Abstract
Periodontitis is a chronic destructive inflammatory disease associated with periodontopathic bacteria. In addition, autoantigens such as collagen and heat shock proteins (hsp) have been suggested to play a role. Established periodontal lesions are characterized by dense infiltrations of immune cells such as cytokine-producing CD4+ and CD8+ T cells. CD4+ T cells specific for Prevotella intermedia can be isolated from lesional gingiva, suggesting an active role for CD4+ T cells in the response to this bacterium. We therefore investigated the characteristics of a panel of 13 P. intermedia-specific CD4+ T cells generated from the peripheral blood of a patient with chronic adult periodontitis. All 13 P. intermedia-specific CD4+ T cells recognized the antigens in the context of HLA-DR. The T cell clones were mainly classified as Th0, producing comparable amounts of interferon-gamma (IFN-γ) and IL-4, and Th2, producing high amounts of IL-4 and almost no IFN-γ. None of the P. intermedia-specific T cell clones recognized antigens of the periodontopathic bacteria Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis and of the autoantigens collagen and hsp. The reactivity profile of the T cell clones to size-fractionated cell envelope antigens of P. intermedia indicated that P. intermedia-specific CD4+ T cell clones recognize probably five different antigen specificities in the context of the MHC class II molecules, DR7 or DR15. These results suggest that a broad panel of cell-associated protein antigens play a role in the induction of P. intermedia-specific CD4+ T cell response.
Keywords: CD4+ T cell clones, Prevotella intermedia, periodontitis, cytokines
INTRODUCTION
Adult periodontitis is a chronic inflammatory disease characterized by severe gingival inflammation, progressive destruction of ligament fibres and alveolar bone loss. The disease is primarily related to chronic plaque accumulation. Putative periodontopathic bacteria such as Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedia have been implicated in the initiation and progression of different stages of this disease [1,2]. In addition to the bacterial antigens, autoantigens such as collagen type I and heat shock proteins (hsp) might play a role [3–6].
Established periodontal lesions are characterized by dense infiltrations of immune cells such as plasma cells, B lymphocytes, macrophages, CD4+ and CD8+ T lymphocytes in the connective tissue, which mainly consists of gingival fibroblasts [1,2]. Moreover, histological sections of the lesions show the presence of CD4+ and CD8+ T cells that produce interferon-gamma (IFN-γ) [6–9]. Probably due to the production of IFN-γ, a great number of MHC class II-expressing cells are present in the lesions [10,11]. After random cloning of T cells isolated from chronic inflammatory periodontal lesions, we found previously that some CD4+ T cell clones are specific for P. intermedia, suggesting an active role for CD4+ T cells in the recognition of this bacterium [7]. Since CD4+ T cells exclusively recognize antigens in association with MHC class II exposed by antigen-presenting cells (APC), activation of CD4+ T cells requires the presence of MHC class II-expressing cells. However, recently we found that MHC class II expression alone is not enough, as gingival fibroblasts fail to present P. intermedia to specific CD4+ T cells [11], suggesting that P. intermedia is only presented by professional APC such as Langerhans cells, macrophages or mononuclear cells [12].
In this study we focused on P. intermedia-specific CD4+ T cells from the peripheral blood (PB) of a patient with chronic adult periodontitis. To gain insight into the characteristics of the CD4+ T cell response, we cloned CD4+ T cells reactive to this bacterium. The CD4+ T cell clones were analysed for antigen specificity, MHC class II restriction, IFN-γ and IL-4 secretion. Six CD4+ T cell clones were further analysed for their reactivity to cell envelope preparations and to fractions of the cell envelopes separated on SDS–PAGE and bound to nitrocellulose.
Our results show the presence of P. intermedia-specific CD4+ T cells which recognize probably five different antigens in the context of the MHC class II molecules, DR7 or DR15.
MATERIALS AND METHODS
Antigens and antibodies
The following antigens, as described previously in detail [7,11], were used: P. gingivalis (HG1491), P. intermedia (HG1490), A. actinomycetemcomitans (HG1492), human collagen type I, recombinant hsp from Mycobacterium bovis (65 kD) and M. tuberculosis (71 kD).
Subclasses of HLA class II restriction determinants were characterized by blocking antigen-specific T cell proliferation with the following MoAbs: anti-HLA-DR (B.8.11.2, ascites used at a dilution of 1:200; a kind gift of Dr F. Koning, Department of Immunohaematology, University of Leiden, The Netherlands), anti-HLA-DQ (SPV L3-8, ascites used at a dilution of 1:200; a kind gift of Dr H. Spits, Netherlands Cancer Institute, Amsterdam, The Netherlands), and anti-HLA-DP (B21/7, purified antibodies used at 10 μg/ml; a kind gift of Dr J. Higgins, St Mary's Hospital Medical School, London, UK).
Generation of P. intermedia-reactive T cell clones
A short-term P. intermedia- reactive T cell line was prepared by using a protocol described previously [11]. In short, peripheral blood mononuclear cells (PBMC) from a patient with chronic adult periodontitis before therapeutic periodontal surgery were isolated by density centrifugation on Lymphoprep (Nycomed, Torshov, Norway) and cultured in 96-well flat-bottomed culture plates (Costar, Cambridge, MA) for 11 days in the presence of whole-cell antigens of P. intermedia (10 μg/well), the last 5 days in the presence of 10 U/ml human recombinant rIL-2 (Cetus, Emeryville, CA). Prevotella intermedia-specific T cells were cloned from the short-term P. intermedia-reactive T cell line. On day 11, T cell cloning was performed by limiting dilution at 0.3 cells/well and non-specific stimulation with 1 μg/ml phytohaemagglutinin (PHA; Difco, Detroit, MI) in the presence of a feeder mixture as described previously [7,11,13]. The obtained P. intermedia-specific T cell clones were expanded and phenotyped by FACS analysis as described previously [7,11,13].
Antigen-specific T cell proliferation assays
Ten days after the last stimulation, T cells were washed three times in Hanks' balanced salt solution (HBSS) and 2% fetal calf serum (FCS; Hyclone, Logan, UT) to remove all rIL-2. Subsequently, antigen-specific proliferation of CD4+ T lymphocyte clones (TLC) was assayed by culturing the cells (4 × 104/well) in 96-well flat-bottomed culture plates in the presence or absence of antigen, using 30 Gy-irradiated autologous PBMC (105/well) as APC. After a 48-h culture period the cells were pulsed for 16 h with 0.3 μCi (= 11.1 kBq)/well of 3H-TdR (Amersham International, Aylesbury, UK). T cell proliferation was determined by the incorporation of 3H-TdR and expressed as mean ct/min and s.d. of triplicate cultures. Data were statistically evaluated using one-way anova.
Cytokine induction and measurements
Ten days after the last stimulation, T cells were washed three times in HBSS + 2% FCS and stimulated in 96-well flat-bottomed culture plates at 4 × 104 cells/well. T cell supernatants were obtained by incubating the TLC for 24 h with a combination of PHA (1 μg/ml) and phorbol myristate acetate (PMA; 1 ng/ml). Alternatively, we collected 24 h supernatants of antigen-specific stimulated TLC cells before adding 3H-TdR. Supernatants were stored in aliquots at −20°C until cytokine production was analysed.
IL-4 and IFN-γ secretion by the cloned T lymphocytes was measured in culture supernatant with specific solid-phase sandwich ELISAs, as described in detail elsewhere [7,13].
Cell envelope preparation P1 and P2 of P. intermedia
Formaldehyde-fixed P. intermedia were centrifuged at 4°C for 15 min at 10 000 g. The pellet was suspended in 2 ml PBS and the cell suspension was disrupted with an ultrasonicator (Sonics and Materials Inc., Danbury, CT) on ice with an output of 200 W for 20 min with four intervals of 4 min. After intact cells were removed by centrifugation at 26 000 g for 30 min, the sonicate supernatant was separated by centrifugation at 100 000 g for 90 min to obtain the cell envelope fraction as sediment. This fraction was washed five times with PBS. The cell envelope fraction (31% of whole cells) was resuspended in 1 ml PBS with (preparation P1) or without (preparation P2) 30 mmβ-octylglucoside (Sigma Chemical Co., St Louis, MO). The β-octylglucoside-containing preparation P1 was incubated for 40 min at room temperature and was dialysed overnight against 5 l of PBS at 4°C. Finally, preparations P1 and P2 were centrifuged at 100 000 g for 90 min. The sediments of P1 and P2 were precipitated by ammonium sulphate precipitation and dialysed overnight against 5 l of PBS at 4°C. Preparations P1 and P2 were subjected to 10% precast SDS–polyacrylamide (PhastGel; Pharmacia Biotech, Uppsala, Sweden) and run under reducing conditions. Gels were stained with coomassie brilliant blue by using a commercial kit (Pharmacia) following the manufacturer's instructions.
Preparation of immunoblots and nitrocellulose particle suspensions
To study the proliferative response of P. intermedia-specific T cell clones to cell envelope proteins, the two different preparations P1 and P2 were separated based on molecular weight by SDS–PAGE as described above. Following electrophoresis, protein was transferred to nitrocellulose (NC 0.45 μm; BioRad, Melville, NY). Both the left and right sides of the nitrocellulose blot were cut off and stained with 0.2% Ponceau S (Sigma) in 3% trichloroacetic acid/3% sulphosalicylic acid to visualize the protein bands. The rest was used to prepare suspensions of nitrocellulose particles containing the separated proteins, as previously described by Abou-Zeid et al. [14]. These nitrocellulose particle suspensions were used at a final concentration of 5 μg/ml in antigen-specific T cell proliferation assays.
RESULTS
Characterization of P. intermedia-specific CD4+T cell clones
From the short-term antigen-reactive T cell line obtained by stimulation of PBMC with P. intermedia, T cell clones were generated. The specificity of the obtained T cell clones was screened in an antigen-specific T cell proliferation assay using autologous irradiated PBMC as a source of APC and a panel of antigens including the 65- and 71-kD hsp, human collagen type I and whole-cell antigens from A. actinomycetemcomitans, P. gingivalis and P. intermedia. We obtained 13 T cell clones with a specificity for P. intermedia and a TCR α/β+ CD3+ CD4+ CD8− phenotype (Table 1). None of the 13 P. intermedia-specific T cell clones reacted with A. actinomycetemcomitans, P. gingivalis, collagen type I, hsp65 and hsp71 (Table 1). The T cell clones were CD4+, implying MHC class II restriction. Blocking of the antigen-specific proliferation showed that all 13 T cell clones recognized P. intermedia presented by DR molecules (data not shown). Table 2 summarizes the results of a detailed analysis of the restriction specificity using autologous and allogeneic PBMC as APC. The data show that out of 11 clones tested, nine clones recognized P. intermedia antigens in the context of HLA-DR15+ and two clones in the context of HLA-DR7+. These results suggest that in this patient HLA-DR15 is a major antigen-presenting molecule in the recognition of P. intermedia by CD4+ T cells.
Table 1.
Proliferative responses of T cell clones to various antigens*
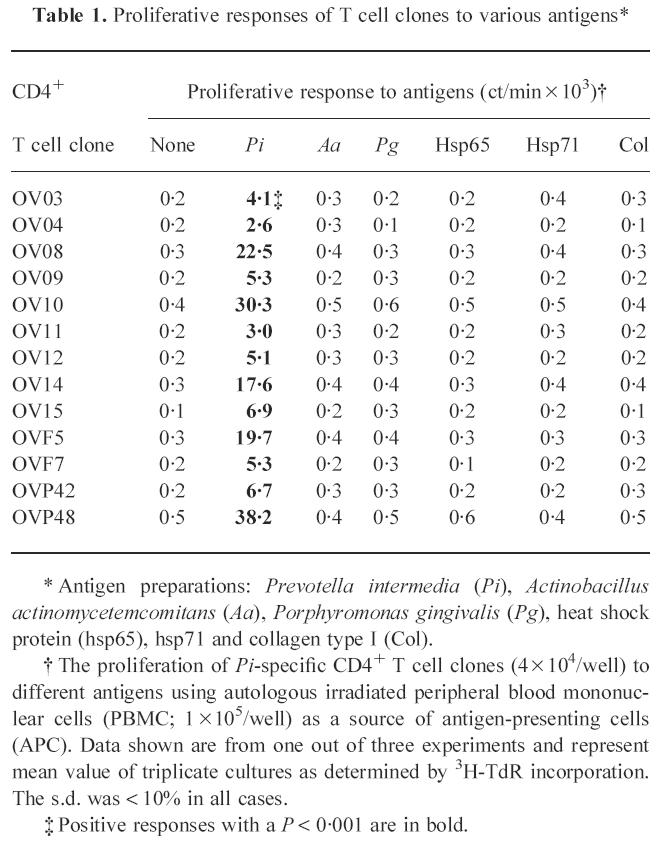
* Antigen preparations: Prevotella intermedia (Pi), Actinobacillus actinomycetemcomitans (Aa), Porphyromonas gingivalis (Pg), heat shock protein (hsp65), hsp71 and collagen type I (Col).
† The proliferation of Pi-specific CD4+ T cell clones (4 × 104/well) to different antigens using autologous irradiated peripheral blood mononuclear cells (PBMC; 1 × 105/well) as a source of antigen-presenting cells (APC). Data shown are from one out of three experiments and represent mean value of triplicate cultures as determined by 3H-TdR incorporation. The s.d. was < 10% in all cases.
‡ Positive responses with a P < 0.001 are in bold.
Table 2.
HLA-DR restriction determinants of Prevotella intermedia-specific CD4+ T cell clones
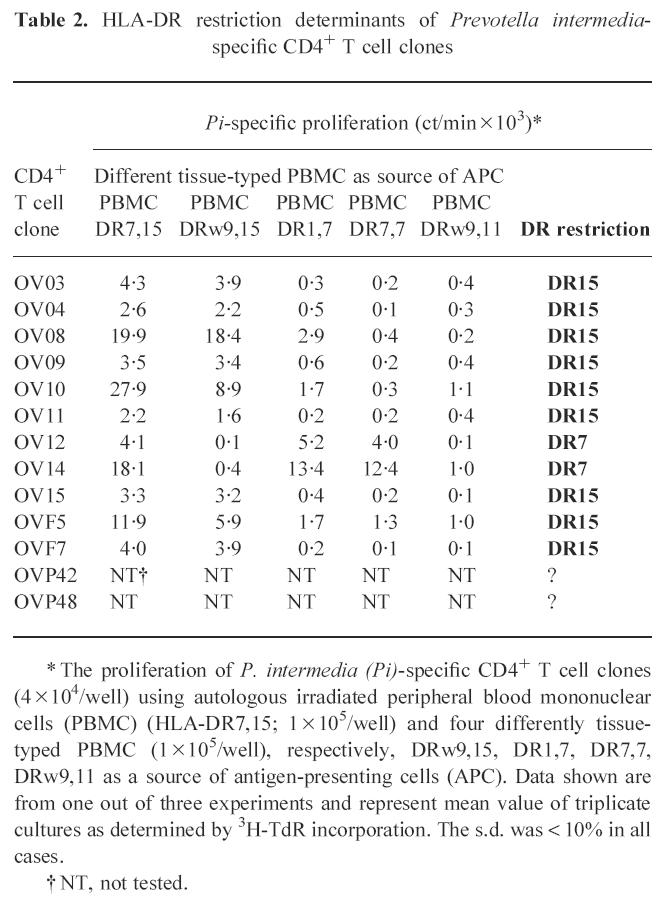
* The proliferation of P. intermedia (Pi)-specific CD4+ T cell clones (4 × 104/well) using autologous irradiated peripheral blood mononuclear cells (PBMC) (HLA-DR7,15; 1 × 105/well) and four differently tissue-typed PBMC (1 × 105/well), respectively, DRw9,15, DR1,7, DR7,7, DRw9,11 as a source of antigen-presenting cells (APC). Data shown are from one out of three experiments and represent mean value of triplicate cultures as determined by 3H-TdR incorporation. The s.d. was < 10% in all cases.
† NT, not tested.
Cytokine profiles of P. intermedia-specific CD4+T cell clones
From the 13 P. intermedia-specific T cell clones, cytokine production was measured after antigen-specific stimulation for 24 h. Defining a production of comparable amounts of IL-4 and IFN-γ as Th0, a production of < 10% IL-4 compared with IFN-γ as Th1 and a production of < 10% IFN-γ compared with IL-4 as Th2 [7], seven TLC were classified as Th0, two as Th1 and four as Th2 (Table 3). Similar cytokine patterns were obtained after mitogenic stimulation with the combination of PHA and PMA (data not shown).
Table 3.
Cytokine profiles of Prevotella intermedia-specific CD4+ T cell clones
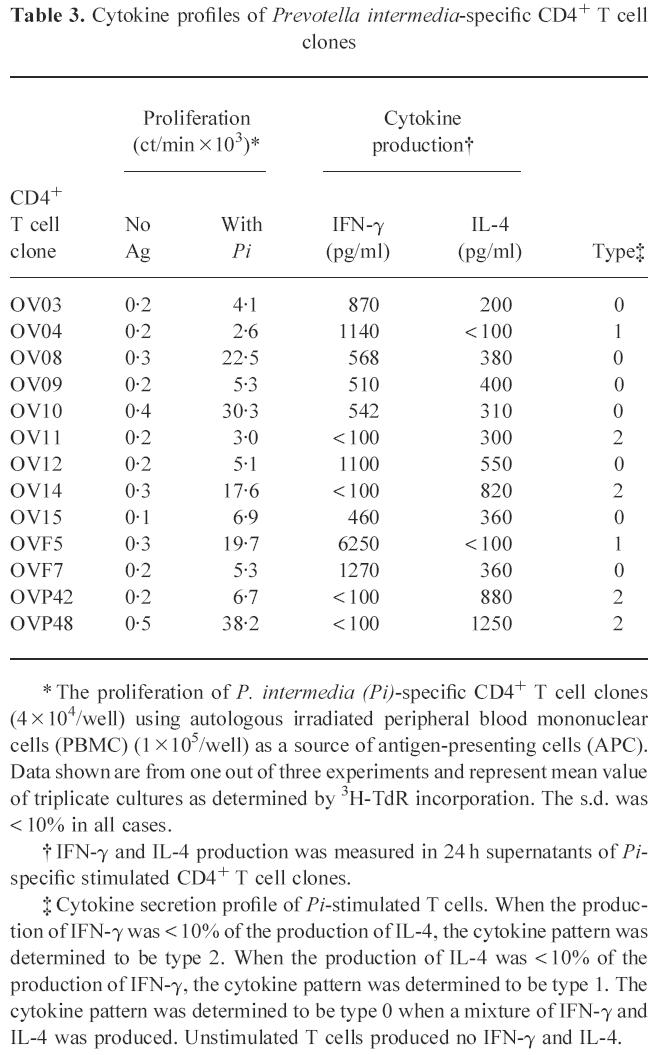
* The proliferation of P. intermedia (Pi)-specific CD4+ T cell clones (4 × 104/well) using autologous irradiated peripheral blood mononuclear cells (PBMC) (1 × 105/well) as a source of antigen-presenting cells (APC). Data shown are from one out of three experiments and represent mean value of triplicate cultures as determined by 3H-TdR incorporation. The s.d. was < 10% in all cases.
† IFN-γ and IL-4 production was measured in 24 h supernatants of Pi-specific stimulated CD4+ T cell clones.
‡ Cytokine secretion profile of Pi-stimulated T cells. When the production of IFN-γ was < 10% of the production of IL-4, the cytokine pattern was determined to be type 2. When the production of IL-4 was < 10% of the production of IFN-γ, the cytokine pattern was determined to be type 1. The cytokine pattern was determined to be type 0 when a mixture of IFN-γ and IL-4 was produced. Unstimulated T cells produced no IFN-γ and IL-4.
Proliferative response of P. intermedia-specific CD4+ T cell clones to heterogeneous mixture and size-fractionated proteins of envelope preparations P1 and P2
Thirteen CD4+ T cell clones were raised against whole-cell antigens of P. intermedia. The cell envelope of such a Gram-negative bacterium is important in generating immune responses and it is likely that the cell envelope is involved in specific T cell recognition. To investigate whether P. intermedia-specific CD4+ T cell clones could recognize cell envelope proteins, two different preparations, P1 and P2, were tested on a panel of six P. intermedia-specific T cell clones. P1 was prepared with the use of β-octylglucoside and P2 was prepared without the use of β-octylglucoside. To gain more insight in the nature of the cell envelope proteins, P1 and P2 were separated by SDS–PAGE under reducing conditions by using a 10% gel. Electrophoresis of P1 resulted in bands of ≈ 67, 43 and 29 kD (Fig. 1). Separation of P2 by electrophoresis resulted in bands of ≈ 94, 74, 67, 61 (very weak) and 55 (very weak) kD. Three T cell clones reacted with P1 and two clones with P2 (Table 4). One T cell clone reacted with none of the preparations.
Fig. 1.
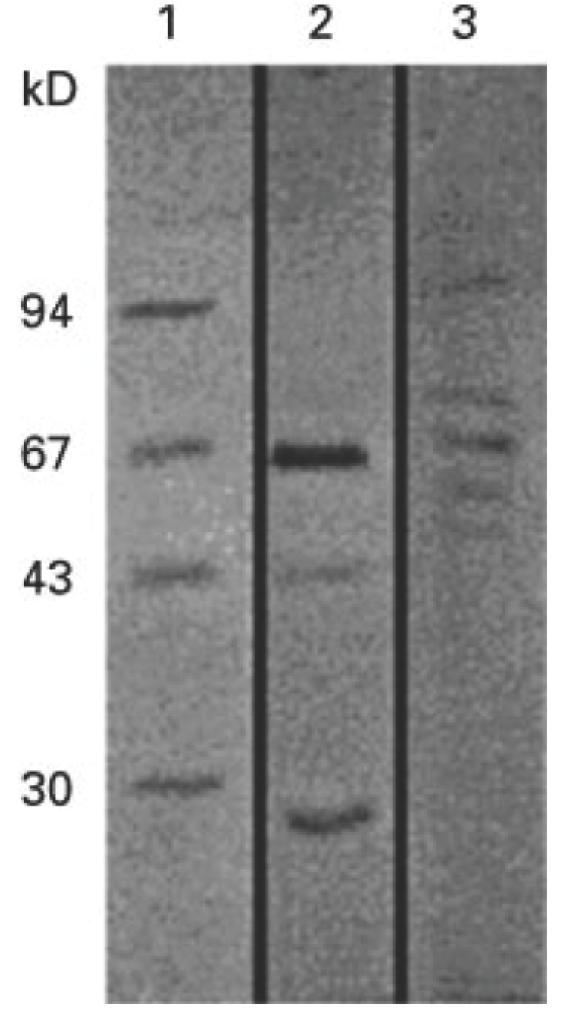
SDS–PAGE of two different cell envelope preparations P1 and P2. Samples were run on a 10% SDS–polyacrylamide gel under reducing conditions. Lane 1, low range molecular weight markers (BioRad); lane 2, P1 (prepared with the use of β-octylglucoside); lane 3, P2 (prepared without the use of β-octylglucoside). Proteins were visualized by staining with coomassie brilliant blue. The molecular weights of the markers are indicated on the left of the figure.
Table 4.
Proliferative response of Prevotella intermedia-specific CD4+ T cell clones to heterogeneous mixture and size-fractionated proteins of cell preparations P1 and P2
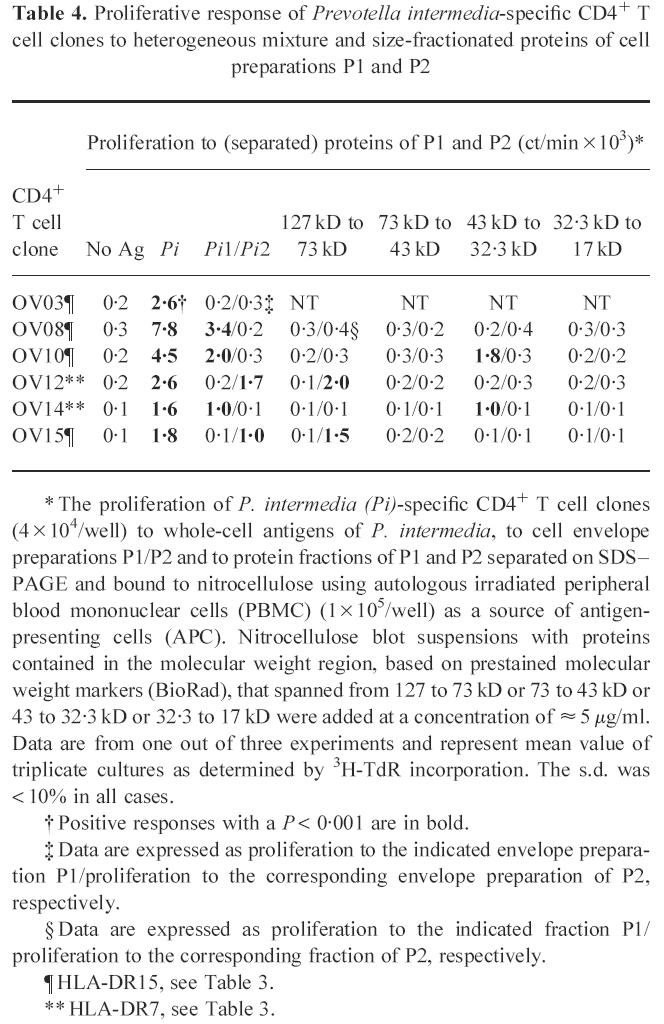
* The proliferation of P. intermedia (Pi)-specific CD4+ T cell clones (4 × 104/well) to whole-cell antigens of P. intermedia, to cell envelope preparations P1/P2 and to protein fractions of P1 and P2 separated on SDS–PAGE and bound to nitrocellulose using autologous irradiated peripheral blood mononuclear cells (PBMC) (1 × 105/well) as a source of antigen-presenting cells (APC). Nitrocellulose blot suspensions with proteins contained in the molecular weight region, based on prestained molecular weight markers (BioRad), that spanned from 127 to 73 kD or 73 to 43 kD or 43 to 32.3 kD or 32.3 to 17 kD were added at a concentration of ≈ 5 μg/ml. Data are from one out of three experiments and represent mean value of triplicate cultures as determined by 3H-TdR incorporation. The s.d. was < 10% in all cases.
† Positive responses with a P < 0.001 are in bold.
‡ Data are expressed as proliferation to the indicated envelope preparation P1/proliferation to the corresponding envelope preparation of P2, respectively.
§ Data are expressed as proliferation to the indicated fraction P1/proliferation to the corresponding fraction of P2, respectively.
¶ HLA-DR15, see Table 3.
** HLA-DR7, see Table 3.
To determine which protein in P1 or P2 was responsible for the antigen-specific T cell proliferation, fractions of P1 or P2 separated on SDS–PAGE were bound to nitrocellulose and screened in an antigen-specific T cell proliferation assay. As shown in Table 4, two CD4+ T cell clones, TLC OV10 and TLC OV14, were reactive to a 43–32-kD fraction of P1. Only one protein of about 43 kD is visible on the gel in this molecular weight range, suggesting that this protein was recognized. In spite of the fact that the two T cell clones were reactive to the same protein fraction, they differed in HLA-DR restriction and cytokine profile. TLC OV10 recognized proteins presented by DR15+ APC and resembled a type 0 cytokine profile. TLC OV14 recognized proteins presented by DR7+ APC and showed a type 2 cytokine profile. One T cell clone, TLC OV08, reacted with P1, but with none of the fractions, suggesting that this clone was reactive to an antigen that was lost in the size-fractionation procedure. Two CD4+ T cell clones, TLC OV12 and TLC OV15, were reactive to a 127–73-kD fraction of P2. In this molecular weight range, two P2 proteins of ≈ 94 and 74 kD are visible on gel, which may be recognized by these clones. Although TLC OV12 and TLC OV15 were reactive to the same protein fraction, they differed in HLA-DR specificity. TLC OV12 and OV15 recognized proteins presented by DR7+ and DR15+, respectively. In addition, the results showed that each T cell clone only reacted with one fraction.
DISCUSSION
Previously, we showed that in chronically inflamed periodontal tissue the infiltration of immune cells is characterized by CD4+ and CD8+ T cells that produce IL-4 and/or IFN-γ [7,13]. After random cloning of T cells from these tissues, some of the CD4+ T cells were shown to be specific for the periodontopathic bacterium P. intermedia, suggesting an active role for T cells in the recognition of this bacterium [7]. In addition, we found that MHC class II-expressing gingival fibroblasts failed to present P. intermedia to specific CD4+ T cells, indicating that cell-associated proteins of this bacterium are presented by professional APC [11]. In addition, in our previous study we showed that CD4+ T cells isolated from periodontal tissue of a single patient represent a mixture of various antigen specificities by recognizing a broad panel of antigens such as A. actinomycetemcomitans, P. gingivalis, P. intermedia and collagen type I. In agreement with the study of Ivanyi et al. [15], these results together suggest that a broad panel of antigens is able to elicit CD4+ T cell responses in periodontitis which is consistent with bacterial diseases such as tuberculosis and leprosy [16,17].
This study shows that P. intermedia is able to induce CD4+ T cell responses in peripheral blood as well. The CD4+ T cell clones obtained from one single patient with chronic adult periodontitis recognize a broad panel of (cell envelope) antigens of P. intermedia presented by PBMC. By using the method of T cell immunoblotting, we were able to identify T cell responses to two protein fractions. Each fraction induced proliferation of two T cell clones. The number of proteins in each fraction is unknown. The 43–32-kD fraction reacted with two T cell clones differing in HLA-DR specificity and cytokine profile. The 127–73-kD fraction reacted with two T cell clones only differing in HLA-DR specificity. The absence of a strict correlation between antigen specificity and HLA restriction has been reported [18,19]. Still, antigen specificity and HLA restriction may be important factors influencing T cell cytokine profiles [20,21].
The cell envelope proteins of P. intermedia have not been thoroughly investigated yet, but recently three proteins were characterized. A 55-kD protein [22], a 57-kD protein with lactoferrin-binding capacity [23] and a protein of ≈ 65 kD with human IgG Fc binding [24] were reported. In addition, Leung et al. [25] showed fimbriae on the surface of P. intermedia, but data on molecular weights of these fimbriae proteins are lacking. At present, it is still unclear whether our T cell clones recognized one of more of these proteins.
In agreement with our previous results [7] on the antigen specificity of local gingival T cell clones, we show that P. intermedia-specific T cells are not reactive with other antigens implicated in the disease, such as the periodontopathic bacteria A. actinomycetemcomitans and P. gingivalis and the autoantigens collagen type I and hsp. This absence of this reactivity makes the earlier postulated molecular mimicry between periodontitis-associated bacteria and autoantigens more unlikely, at least for P. intermedia [3–5]. The cytokine profiles of P. intermedia-specific CD4+ T cells obtained from PB as described in this study showed a more diverse pattern than P. intermedia-specific T cells isolated from the inflamed lesions as described in a previous study [7]. However, most of the P. intermedia-specific T cells showed a Th0 and Th2 cytokine pattern consistent with the reported general cytokine pattern of local T cells within the inflamed periodontal lesion [6–9,26]. In comparison with P. intermedia-specific CD4+ T cells from chronically inflamed gingival tissue, we also observed Th2 and a few Th1 patterns. It is difficult to make a truly conclusive comparison between T cells from blood and those from gingiva because the clones from these different sources were selected by different procedures. Supporting our data, a study by Howe et al. [27] showed differences between cytokine profiles of T cell clones isolated from blood and skin obtained from leprosy patients. In contrast, van der Heijden et al. [28] reported similar profiles of house dust mite-specific CD4+ T cell clones isolated from blood and lesional skin in atopic dermatitis. Nonetheless, it is tempting to speculate that there may be important tissue-specific components that influence the differentiation and outgrowth of Th0, Th1 and Th2 cells. Upon activation, Th cells are optimally susceptible to modulatory actions of cytokine profile-skewing factors derived from various types of APC or accessory cells. Several components in the local microenvironment, most of them cytokines themselves, may specifically promote Th1 or Th2 outgrowth. IFN-γ and IL-12 strongly promote the generation of Th1 cells, whereas IL-4, IL-10 and prostaglandin E2(PGE2) promote the generation of Th2 cells [29–33].
From the rat model it was suggested that Th2 cells are protective against the development of periodontal disease [34,35]. However, in the PB of a chronic adult periodontitis patient we found Th0 and Th2 cells predominant. If Th2 cells are truly protective in periodontitis, they may act by slowing the disease. Indeed, the Th2 product IL-4 is important in B cell activation, up-regulation of CD23 expression on B cells as well as IgG4 synthesis, while the number of CD23+ activated B cells is increased in periodontitis lesions compared with gingivitis [36]. In addition, increased levels of IgG4 in serum or gingival crevicular fluid from patients with active periodontitis sites have been reported [37]. Furthermore, IL-4 down-regulates Th1 cells by blocking IL-2-dependent proliferation and IFN-γ secretion and thus has an indirect anti-inflammatory effect. IL-4 may then help to reduce tissue damage to a slow ongoing process instead of a local DTH reaction with increased local tissue destruction. Disturbing the local IL-4 balance might inflame the disease and induce progression of the disease.
The present findings in the PB of a patient with chronic adult periodontitis confirm previous results and suggest that a broad panel of (cell envelope) antigens can be important in inducing P. intermedia CD4+ T cell responses which are mainly skewed to a Th0 and Th2 cytokine profile.
REFERENCES
- 1.Listgarten MA. Pathogenesis of periodontitis. J Clin Periodontol. 1989;13:418–30. doi: 10.1111/j.1600-051x.1986.tb01485.x. [DOI] [PubMed] [Google Scholar]
- 2.Seymour GJ, Gemmell E, Reinhardt RA, Eastcott J, Taubman MA. Immunopathogenesis of chronic inflammatory disease: cellular and molecular mechanisms. J Periodont Res. 1993;28:478–86. doi: 10.1111/j.1600-0765.1993.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 3.Anusaksathien O, Dolby AE. Autoimmunity in periodontal diseases. J Oral Pathol Med. 1991;20:101–7. doi: 10.1111/j.1600-0714.1991.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 4.Anusaksathien O, Singh G, Matthews N, Dolby AE. Autoimmunity to collagen in adult periodontitis disease: immunoglobulin classes in sera and tissue. J Periodont Res. 1992;27:55–61. doi: 10.1111/j.1600-0765.1992.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 5.Ando T, Kato T, Ishihara K, Ogiuchi H, Okuda K. Heat shock proteins in the human periodontal disease process. Microbiol Immunol. 1995;39:321–6. doi: 10.1111/j.1348-0421.1995.tb02208.x. [DOI] [PubMed] [Google Scholar]
- 6.Lundqvist C, Baranov V, Teglund S, Hammarström S, Hammarström M. Cytokine profile and ultrastructure of intraepithelial γδ T cells in chronically inflamed human gingiva suggest a cytotoxic effector function. J Immunol. 1994;153:2302–12. [PubMed] [Google Scholar]
- 7.Wassenaar A, Reinhardus C, Thepen T, Abraham-Inpijn L, Kievits F. Cloning, characterization, and antigen specificity of T-lymphocyte subsets extracted from gingival tissue of chronic adult periodontitis patients. Infect Immun. 1995;63:2147–53. doi: 10.1128/iai.63.6.2147-2153.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazaki K, Nakajima T, Hara K. Immunohistological analysis of T cell functional subsets in chronic inflammatory disease. Clin Exp Immunol. 1995;99:384–91. doi: 10.1111/j.1365-2249.1995.tb05562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujihashi K, Yamamoto M, Hiroi T, Bamberg TV, McGhee JR, Kiyono H. Selected Th1 and Th2 cytokine mRNA expression by CD4+ T cells isolated from inflamed human gingival tissue. Clin Exp Immunol. 1996;103:422–8. doi: 10.1111/j.1365-2249.1996.tb08297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawford JM. Distribution of ICAM-1, LFA-3 and HLA-DR in healthy and diseased gingival tissues. J Periodont Res. 1992;27:291–8. doi: 10.1111/j.1600-0765.1992.tb01680.x. [DOI] [PubMed] [Google Scholar]
- 11.Wassenaar A, Snijders A, Abraham-Inpijn L, Kapsenberg ML, Kievits F. Antigen-presenting properties of gingival fibroblasts in chronic adult periodontitis. Clin Exp Immunol. 1997;110:277–84. doi: 10.1111/j.1365-2249.1997.tb08328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett AW, Cruchley AT, Williams DM. Oral mucosal Langerhans cells. Crit Rev Oral Biol Med. 1996;7:36–58. doi: 10.1177/10454411960070010301. [DOI] [PubMed] [Google Scholar]
- 13.Wassenaar A, Reinhardus C, Abraham-Inpijn L, Kievits F. Type-1 and type-2 CD8+ T-cell subsets isolated from chronic adult periodontitis tissue differ in surface phenotype and biological functions. Immunology. 1996;87:113–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Abou-Zeid C, Filley E, Steele J, Rook GAW. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J Immunol Methods. 1987;98:5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- 15.Ivanyi L, Newman HN, Marsh PD. T cell proliferative responses to molecular fractions of periodontopathic bacteria. Clin Exp Immunol. 1991;83:108–11. doi: 10.1111/j.1365-2249.1991.tb05597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SP, Stocker NG, Grant KA, Handzel ZT, Hussain R, McAdam Kpwj, Dockrell HM. Cellular immune responses of leprosy contacts to fractionated Mycobacterium leprae antigens. Infect Immun. 1989;57:2475–80. doi: 10.1128/iai.57.8.2475-2480.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orme IM, Andersen P, Boom WH. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–97. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 18.Carballido JM, Carballido-Perrig N, Kagi MK, Meloen RH, Wurtrich B, Heusser CH, Blaser K. T cell epitope specificity in human allergic and nonallergic subjects to bee venom phospholipase A2. J Immunol. 1993;50:3582–91. [PubMed] [Google Scholar]
- 19.van Neerven RJJ, de Pol MM, Wierenga EA, Aalberse RC, Jansen HM, Kapsenberg ML. Peptide-specificity and HLA-restriction do not dictate lymphokine production by allergen-specific T lymphocyte clones. Immunology. 1994;82:351–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Soloway P, Fish S, Passmore H, Gefter M, Coffe R, Manser T. Regulation of the immune response to peptide antigens: differential induction of immediate-type hypersensitivity and T cell proliferation due to changes in either peptide structure or Major Histocompatibility Complex haplotype. J Exp Med. 1991;174:847–58. doi: 10.1084/jem.174.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray JS, Madri J, Tite J, Carding SR, Bottomly K. MHC control of CD4+ T cell subset activation. J Exp Med. 1989;170:2135–40. doi: 10.1084/jem.170.6.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsushita K, Hagaoka S, Arakaki R, Kawabata Y, Iki K, Kawagoe M, Takada H. Immunobiological activities of a 55-kilodalton cell surface protein of P. intermedia ATCC 25611. Infect Immun. 1994;62:2459–69. doi: 10.1128/iai.62.6.2459-2469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alugupalli KR, Kalfas S, Edwardsson S, Forsgren A, Arnold RR, Naidu AS. Effect of lactoferrin on the interaction of Prevotella intermedia with plasma and subepithelial matrix. Oral Microbiol Immunol. 1994;9:174–9. doi: 10.1111/j.1399-302x.1994.tb00055.x. [DOI] [PubMed] [Google Scholar]
- 24.Labbe S, Grenier D. Characterization of the human immunoglobulin G Fc-binding activity in Prevotella intermedia. Infect Immun. 1995;63:2785–9. doi: 10.1128/iai.63.7.2785-2789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung K, Fukushima H, Nesbitt WE, Clark WB. Prevotella intermedia fimbriae mediate hemagglutination. Oral Microbiol Immunol. 1996;11:42–50. doi: 10.1111/j.1399-302x.1996.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 26.Tokoro Y, Matsuki Y, Yamamoto T, Suzuki T, Hara K. Relevance of local Th2-type cytokine mRNA expression in immunocompetent infiltrates in inflamed gingival tissue to periodontal diseases. Clin Exp Immunol. 1997;107:166–74. doi: 10.1046/j.1365-2249.1997.d01-880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howe RC, Wondimu A, Demissee A, Frommel D. Functional heterogeneity among CD4+ T-cell clones from blood skin lesions of leprosy patients. Identification of T-clones distinct from Th0, Th1 and Th2. Immunology. 1995;84:585–94. [PMC free article] [PubMed] [Google Scholar]
- 28.van der Heijden FL, Wierenga EA, Bos JD, Kapsenberg ML. High frequency of IL-4-producing CD4+ allergen-specific T lymphocytes in atopic dermatitis lesional skin. J Invest Dermatol. 1991;97:389–94. doi: 10.1111/1523-1747.ep12480966. [DOI] [PubMed] [Google Scholar]
- 29.Steinmann RM. The dendritic cell system and its role in immunogenicity. Ann Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 30.Maggi E, Parronchi P, Manetti R, et al. Reciprocal regulatory effects of IFN-γ and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992;148:2142–7. [PubMed] [Google Scholar]
- 31.Abehsira-Amar O, Gibert M, Joliy M, Theze J, Jancovic DL. Il-4 plays a dominant role in the differential development of Th0 into Th1 and Th2 cells. J Immunol. 1992;148:3820–9. [PubMed] [Google Scholar]
- 32.Manetti R, Parroncji P, Giudizi MG, Piccini MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin-12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:199–204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snijdewint FGM, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993;150:5321–9. [PubMed] [Google Scholar]
- 34.Eastcott JW, Yamashita K, Taubman A, Smith DJ. Characterization of rat T-cell clones with bacterial specificity. Immunology. 1990;71:120–6. [PMC free article] [PubMed] [Google Scholar]
- 35.Yamashita K, Eastcott JW, Taubman MA, Smith DJ, Cox DS. Effect of adoptive transfer of cloned Actinobacillus actinomycetemcomitans-specific T helper cells on periodontal disease. Infect Immun. 1991;59:1529–34. doi: 10.1128/iai.59.4.1529-1534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamazaki K, Nakajima T, Aoyagi T, Hara K. Immunohistological analysis of memory T lymphocytes and activated B lymphocytes in tissues with periodontal disease. J Periodont Res. 1993;28:324–34. doi: 10.1111/j.1600-0765.1993.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 37.Reinhardt RA, McDonald TL, Bolton RW, Dubois LM, Kaldahl WB. IgG subclasses in gingival crevicular fluid from active versus stable periodontitis. J Periodontol. 1989;60:44–50. doi: 10.1902/jop.1989.60.1.44. [DOI] [PubMed] [Google Scholar]


