Abstract
Grave's disease (GD) is characterized by pathogenic autoantibodies to the human thyrotropin receptor (hTSH-R), and is frequently associated with a lymphocytic infiltrate of the thyroid gland. In attempts to establish a murine model of GD, we and others have previously shown that immunization of mice with recombinant preparations of the hTSH-R ectodomain induces high titres of specific antibodies, which, however, are not pathogenic, nor is the response accompanied by the development of thyroiditis. Since earlier reports identified the serological immunodominant determinants within the N- and C-terminal regions of hTSH-R ectodomain, we reasoned that immunization of mice with truncated fragments of ectodomain lacking these dominant regions might result in skewing of the response to other determinants of the molecule, with consequent induction of immunopathological features present in GD. We show here that multiple challenge of BALB/c mice with an amino acid fragment of residues 43–282 generates antibodies directed at hTSH-R peptides 37–56, 157–176, 217–236 and 232–251. This reactivity pattern is distinct from that induced previously with the whole ectodomain of hTSH-R in BALB/c animals. Thyroid function remained unaffected in these mice, suggesting that pathogenic antibodies were not being induced. Interestingly, some animals developed lymphocytic infiltration of the thyroid gland, clearly indicating the presence of pathogenic T cell determinants within the 43–282 fragment. Challenge with the related fragment 43–316 produced the same pattern of serological response to the synthetic peptides as fragment 43–282, but was not accompanied by thyroiditis. The results demonstrate: (i) the presence of thyroiditogenic determinants within hTSH-R, and (ii) that these pathogenic determinants are likely to be cryptic, as their effect is exhibited only when the hierarchy of immunodominance within hTSH-R is drastically altered.
Keywords: thyrotropin receptor, cryptic determinants, thyroiditis, autoimmune disease
INTRODUCTION
The pituitary hormone thyrotropin (TSH) regulates the growth and function of thyroid cells by interacting with thyrotropin receptor (TSH-R) on the basal membrane surface [1]. The human TSH-R (hTSH-R) is a G-binding receptor protein that contains a large ectodomain of 418 amino acids that is responsible for binding TSH, together with seven transmembrane regions and a cytoplasmic region [2]. Autoimmunity to hTSH-R is a common occurrence in human disease. Grave's disease (GD) is characterized by autoantibodies and T cell reactivity to hTSH-R and usually accompanied by a lymphocytic infiltrate of the thyroid gland [3,4]. In GD, thyroid-stimulating antibodies (TSAbs) bind to the hTSH-R and stimulate the cAMP cascade leading to hyperthyroidism. Thyroid-stimulating blocking antibodies (TSBAbs) that block the effects of TSH-mediated cAMP stimulation in thyroid cells are also present and are likely to have a pathogenic role in some patients with Hashimoto's thyroiditis [3–5]. TSH receptor-specific antibodies are frequently assessed in in vitro radioreceptor assays, where they are referred to as TSH binding inhibitory immunoglobulins (TBII) [3,5].
Remarkably, hyperthyroid GD is confined only to humans, since no other species is known so far to spontaneously develop hyperthyroidism mediated by TSAbs [6,7]. Disease induction has been attempted via the transfer of peripheral blood lymphocytes and autologous GD thyroid tissue xenografts into athymic nude or into severe combined immunodeficient (SCID) mice, but resulted only in a transient hyperthyroxinaemia [8–10]. Efforts by various laboratories to develop an animal model of GD by immunizing mice or rabbits with recombinant preparations of hTSH-R ectodomain in adjuvant have largely been unsuccessful [11–17]. Commonly, the antigen preparations in these studies were highly immunogenic, eliciting specific antibodies with high titres but completely non-pathogenic by hormonal or histopathologic criteria. Recently, hyperthyroidism has been induced in mice after challenge with fibroblasts expressing hTSH-R plus MHC class II molecules, leading to production of TSAbs in a small proportion of the injected animals; however, lymphocytic inflammation of the thyroid was not detected [18]. Conversely, immune challenge of mice with a full length hTSH-R [19] or with fusion protein ectodomain of hTSH-R produced in Escherichia coli elicited an inflammatory infiltrate; however, there was no apprent induction of TSAbs in these animals [20,21].
Several studies has shown that following immunization of mice or rabbits with the ectodomain of hTSH-R in adjuvant, the serologically immunodominant determinants appear to reside at the N- and C-terminal regions of the ectodomain [12,15–17,22]. In the present study, we set out to examine the immunopathogenic properties of large truncated hTSH-R fragments lacking these dominant epitopes. We reasoned that deletion of these regions might allow us to look for the presence of cryptic pathogenic epitopes that might not be generated during intracellular processing of the intact ectodomain.
MATERIALS AND METHODS
Expression of hTSH-R fragments lacking serologically dominant regions
Two hTSH-R fragments were expressed as recombinant proteins in Escherichia coli with a C-terminal six-histidine tag to facilitate purification and to ensure that only full length protein products were purified. Fragment 1 (Fr 1) comprised amino acid residues 43–282 and fragment 2 (Fr 2) residues 43–366 (Fig. 1). Murine MoAb A9 recognizing an epitope within the amino acid 217–221 segment (Fig. 1) [22] was used for the identification of these fragments following SDS–PAGE and Western blotting of the inclusion bodies. MoAbs A7 or A10 which recognize epitopes in deleted regions (Fig. 1) were used as controls [22].
Fig. 1.
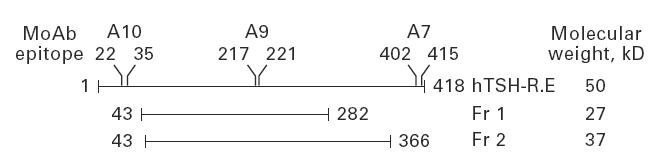
Schematic diagram of the ectodomain of human thyrotropin receptor (hTSH-R) and the two large fragments, Fr 1 and Fr 2 in which the major N- and the C-terminal immunodominant regions of the molecule have been selectively deleted and the recombinant proteins used for immunization. The molecular weights are indicated and the amino acid regions recognized by the MoAbs on the ectodomain of hTSH-R are labelled. (hTSH-R.E refers to the ectodomain of hTSH-R.)
The fragments were generated by polymerase chain reaction (PCR) using hTSH-R cDNA as a template [23]. Oligonucleotide primers were designed so that the resulting PCR products contained an SphI restriction site at the 5′ end, followed by the hTSH-R coding sequence, and a BamHI restriction site at the 3′ end to allow in-frame cloning with the 6xHis tag in the pQE70 expression vector (Qiagen, Crawley, UK). For cDNA coding for residues 43–282 (Fr 1), the forward and reverse primers were: 5′-CAGAGTCGCATGCAGGATATTCAACGCATCCCCAGCTTA -3′ (primer 1) and 5′-AAAAGCGGATCCGTGGCTTGGGTAAGAAAGGTC-3′ (primer 2), respectively. For cDNA coding for residues 43–366 (Fr 2), the forward primer was primer 1 and the reverse primer was 5′-GAGCTCGGATC CAAAACCAATGATCTCATCCTC-3′ (primer 3).
For PCR, to 1 μl of diluted cDNA (10 ng/μl) were added 20 pmol of each primer, 1.5 mm MgCl2 and 2.5 U Taq polymerase in final volume of 50 μl. PCR was performed in a thermal cycler (Perkin Elmer Cetus, Bucks, UK) for 30 cycles, where each cycle consisted of 94°C for 1 min, 55°C for 2 min and 72°C for 2 min. The PCR amplified products were digested with SphI and BamHI, gel-purified and ligated into SphI/BamHI-linearized pQE70 vector. Expression of recombinant protein was induced with 2 mm isopropylthio-β-d-galactose (IPTG) for 5 h and the bacterial suspension pelleted by centrifugation and stored at −70°C. Expression of recombinant hTSH-R fragment protein was confirmed by Western blotting with MoAb A9 [22]. Recombinant proteins were purified using Ni-NTA-resin chromatography (Qiagen) after solubilization in 6 m guanidine–HCl and the protein concentration was measured by the bicinchoninic acid protein assay.
Immunization protocols
For immunizations, female 8-week-old BALB/c mice were used since previous studies showed this strain to be a high responder to hTSH-R ectodomain immunizations [14]. All mice (four animals per group) were subcutaneously challenged at the base of the tail with 50 μg purified Fr 1 or Fr 2 emulsified in Freund's complete adjuvant (FCA) containing Mycobacterium tuberculosis (Sigma Chemical Co, Poole, UK). Three weeks later, they were boosted subcutaneously with the same dose of the receptor fragment in Freund's incomplete adjuvant (FIA). Subsequently, the animals were rechallenged three times every 2 weeks with the same dose of protein in FIA. Control animals were injected with ovalbumin (OVA; 50 μg/injection) in adjuvant at the same time intervals as the hTSH-R fragment-injected animals. A preimmune bleed was collected from the tail 1 day before the first injection and before all injections. One week after the last injection (week 10), mice were killed by CO2 inhalation, blood collected by cardiac puncture and the thyroid glands removed for histology.
Histology
Thyroid glands were fixed in 10% formalin for histological analysis of mononuclear infiltration. The lobes were dissected from the trachea and embedded in methacrylate. Approximately 40 sections, 3.0 μm thick, were collected at 36-μm intervals through each gland, and stained with haematoxylin and eosin for microscopic examination. Scoring was performed blind to the animal groups, as follows: 0 = no infiltration; 0.5 = interstitial accumulation of inflammatory cells distributed between two or more follicles; 1 = one or two foci of inflammatory cells at least the size of one follicle; 2 = extensive infiltration, 10–40% of the total area; 4 = extensive infiltration, > 80% of the total area [24]. The highest inflammatory score observed per gland was assigned to each mouse.
Serological and hormonal analysis
The presence of serum IgG specific for hTSH-R was tested using an ELISA using purified recombinant hTSH-R ectodomain, as described [25]. For measurement of antibodies to synthetic peptides of hTSH-R, ELISA was performed as described [16,17], using a set of 26 overlapping synthetic peptides encompassing hTSH-R ectodomain (a gift from Dr J. Morris, Minneapolis, USA) [26]. The lyophilized hTSH-R peptides were diluted at 10 μg/ml in carbonate bicarbonate buffer for coating ELISA plates. Pooled sera of day 70 post-immunization from all the animals in each group (Fr 1 and Fr 2) were used to map the epitopes of the induced antibodies. For controls, pooled serum from OVA-injected animals from day 70 post-immunization were used. TBII activity was measured by the ability of antibodies to block the binding of 125I-labelled TSH to native TSH-R in porcine thyroid membranes using 50 μl neat mouse serum using a commercial kit [3,14] (TRAK kit; Brahms Diagnostica, Berlin, Germany). Serum from individual mice, obtained on day 70 post-immunization, was used; due to the large volume of serum required, the assay was performed in a single tube assay. TSAbs and TSBAbs were measured in individual sera of day 70 post-immunization using neat serum and chinese hamster ovary (CHO) cells (JP09 cells) as described [27,28]. Due to the use of serum from individual mice in the bioassays, all assays were performed as single determinations. Total serum thyroxine (T4) was measured by radioimmunoassay (DYNOtest; Brahms Diagnostica) [14].
RESULTS
Expression and purification of hTSH-R fragments
Following purification by Ni-NTA-resin chromatography, the hTSH-R Fr 1 (43–282) and Fr 2 (43–366) were assessed for purity by SDS–PAGE and Western blotting with MoAb A9 that recognizes an epitope internal to these sequences (Fig. 1). Purified Fr 1 on coomassie staining gave a strong band at the expected molecular weight of 27 kD, together with a number of other minor bands (Fig. 2a, lane 1). Western blotting with MoAb A9 confirmed that the 27-kD band was a fragment of TSH-R (Fig. 2a, lane 3). Purified Fr 2 on coomassie staining gave a strong band at the expected molecular weight of 37 kD, together with a number of other minor bands, including a strong band at 30 kD (Fig. 2b, lane 1). Western blotting with MoAb A9 confirmed that the 37-kD band was a fragment of TSH-R, but interestingly, also showed reactivity with the 30-kD band, suggesting that this band was likely to be a degradation product of the 37-kD TSH-R fragment (Fig. 2b, lane 3). No products were detected in Western blotting with the control MoAbs A10 and A7 (Fig 1) that react with epitopes localized in the truncated regions (not shown).
Fig. 2.
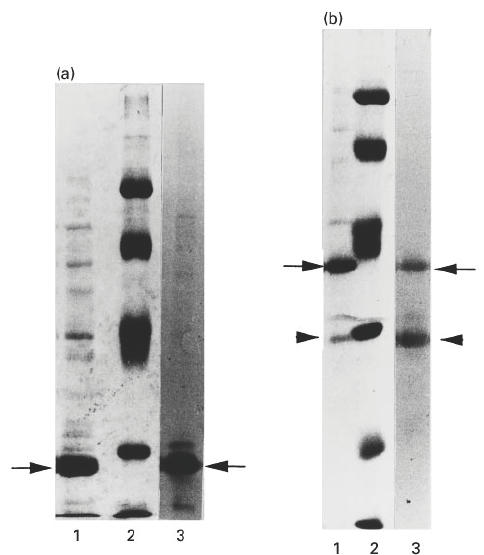
SDS–PAGE analysis of the purified fragments, Fr 1 (a) and Fr 2 (b) used for immunization. Lane 1, purified recombinant fragments; lane 2, molecular weight marker proteins (from top of gel) 93, 67, 43, 30, 20 and 14 kD; lane 3, Western blot with MoAb A9 to visualize the human thyrotropin receptor (hTSH-R) fragments. Lanes 1 and 2 are coomassie blue-stained gels and lane 3 represents nitrocellulose blot developed with horseradish peroxidase (HRP)-labelled anti-mouse IgG. Arrows in (a) and (b) indicate Fr 1 or Fr 2 proteins, respectively; arrowheads in (b) indicate the degraded form of Fr 2.
Analysis of induced antibodies to hTSH-R fragments
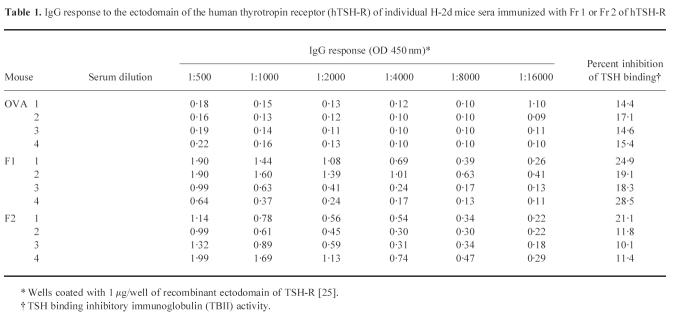
Animals were primed and boosted with 50 μg of Fr 1 or Fr 2 per injection as described in Materials and Methods. On day 70 after the initial challenge, individual animal sera were obtained and tested for the presence of IgG antibodies to hTSH-R ectodomain. Strong hTSH-R-specific responses were observed in all mice that received Fr 1 or Fr 2, whereas OVA-challenged controls were unresponsive (Table 1). However, the overall TSH binding inhibitory activity in sera of mice challenged with Fr 1 or Fr 2 was not significantly different from that of control OVA-primed mice as assessed by the in vitro radioreceptor assay monitoring the binding of radiolabelled TSH to porcine thyroid membranes (Table 1). Serum samples from individual mice were also tested for TSAb and TSBAb activity using JP09 cells. Using undiluted serum, there was no significant TSAb or TSBAb activity in any of the sera from animals immunized with Fr 1 or Fr 2 in comparison with control, OVA-injected mice (not shown).
Table 1.
IgG response to the ectodomain of the human thyrotropin receptor (hTSH-R) of individual H-2d mice sera immunized with Fr 1 or Fr 2 of hTSH-R
* Wells coated with 1 μg/well of recombinant ectodomain of TSH-R [25].
† TSH binding inhibitory immunoglobulin (TBII) activity.
Epitope mapping of antibodies to large fragments of hTSH-R ectodomain
We next proceeded to examine by ELISA the fine specificity of the serological IgG response elicited by Fr 1 versus Fr 2 using a panel of synthetic overlapping peptides covering the whole hTSH-R ectodomain as target antigens. Pooled immune sera from day 70 post-immunization were used (four mice per group). A similar reactivity pattern was observed in sera from either Fr 1- or Fr 2-challenged mice, as IgG bound specifically to peptides 37–56, 157–176 and two overlapping peptides 217–236 and 232–251 (Fig. 3). Control sera from OVA-injected animals did not recognize any of the peptides (not shown). This reactivity pattern was distinct from that observed after challenge of BALB/c with the whole hTSH-R ectodomain reported earlier, in which the response was directed to N-terminus peptide 22–41 and to the C-terminus peptides 352–371 and 367–386 [15,16].
Fig. 3.
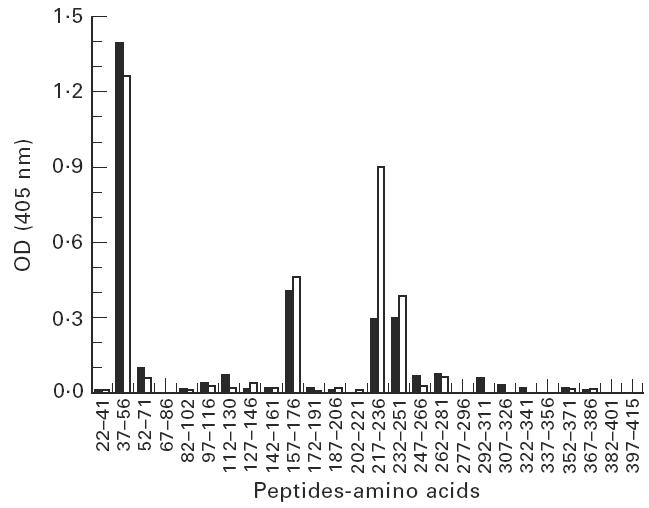
Epitope mapping of the antibodies induced in BALB/c animals with Fr 1 (□) and Fr 2 (▪) of human thyrotropin receptor (hTSH-R) using overlapping synthetic peptides to the ectodomain of hTSH-R in ELISA. The amino acid numbers of the peptide are indicated on the abscissa and the absorbance at 405 nm on the ordinate.
Thyroiditis induction with the hTSH-R fragment 43–282
To assess whether the immunization regimen with the truncated hTSH-R fragments elicited lymphocytic infiltration of the thyroid, histological examination was performed on day 70 after the initial challenge. It was observed that two out of four animals of the group challeged with Fr 1 presented with moderate to severe thyroiditis in both thyroid lobes (infiltration index score 1–2) (Fig. 4a). In contrast, mononuclear infiltration was not detected in the glands from all mice challenged with Fr 2 which had similar morphology to those of normal mice (Fig. 4b), which was indistinguishable from thyroid glands from OVA-primed controls (not shown). These results were surprising in view of earlier data documenting lack of thyroiditis induction by whole recombinant hTSH-R ectodomain in BALB/c animals [11,14,16], and they strongly suggest the presence of cryptic thyroiditogenic determinants within the ectodomain of hTSH-R.
Fig. 4.
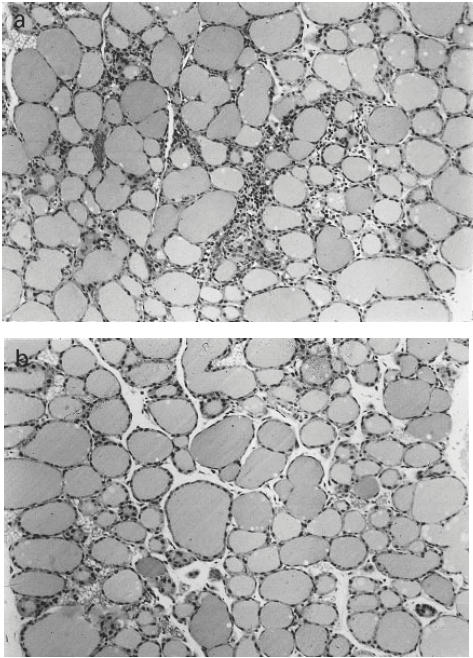
Representative histology of thyroid glands, after formalin fixation and haematoxylin and eosin staining. BALB/c mice were immunized with 50 μg purified protein in Freund's adjuvant. (a) Thyroid from mouse that received Fr 1 of human thyrotropin receptor (hTSH-R). (b) Normal thyroid gland. The focal infiltration (infiltration index 1–2) following challenge with Fr 1 was present in both lobes of the animals that developed thyroiditis. (Mag. × 100.)
Assessment of total serum thyroxine
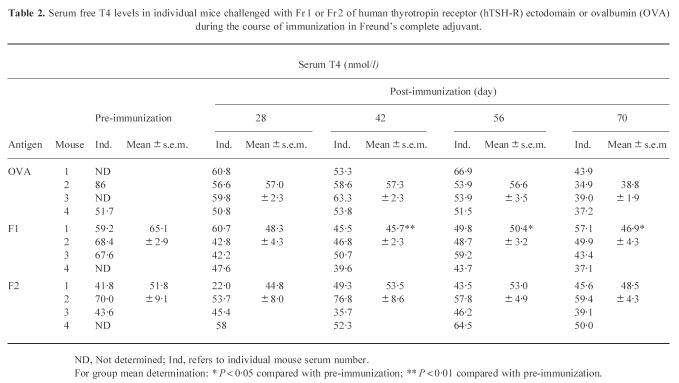
The possible effects on thyroid function were ascertained by measuring total serum T4 levels (T4) in individual mice challenged with Fr 1, Fr 2 or OVA during the course of immunizations (Table 2). In preimmune sera, a large variation in the basal levels of total T4 was observed, varying from 41.8 to 86 nmol/l (Table 2). This large variation in serum total T4 concentrations [29] made interpretation of the data difficult, although some of the group means at different time points in the Fr 1-injected animals showed statistically significant differences (Table 2). Furthermore, the lack of T4 values in preimmune sera of half the animals injected with the control antigen, OVA, made it difficult to compare the effect of immunization in this control group.
Table 2.
Serum free T4 levels in individual mice challenged with Fr 1 or Fr 2 of human thyrotropin receptor (hTSH-R) ectodomain or ovalbumin (OVA) during the course of immunization in Freund's complete adjuvant.
ND, Not determined; Ind, refers to individual mouse serum number.
For group mean determination: * P < 0.05 compared with pre-immunization; ** P < 0.01 compared with pre-immunization.
DISCUSSION
It has been clear from a large number of studies that injection of the whole ectodomain of hTSH-R into mice or rabbits generates antibodies to hTSH-R that are non-pathogenic, even though the induced antibodies are capable of inhibiting the binding of TSH to its receptor [11–17]. The dominant serological regions have been mapped at the N- and the C-terminal ends of the ectodomain [12,15–17,22], and in this study we proceeded to examine the immune response to hTSH-R fragments lacking these regions. Our rationale was that this approach might facilitate induction of specific antibodies with biological action or reveal the presence of thyroiditogenic determinants because of the alteration in the hierarchy of immunodominant epitopes at the B or T cell level.
BALB/c mice immunized with Fr 1 or Fr 2 elicited IgG antibodies that exhibited a very similar pattern of reactivity against the panel of synthetic peptides tested. The strong response against peptide 37–56 suggested the presence of a serological epitope within the 43–56 region, since both fragments lacked amino acids 37–42. Significant reactivity was also detectable to internal regions, as highlighted by the response to peptides 157–176, 217–236 and 232–251. These results demonstrate clearly an alteration in the hierarchy of serologically dominant epitopes, because IgG antibodies have not been elicited to any of these peptides following challenge of BALB/c mice with the whole ectodomain of TSH-R [15,16]. Interestingly, neither Fr 1 nor Fr 2 induced IgG to C-terminal epitopes of the fragments, despite the presence of a longer carboxyl region 283–366 in Fr 2 than in Fr 1. This again contrasts with findings from immunizations with the whole ectodomain [12,15–17,22] and suggests that expression of the region 367–418 is required for the induction of IgG specific for C-terminal epitopes.
We next assessed the relevance of these induced antibodies to different peptides of hTSH-R. Previous studies using mouse and rabbit antisera to the ectodomain of hTSH-R coupled with inhibitory experiments with synthetic peptides have indicated the importance of the N- and the C-terminus regions in the action of the antibodies with TBII activity [11,12,14,15]. Our data showing that antibodies induced to Fr 1 or Fr 2 in BALB/c animals do not show any significant TBII activity give compelling evidence that antibodies with such function recognize the N- and the C-terminus regions of the ectodomain of hTSH-R.
We also examined the biological relevance of the induced antibodies to Fr 1- and Fr 2-immunized BALB/c mice by bioassays. Individual sera from Fr 1- or Fr 2-challenged mice did not mediate a significant increase in cAMP production, nor did they contain significant levels of TSH blocking antibodies. It is possible that using denatured, non-glycosylated fragments such as Fr 1 or Fr 2 does not lead to the production of the correct conformational specific antibodies capable of activating the TSH-R; on the other hand, if such antibodies are induced, the sensitivity of the assay may be insufficient to measure the TSAbs and TSBAbs in serum.
One way to measure the effect on thyroid function of the induced antibodies in BALB/c mice injected with Fr 1 and Fr 2 is by assessment of the levels of total serum T4. Assessment of thyroid function by measuring total serum T4 levels gave equivocal results. On one hand, Fr 1-immunized mice exhibited significantly reduced levels of T4, which were not transient but were maintained from day 42 post-immunization until the end of the experiment. Reduction in T4 levels was not observed in sera of mice challenged with OVA or Fr 2. On the other hand, the biological significance of these data could not be easily ascertained because the T4 levels among preimmune sera showed considerable variation. Measurement of serum TSH [14] might have been a more accurate indicator of thyroid function in the injected mice.
Detection of mononuclear cell infiltration in the thyroids of mice challenged with Fr 1 (43–282) was a surprising and serendipitous finding of the present study. It contrasts with previous observations that BALB/c challenged with the whole hTSH-R ectodomain do not develop thyroiditis [11, 14, 16] as well as with the current data demonstrating that the longer Fr 2 (43–366) is not thyroiditogenic. These results strongly suggest the presence of cryptic pathogenic determinants within amino acids 43–282 of the hTSH-R, which are either not generated during processing of the intact TSH-R ectodomain in antigen-presenting cells (APC) or are generated but competed out by high-affinity MHC-binding peptides localized between amino acids 283 and 418. These pathogenic determinants should be distinct from epitopes recognized by helper T cells involved in the induction of the IgG response, since Fr 1 and Fr 2 both elicit strong IgG responses to the same regions of the TSH-R. Crypticity of pathogenic determinants within hTSH-R could also explain the reported thyroiditogenicity of the full length receptor protein [19] or a fusion protein of the TSH-R ectodomain [20,21], since the additional peptidic regions in these antigens might influence intracellular processing to allow generation of cryptic epitopes. It is of interest that in the studies with the recombinant TSH-R ectodomain fusion protein immunizations [20,21], the dose of the TSH-R moiety in the fusion protein was similar to the dose of the truncated fragments (Fr 1 and Fr 2) used in this study. Since BALB/c animals were challenged in both studies it is unlikely that differences in antigen dose or MHC backgrounds were responsible for the presence of thyroiditis in these studies.
Finally, it is interesting that the salient features of GD, i.e. hyperthyroidism and thyroid infiltration by mononuclear cells, can be reproduced as independent entities in experimental animal models ([18–20] and this study). Although a reliable methodology that can consistently reproduce all GD symptoms in one animal model is still lacking, the accumulated data so far suggest that the lymphocytic infiltration of the thyroid in GD can be brought about via the immune recognition of pathogenic determinants within hTSH-R. Thus, thyroid inflammation in GD may not be an epiphenomenon mediated by spreading of the immune response to other thyroid autoantigens such as thyroglobulin [30] or thyroid peroxidase [31], but it might be precipitated by effector cells responding to thyroiditogenic hTSH-R epitopes as shown in this study. Mapping of these epitopes will provide an impetus towards the establishment of an animal model for GD.
Acknowledgments
This study was supported by a grant from the Medical Research Council of UK (J.P.B. and A.M.M.) and a NATO collaborative grant (to J.P.B. and G.C.). We are grateful to Professor Andrej Gardas for valuable help in the purification of the recombinant ectodomain of hTSH-R from insect cell cultures and to Dr John Morris for the provision of synthetic peptides to hTSH-R. Our thanks also to Brahms Diagnostica, Berlin, Germany, for gifts of antibody and hormone kits.
REFERENCES
- 1.Rapoport B, Spaulding SW. Mechanism of action of thyrotropin and other thyroid growth factors, The thyroid. In: Braverman LE, Utiger RD, editors. 7. Philadelphia: Lippincott-Raven; 1996. pp. 207–19. [Google Scholar]
- 2.Nagayama Y, Rapoport B. The thyrotropin receptor 25 years after its discovery: new insight after its molecular cloning. Mol Endocrinol. 1992;6:145–56. doi: 10.1210/mend.6.2.1569961. [DOI] [PubMed] [Google Scholar]
- 3.Rees Smith B, McLachlan SM, Furmaniak J. Autoantibodies to the thyrotropin receptor. Endocr Rev. 1988;9:106–0. doi: 10.1210/edrv-9-1-106. [DOI] [PubMed] [Google Scholar]
- 4.Weetman AP, McGregor AM. Autoimmune thyroid disease: further developments in our understanding. Endocrine Rev. 1994;15:788–830. doi: 10.1210/edrv-15-6-788. [DOI] [PubMed] [Google Scholar]
- 5.McGregor AM. Autoantibodies to the TSH receptor in patients with autoimmune thyroid disease. Clin Endocrinol (Oxf) 1990;33:683–5. doi: 10.1111/j.1365-2265.1990.tb03905.x. [DOI] [PubMed] [Google Scholar]
- 6.Banga JP. Immunopathogenicity of thyrotropin receptor—ability to induce autoimmune thyroid disease in an experimental animal model. Exp Clin Endocrinol Diabetes. 1996;104:32–34. doi: 10.1055/s-0029-1211678. [DOI] [PubMed] [Google Scholar]
- 7.Volpe R. Has an experimental model for Grave's disease been established? Eur J Endocrinol. 1997;136:150–2. doi: 10.1530/eje.0.1360150. [DOI] [PubMed] [Google Scholar]
- 8.Morita T, Akasu F, Yoshikawa N, et al. Studies of thyroid xenographts from patients with Grave's disease in severe combined immunodeficient mice. J Clin Endocrinol Metab. 1993;77:255–61. doi: 10.1210/jcem.77.1.8100830. [DOI] [PubMed] [Google Scholar]
- 9.Martin A, Valentine M, Unger P, et al. Preservation of functioning human thyroid organoids in the scid mouse: 1. System characterisation. J Clin Endocrinol Metab. 1993;77:305–10. doi: 10.1210/jcem.77.2.8345031. [DOI] [PubMed] [Google Scholar]
- 10.Yoshikawa N, Nishikawa M, Mori S, et al. Simultaneous xenotransplantation of human Grave's thyroid tissue and autologous bone marrow cells in severe combined immunodeficient mice: successful reconstitution of human Grave's hyperthyroidism. Eur J Endocrinol. 1997;136:213–22. doi: 10.1530/eje.0.1360213. [DOI] [PubMed] [Google Scholar]
- 11.Wagle NM, Dallas JS, Seetharamaiah GS, et al. Induction of hyperthyroxinemia in BALB/c but not in several other strains of mice. Autoimmun. 1994;18:103–12. doi: 10.3109/08916939409007983. [DOI] [PubMed] [Google Scholar]
- 12.Dallas JS, Desai RK, Cunningham SJ, et al. Thyrotropin (TSH) interacts with multiple discrete regions of the TSH receptor: polyclonal rabbit antibodies to one or more of these regions can inhibit TSH binding and function. Endocrinol. 1994;134:1437–45. doi: 10.1210/endo.134.3.8119184. [DOI] [PubMed] [Google Scholar]
- 13.Harfst E, Ross MS, Nussey SS, Johnstone AP. Production of antibodies to the human thyrotropin receptor and their use in characterising eukaryotically expressed functional receptor. Mol Cell Endocrinol. 1994;102:77–84. doi: 10.1016/0303-7207(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 14.Carayanniotis G, Huang GC, Nicholson LB, et al. Unaltered thyroid function in mice responding to a highly immunogenic thyrotropin receptor: implications for the establishment of a mouse model for Grave's disease. Clin Exp Immunol. 1995;99:294–302. doi: 10.1111/j.1365-2249.1995.tb05548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagle NM, Patibanda SA, Dallas JS, et al. Thyrotropin receptor specific antibodies in BALB/cJ mice with experimental hyperthyroxinemia show a restricted binding specificity and belong to the immunoglobulin G1 subclass. Endocrinol. 1995;136:3461–9. doi: 10.1210/endo.136.8.7628382. [DOI] [PubMed] [Google Scholar]
- 16.Vlase H, Nakashima M, Graves PN, et al. Defining the major antibody epitopes on the human thyrotropin receptor in immunised mice: evidence for intramolecular epitope spreading. Endocrinol. 1995;136:4415–23. doi: 10.1210/endo.136.10.7664661. [DOI] [PubMed] [Google Scholar]
- 17.Morgenthaler NG, Kim MR, Gardas A, et al. Characterisation of the antibody response to the extracellular region of recombinant thyrotropin receptor. Autoimmun. 1997;26:75–84. doi: 10.3109/08916939709003850. [DOI] [PubMed] [Google Scholar]
- 18.Shimojo N, Kohno Y, Yamaguchi K, et al. Induction of Graves-like disease in mice by immunisation with fibroblasts transfected with the thyrotropin receptor and a class II molecule. Proc Natl Acad Sci USA. 1996;93:11074–9. doi: 10.1073/pnas.93.20.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marion S, Braun JM, Ropars A, et al. Induction of autoimmunity by immunisation of mice with human thyrotropin receptor. Cell Immunol. 1994;158:329–41. doi: 10.1006/cimm.1994.1280. [DOI] [PubMed] [Google Scholar]
- 20.Costagliola S, Many MC, Stalmans-Falys M, et al. Recombinant thyrotropin receptor and the induction of autoimmune thyroid disease in BALB/c mice: a new animal model. Endocrinol. 1994;135:2150–9. doi: 10.1210/endo.135.5.7956939. [DOI] [PubMed] [Google Scholar]
- 21.Costagliola S, Many MC, Stalmans-Falys M, et al. The autoimmune response induced by immunising female mice with recombinant human thyrotropin receptor varies with the genetic background. Mol Cell Endocrinol. 1995;115:199–206. doi: 10.1016/0303-7207(95)03691-1. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson LB, Vlase H, Graves P, et al. Monoclonal antibodies to the human thyrotropin receptor: epitope mapping and binding to the native receptor on the basolateral plasma membrane of thyroid follicular cells. J Mol Endocrinol. 1996;16:159–70. doi: 10.1677/jme.0.0160159. [DOI] [PubMed] [Google Scholar]
- 23.Huang GC, Collison KS, Page MJ, et al. Expression of human thyrotropin receptor in E. coli, purification of recombinant protein and its interaction with autoantibodies from Grave's disease patients. J Mol Endocrinol. 1992;8:137–44. doi: 10.1677/jme.0.0080137. [DOI] [PubMed] [Google Scholar]
- 24.Carayanniotis G, Masters SR, Noelle RJ. Suppression of murine thyroiditis via blockade of the CD40–CD40L interaction. Immunol. 1997;90:421–6. doi: 10.1111/j.1365-2567.1997.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang GC, Page MJ, Nicholson LB, et al. The thyrotropin hormone receptor of Graves disease: overexpression of the extracellular domain in insect cells using recombinant baculovirus, immunoaffinity purification and analysis of autoantibody binding. J Mol Endocrinol. 1993;10:127–42. doi: 10.1677/jme.0.0100127. [DOI] [PubMed] [Google Scholar]
- 26.Morris JC, Bergert ER, McCormick DJ. Structure–function studies of the human thyrotropin receptor. J Biol Chem. 1993;268:10900–5. [PubMed] [Google Scholar]
- 27.Perret J, Ludgate M, Libert F, et al. Stable expression of the human TSH receptor in CHO cells and characterisation of differentially expressing clones. Biochem Biophys Res Commun. 1990;171:1044–50. doi: 10.1016/0006-291x(90)90789-p. [DOI] [PubMed] [Google Scholar]
- 28.Morgenthaler NG, Kim MR, Tremble J, et al. Human IgG autoantibodies to the thyrotropin receptor from Epstein–Barr virus transformed B lymphocytes: characterisation by immunoprecipitation with recombinant antigen and biological activity. J Clin Endocrinol Metab. 1996;81:3155–61. doi: 10.1210/jcem.81.9.8784060. [DOI] [PubMed] [Google Scholar]
- 29.Depaolo LV, Masoro EJ. Endocrine hormones in laboratory animals. In: Loeb WF, Quimby FW, editors. Clinical chemistry of laboratory animals, Oxford: Pergamon Press; 1988. pp. 279–308. [Google Scholar]
- 30.Carayanniotis G, Rao VP. Searching for pathogenic epitopes in thyroglobulin: parameters and caveats. Immunol Today. 1997;18:83–88. doi: 10.1016/s0167-5699(96)10073-6. [DOI] [PubMed] [Google Scholar]
- 31.Banga JP, Barnett PS, McGregor AM. Immunological and molecular characteristics of the thyroid peroxidase autoantigen. Autoimmun. 1991;8:335–43. doi: 10.3109/08916939109007642. [DOI] [PubMed] [Google Scholar]