- 24.Margolin S, Margolin B, Margolin D. Removal of interstitial pulmonary fibrosis (Asvestos-induced) by oral chemotherapy with pirfenidone. Fed Proc. 1982;41:1550. [Google Scholar]
- 25.Suga H, Teraoka S, Ota K, et al. Preventive effect of pirfenidone against experimental sclerosing peritonitis in rats. Exp Toxicol Pathol. 1995;47:287–91. doi: 10.1016/s0940-2993(11)80261-7. [DOI] [PubMed] [Google Scholar]
- 26.Iyer SN, Wild JS, Schiedt MJ, et al. Dietary intake of pirfenidone ameliorates bleomycin induced lung fibrosis in hamster. J Lab Clin Med. 1995;125:779–85. [PubMed] [Google Scholar]
- 27.Miyasaka N, Inoue H, Totsuka T, et al. An immunomodulatory protein, Ling Zhi-8, facilitates cellular interaction through modulation of adhesion molecules. Biochem Biophys Res Commun. 1992;186:385–90. doi: 10.1016/s0006-291x(05)80819-8. [DOI] [PubMed] [Google Scholar]
- 28.Inoue H, Saito I, Nakazawa R, et al. Expression of inflammatory cytokines and adhesion molecules in haemodialysis-associated amyloidosis. Nephrol Dial Transplant. 1995;10:2077–82. [PubMed] [Google Scholar]
- 29.Hayashi J, Saito I, Ishikawa I, et al. Effects of cytokines and periodontopathic bacteria on the leukocyte function-associated antigen 1/intercellular adhesion molecule 1 pathway in gingival fibroblasts in adult periodontitis. Infect Immun. 1994;62:5205–12. doi: 10.1128/iai.62.12.5205-5212.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bevilacqua MP, Pober JS, Mendrick DL, et al. Identification of an inducible endothelial–leukocyte adhesion molecule. Proc Natl Acac Sci USA. 1987;84:9238–42. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]


