Abstract
C8/119S is a mutant of recombinant Der f 2 (rDer f 2), and lacks a disulphide bond possessed by wild-type rDer f 2. In humans and mice, C8/119S has a very weak IgE-binding capacity compared with the wild-type, but possesses a T cell reactivity comparable to that of the wild-type. C8/119S may thus be a safe immunotherapeutic agent for house dust mite allergy. The aim of the present study was to evaluate whether the intranasal administration of C8/119S could suppress an immediate allergic reaction in mice sensitized with wild-type rDer f 2, possessing an allergic activity comparable to native counterparts purified from mite extract. Seven-week-old male A/J mice were immunized with wild-type rDer f 2 four times, and then intranasally administered 0.2–2 μg of wild-type, 0.2–20 μg of C8/119S, or PBS alone, three times a week for 4 weeks. Seven days after the last administration, the mice were examined for an immediate allergic reaction. The animals administered 2 μg of C8/119S (C2.0 group) showed significantly reduced immediate bronchoconstriction provoked by the i.v. injection of 1 and 10 μg of wild-type rDer f 2, compared with the PBS-treated mice. Similar results were obtained when we examined mice 10 weeks after the last administration. The reactions in the other groups given wild-type or C8/119S also tended to decrease in severity in comparison with the animals of the PBS group. The allergic phenotypes of the T cells, B cells, and basophils in the C2.0 group were shifted to that of naive mice without immunization. We conclude that C8/119S has hyposensitizing activities in mice sensitized with wild-type rDer f 2. C8/119S may be useful for immunotherapy of house dust mite allergy.
Keywords: mite, recombinant Der f 2, mutant, hyposensitization, mouse
INTRODUCTION
Specific immunotherapy for the treatment of patients with respiratory allergy has been conducted for more than 80 years [1], and the use of extracts of several pollens, house dust mites, bee venoms and other substances has been demonstrated to be effective [2–4]. Standardizations of such extracts have been carried out to obtain maximal efficacy and to eliminate side-effects, including systemic anaphylaxis in immunotherapy [5–7]. Many clinically important allergens have been purified from the extracts [8–10], and genes for such allergens have been cloned with a view to their use in immunotherapy [11]. To date, derivatives of several purified and recombinant allergens, including chemically modified allergens [12,13], synthetic peptides [14,15], and mutants of wild-type recombinant allergens [16,17], have also been developed. Since these derivatives often possess weaker IgE-binding capacity than the original allergens, they will not trigger a chemical mediator release from basophils and mast cells by the cross-linking of IgE–IgE receptor complex on these cells [18], and may be safe immunotherapeutic agents for treating allergic diseases. Recently, clinical trials of allergen-specific immunotherapy using synthetic peptides of short length containing T cell epitopes of a major cat allergen, Fel d 1, have been reported [19,20].
Concerning house dust mite allergens, genes for the allergens Der f 1, 2, 3 found in Dermatophagoides farinae, and Der p 1, 2, 3, 5 in Dermatophagoides pteronyssinus have been cloned [21–23]. Among others, the product of Der f 2 gene (recombinant Der f 2 (rDer f 2)) has been shown to exhibit allergic activity in humans comparable with native Der f 2 prepared from crude mite extract [24, 25]. We previously reported the development of murine allergic models by using rDer f 2 as the sensitizing antigen and hyposensitization to the allergic reaction in rDer f 2-sensitized mice by oral administration of rDer f 2 [26,27]. Takai et al. recently reported the construction of a mutant of rDer f 2, C8/119S, which retained complete activity to stimulate T cell proliferation and markedly reduced binding capacity to Der f 2-specific IgE in humans [28]. In the present study, to evaluate the suitability of C8/119S as an immunotherapeutic agent for house dust mite allergy, we investigated the effect of the intranasal (i.n.) administration of C8/119S on the allergic reaction in A/J mice immunized with wild-type rDer f 2. For comparison, we also used wild-type rDer f 2 for administering intranasally into sensitized mice.
MATERIALS AND METHODS
Antigen
Wild-type rDer f 2 was kindly supplied by Nikka Whisky Distilling Co., Ltd (Chiba, Japan). C8/119S was prepared in our laboratory as described elsewhere [28]. Two cysteine residues at the 8th and 119th positions in the wild-type were converted to serine residues in C8/119S, so that the number of disulphide bonds was reduced from three to two in C8/119S [28]. Wild-type and mutant rDer f 2 were dissolved in PBS before use.
Animals
Male A/J mice and Sprague-Dawley (SD) rats were purchased from Japan SLC (Shizuoka, Japan). The animals were maintained under controlled conditions with 12 h alternating light/dark cycles. Water and commercial food were available ad libitum. They were kept without treatment for at least 1 week after arrival at our laboratory.
Immunization
Seven-week-old A/J mice were immunized with wild-type rDer f 2 by the intraperitoneal (i.p.) injection of 10 μg of wild-type adsorbed in 4 mg of aluminium hydroxide four times at intervals of 2 weeks.
Experimental groups
On the day of the last (4th) immunization, the mice were divided into six groups: ‘2 μg wild-type rDer f 2’ (referred to as W2.0), ‘0.2 μg wild-type rDer f 2’ (W0.2), ‘20 μg C8/119S’ (C20), ‘2 μg C8/119S’ (C2.0), ‘0.2 μg C8/119S’ (C0.2), and PBS (control). Each group consisted of 7–12 mice. Several animals which were not immunized (‘naive mice’) were also used as reaction-negative controls in the experiments.
Intranasal administration of wild-type rDer f 2 and C8/119S
On the day of the last immunization, the i.n. treatment was started and performed three times a week for 4 weeks. The mice were lightly anaesthetized with ethyl ether, and a PE-10 polyethylene tube was inserted into the left nasal cavity. Twenty microlitres of antigen solution or PBS were slowly injected into the cavity from a 50-μl microsyringe through the tube. The mice in the W2.0, W0.2, C20, C2.0, and C0.2 groups were given 2 μg of wild-type, 0.2 μg of wild-type, 20 μg of C8/119S, 2 μg of C8/119S, and 0.2 μg of C8/119S per administration, respectively. The control mice were treated with PBS alone. The naive mice were given no treatment.
Determination of plasma antibody levels in hyposensitization experiments
Mice were bled on the first and last days of the i.n. administration for the determination of plasma antibody levels. Heparinized blood was collected from the retroorbital plexus of individual mice. Plasma (about 20 μl/animal) was separated from the whole blood and frozen until the determination of the antibody titre. Total IgE concentration was determined by ELISA using a rat anti-mouse IgE MoAb (MCA419; Serotec Ltd, Oxford, UK) as a capturing antibody and a biotinylated antibody (MCA420B; Serotec) as a detecting antibody. The levels of anti-wild-type IgG1 and IgG2a were determined by ELISA using wild-type rDer f 2 as a capturing antigen and the following detecting antibodies: biotinylated rat MoAbs, anti-mouse IgG1 (no. PM-05002D; Pharmingen, San Diego, CA) and anti-mouse IgG2a (no. PM-05022D; Pharmingen).
In addition to the measurement of antibody in each plasma sample by ELISA, plasma was pooled for each group to determine anti-wild-type rDer f 2 IgE levels by mouse–rat heterogeneous 72-h passive cutaneous anaphylaxis (PCA) using SD rats at 8 weeks of age as recipients [29]. Plasma was serially diluted (1:80–1:2560), and the dilutions were intracutaneously injected into the back of recipient SD rats. After 72 h, Evans Blue and 0.1 μg of provoking antigen were intravenously injected into the rats to provoke a PCA reaction. After 30 min, the maximum dilution at which a blue spot larger than 5 mm in diameter was induced was determined as the PCA titre.
Determination of airway constriction
Antigen provocation tests were carried out by the Konzett–Rössler method either 7 days or 10 weeks after the last i.n. administration. The immediate airway response was provoked by the i.v. injection of wild-type rDer f 2. The severity of airway constriction was expressed by ventilation overflow (VO) defined by the Konzett–Rössler method [30,31].
Mice were anaesthetized with pentobarbital sodium (50 mg/kg, i.p.) and immobilized with pancuronium bromide (0.5 mg/kg, i.v.). Tracheal cannulation was performed with an 18 G needle connected to an animal respirator. The lung was inflated with a fixed volume of air under 5 cm H2O pressure at a rate of 60 breaths/min, and the VO was continuously measured by a pneumotachograph. The ventilation volume (7–10 ml/kg) was adjusted so that the VO was about 3 ml/min. When the VO was stabilized, the antigen challenge was started: 0.05 ml of each wild-type rDer f 2 solution, containing 1 μg and 10 μg of the antigen, was sequentially injected into the femoral vein at an interval of 6 min. The VO was recorded for at least 12 min from the administration of the 1 μg wild-type solution. The ΔVO, i.e. the difference between the maximal VO observed after challenge with 1 μg or 10 μg of the antigen and the base VO before the injection of 1 μg of rDer f 2, was used as an index of the degree of immediate airway constriction.
Determination of histamine concentration in the whole blood
Immediately after the measurement of bronchoconstriction provoked by the i.v. injection of 10 μg of wild-type rDer f 2, heparinized whole blood was obtained from the inferior vein of each mouse. The histamine content in the plasma was determined by a fluorescent assay using o-phthaldialdehyde.
Flow cytometric analyses of peripheral blood leucocytes
In the mice of the C0.2, C2.0 and PBS groups, which were not examined for immediate reaction, leucocyte phenotypes were determined. Heparinized blood was collected from the inferior vein of each mouse 7 days after the last i.n. administration.
Peripheral blood leucocytes were separated from whole blood by dextran sedimentation for the analysis of CD23 expression on B cells, CD11b (Mac-1), and the IgE molecules on basophils. The leucocytes were washed with PBS and then treated with FITC-conjugated rat anti-mouse B220 (no. 32554A; Pharmingen) + biotinylated rat anti-mouse CD23 (no. 32554A; Pharmingen), or FITC-conjugated rat anti-mouse CD11b (no. 32554A; Pharmingen) + biotinylated rat anti-mouse IgE (no. 32554A; Pharmingen). Biotinylated antibodies were labelled with Cychrome-conjugated avidin (no. 32554A; Pharmingen). The flow cytometric analyses were performed with the use of FACScan (Becton Dickinson, San Jose, CA).
Determination of IL-6 and interferon-gamma production from spleen cells
Spleen cells of mice which were subjected to the flow cytometric analyses of peripheral blood leucocytes were examined for cytokine production. Spleen cells were obtained immediately after bleeding, and incubated in serum-free medium, AIM-V (Gibco, Grand Island, NY) in the presence or absence of 10 μg/ml of wild-type rDer f 2 for 72 h. IL-6 and interferon-gamma (IFN-γ) contents of the culture supernatants were determined by ELISA using the following capturing and detecting antibodies: rat monoclonal anti-mouse IL-6 (no. PM-18071D; Pharmingen) and biotinylated rat monoclonal anti-mouse IL-6 (no. PM-18082D; Pharmingen); rat monoclonal anti-mouse IFN-γ (no. PM-18181D; Pharmingen) and biotinylated rat monoclonal anti-mouse IFN-γ (no. PM-18112D; Pharmingen). The detecting antibodies were labelled by avidin-conjugated β-galactosidase (no. A-2300; Vector Labs, Burlingame, CA) for fluorescence development. Standard curves for cytokines were made using recombinant mouse IL-6 (no. PM-19251V; Pharmingen), and IFN-γ (no. PM-19301U; Pharmingen).
Statistical analysis
All of the data obtained are expressed as means with s.e.m. The cell surface molecule and antibody level data were log-transformed before analysis. Student's paired t-test was used to compare differences in the measurements of airway reactions, leucocyte analyses, and plasma antibodies between pairs of groups. Changes in antibody levels before and after the oral treatments were also assessed with the paired t-test. P < 0.05 was considered significant.
RESULTS
Comparison of allergic activity of wild-type rDer f 2 and C8/119S in mice
Before examining the hyposensitizing activity of C8/119S, we compared the allergic activity of C8/119S with that of wild-type rDer f 2 in mice. In humans, the binding activity of C8/119S to Der f 2-specific IgE was 1/100–1/10 of that of wild-type rDer f 2 or native Der f 2, as determined by a skin-prick test and a basophil histamine release assay. Two rats intracutaneously sensitized with serial dilutions of hyperimmune plasma (PCA titre = 640, obtained from sensitized A/J mice) were challenged by either wild-type or mutant rDer f 2. Blue spots appeared at the site of sensitization, as shown in Fig. 1a. The PCA reaction induced by C8/119S was much weaker than that by wild-type rDer f 2. The IgE binding capacity of C8/119S was estimated to be 1/30–1/10 of wild-type.
Fig. 1.
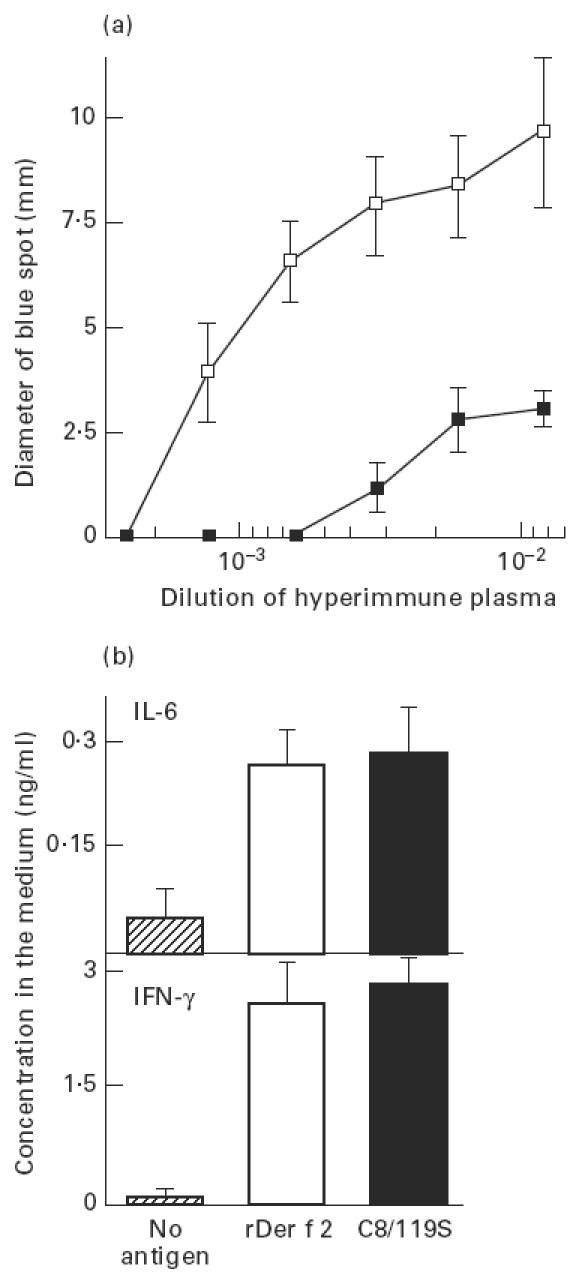
Comparison of allergic activities of wild-type rDer f 2 and C8/119S in A/J mice immunized with wild-type rDer f 2. (a) Passive cutaneous anaphylaxis (PCA) reaction provoked with wild-type rDer f 2 (□) and C8/119S (▪). Two rats intracutaneously sensitized with serial dilutions of hyperimmune plasma were challenged by either wild-type rDer f 2 or C8/119S. (b) IL-6 and IFN-γ production by spleen cells cultured for 72 h in the presence or absence of 10 μg/ml of either wild-type rDer f 2 or C8/119S. Spleen cells were prepared from three sensitized mice. Vertical bars represent s.e.m.
We then determined the T cell reactivity of wild-type and mutant rDer f 2 using spleen cells that were obtained from mice immunized four times with wild-type rDer f 2. After the spleen cells were cultured in the presence or absence of 10 μg/ml of either wild-type or mutant rDer f 2 for 72 h, the concentrations of IL-6 and IFN-γ in the supernatant of the cell culture were measured by ELISA (Fig. 1b). The cytokine production by sensitized spleen cells after stimulation with C8/119S was comparable to that with wild-type. The concentration of IL-4 could not be determined in this study. IL-6 and IFN-γ production by naive spleen cells after incubation with wild-type rDer f 2 and C8/119S was negligible (data not shown).
Effect of i.n. administration of wild-type rDer f 2 or C8/119S on immediate response
Since we confirmed that C8/119S possessed adequate T cell reactivity and very weak IgE-binding capacity in mice, we performed a hyposensitization study in sensitized mice by using C8/119S and wild-type rDer f 2.
Animals were intranasally given 0–20 μg of either wild-type or C8/119S three times a week for 4 weeks, and had their bronchoconstriction evaluated upon antigen provocation 7 days after the last administration. As shown in Fig. 2a, the sequential i.v. injection of 1 and 10 μg of rDer f 2 to mice in the PBS group provoked immediate airway constriction in a dose-dependent manner, whereas no such reaction was observed in the naive mice. The responses were weaker in the W0.2 and W2.0 groups, given 0.2 and 2.0 μg/administration of wild-type rDer f 2, respectively, than in the PBS group. However, no significant difference compared with the PBS group was obtained, and the values of the W0.2 and W2.0 groups were comparable. This was not the case for the C0.2 and C2.0 groups, given 0.2 and 2.0 μg/administration of C8/119S, respectively. The immediate reaction in the C2.0 group was significantly lower than that in the PBS group, and the C0.2 group exhibited a value intermediate between those of the PBS and C2.0 groups. Since the IgE-binding capacity of C8/119S was much lower than that of wild-type, we prepared a C20 group given 20 μg/administration of C8/119S. The airway response in the C20 group was not suppressed as clearly as in the C2.0 group, however (Fig. 2a). The histamine level in the plasma immediately after measurement of the bronchoconstriction was almost proportional to the increase in VO after the i.v. injection of 10 μg of wild-type (Fig. 2b). To determine the continuance of the hyposensitization, the animals in the PBS, C0.2 and C2.0 groups were examined for bronchoconstriction 10 weeks after the last administration. The results were similar to those from day 7 (Fig. 2a).
Fig. 2.
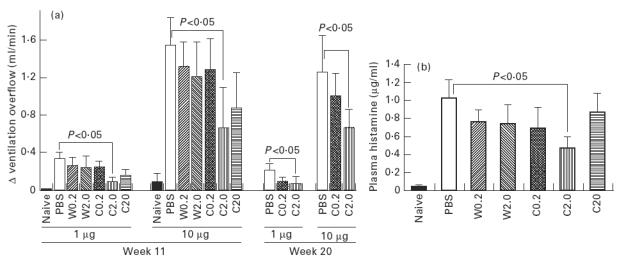
Comparison of the immediate reaction provoked by the i.v. injection of wild-type rDer f 2 among the experimental groups. (a) Increase in the ventilation overflow (VO) after the injections of 1 and 10 μg of wild-type rDer f 2. Antigen provocations were performed 7 days (week 11) or 10 weeks (week 20) after the last intranasal treatment. The numbers of mice were as follows; PBS, n = 10; W0.2, n = 7; W2.0. n = 7; C0.2, n = 9; C2.0, n = 10; C20, n = 7; and naive, n = 4 at week 11; PBS, n = 8; C0.2, n = 8; and C2.0, n = 8 at week 20. (b) Plasma histamine concentration immediately after the determination of bronchoconstriction 7 days after the last intranasal treatment. The same mice as in (a) were examined.
Changes in plasma IgE, IgG1 and IgG2a levels
The total IgE, wild-type rDer f 2-specific IgG1 and IgG2a levels before and after the administration period are shown in Fig. 3. The levels of total IgE and that of anti-wild-type IgG1 were slightly decreased and anti-wild-type IgG2a was markedly decreased after the administration in all groups except the W2.0 and C20 groups. In these groups, the total IgE and anti-wild-type IgG1 levels were slightly increased after 4 weeks of i.n. administration. The anti-wild type PCA titre was 320 in all groups over the experimental period. No significant difference was observed in antibody levels among the groups or between before and after the administration. The antibody levels did not correlate with the immediate reaction expressed by the increase in VO, or with plasma histamine levels (data not shown). Neither a drop in the level of reaginic antibody nor an increase in the level of blocking antibody were found.
Fig. 3.
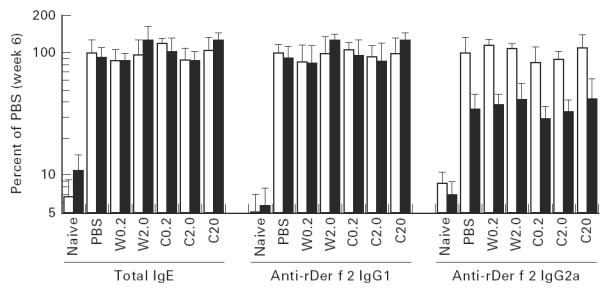
Plasma total IgE concentration, anti-wild-type rDer f 2 IgG1, and IgG2a levels before (□) and after (▪) intranasal treatments in mice that were examined for immediate reaction 7 days after the last treatment. Regarding the total IgE concentration, ‘100%’ was 2.35 μg/ml.
Flow cytometric analysis of peripheral blood leucocytes
Mice in the PBS, C0.2 and C2.0 groups in which no immediate reaction was provoked were examined for the phenotypes of peripheral blood leucocytes and spleen cells 7 days after the last i.n. administration.
B cells were distinguished from the other cell types by B220 expression and cellular morphology. CD23 expression on B cells in the PBS group mice as determined by flow cytometry was two-fold higher than that in the naive mice. The CD23 values of the C2.0 group were significantly lower than those of the PBS group, while the C0.2 group showed values comparable to those of the PBS group (Fig. 4).
Fig. 4.
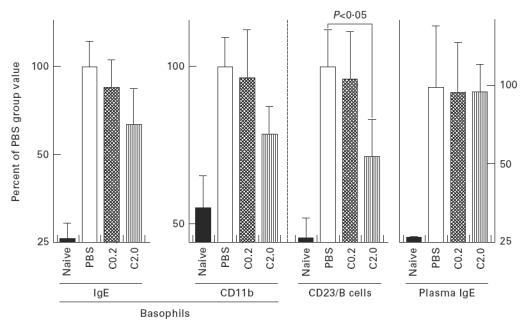
Plasma total IgE content, IgE and CD11b molecules on basophils, and CD23 expression on B cells. The numbers of mice were as follows: PBS, n = 10; C0.2, n = 10; C2.0, n = 9; and naive, n = 7. Data from naive mice whose basophils could not be captured by FACS analysis were omitted.
Basophils were distinguished by surface IgE and cellular morphology. Since the naive A/J mice had a small amount of IgE in their plasma, their basophils could also be captured by higher signals for IgE molecules, in seven of nine animals examined. The basophils counted by flow cytometry were about 0.7% of whole leucocytes in the PBS, C0.2 and C2.0 groups (without significant differences among them), and 0.3% in the naive mice. These values were comparable to the basophil counts determined by histochemical analysis (data not shown). The CD11b and IgE molecules on basophils were 2–4-fold more numerous in the PBS group than in the naive mice, while the numbers of these molecules in the C0.2 and C2.0 groups were decreased compared with the PBS group, in a dose-dependent manner (Fig. 4). No significant difference was obtained between the PBS and C8/119S-administered groups, however. The plasma total IgE levels in the PBS, C0.2 and C2.0 groups were comparable, and thus the numbers of IgE molecules on the basophils were considered to reflect the numbers of IgE receptors, except in the naive mice.
We also examined the expressions of CD44, CD49d and CD117 on basophils. No differences were observed in CD44 and CD49d between the naive and sensitized mice, and CD117 was not detected on the basophils (data not shown).
IL-6 and IFN-γ production by spleen cells
Spleen cells obtained from mice in the PBS, C0.2 and C2.0 groups and naive mice were cultured with or without 10 μg/ml of wild-type rDer f 2 for 72 h. The concentrations of IL-6 and IFN-γ in the supernatant of the cultures without antigen were < 0.1 ngl/ml and 1 ng/ml in all groups, respectively (data not shown). The IL-6 and IFN-γ levels after stimulation with wild-type rDer f 2 in the PBS group were two to four times higher than in the naive mice (Fig. 5). IL-6 and IFN-γ production in the C8/119S-administered groups was decreased in a dose-dependent manner, and a significant difference was obtained in IL-6 production between the PBS and C2.0 groups. IL-4 in the culture supernatant could not be determined.
Fig. 5.
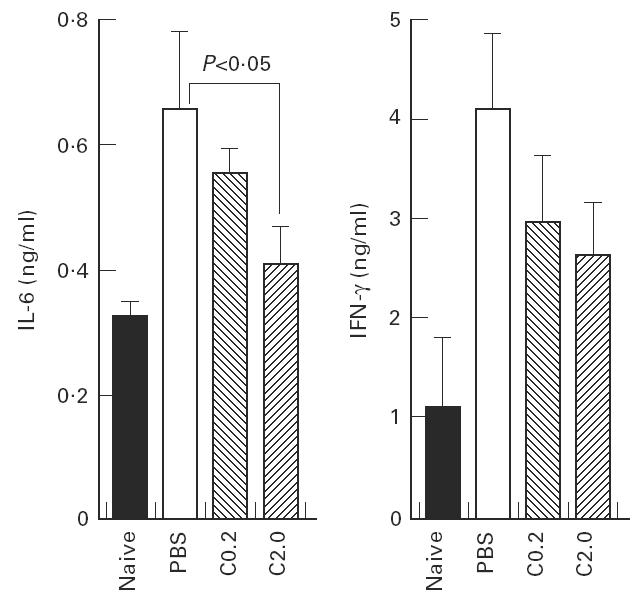
IL-6 and IFN-γ production by spleen cells cultured for 72 h in the presence of 10 μg/ml of wild-type rDer f 2. Spleen cells were prepared from mice that were examined for phenotypes of basophils and B cells.
DISCUSSION
Immunotherapy with crude extracts of allergenic substances has been widely performed in the treatment of allergic diseases in the airway, and many affirmative results concerning the therapy have been obtained in clinical and experimental studies. Despite the beneficial effect of immunotherapy, however, several problems remain in the use of crude extracts. First, although only a few allergens that sensitize patients are necessary in the immunotherapy, crude allergens often contain unnecessary substances [32,33]. The effect of the administration of these substances on patients’ health is still unclear. Second, allergens in the extract have strong IgE-binding activity, and side-effects such as systemic anaphylaxis may be caused by the injection of a crude extract if the dose of the extract is incorrect or the patient's physiologic status is not good [34,35]. The international standardization of allergen extracts has ameliorated these problems to some extent, but not completely. Recent advances in biochemistry and immunology have further improved the situation. Several recombinant forms of clinically important allergens with high purity are now available in large quantities [11,25], and derivatives of wild-type allergens including synthetic peptides and mutants of recombinant allergens have been developed from the original allergens. Several of these derivatives have been shown to contain T cell epitope and possess little or no IgE-binding capacity [16,20]. Since it seems that interactions between T cells and injected allergens play a pivotal role in the hyposensitization of patients to allergens, such derivatives have the potential to be safe immunotherapeutic agents [36].
Concerning house dust mite allergens, the genes for group 1 (Der f 1, Der p 1) and 2 (Der f 2, Der p 2) major allergens, both of which have been known to sensitize 60–80% of patients with house dust mite allergy [37,38], have been cloned, and T and B cell epitopes of these allergens have been well studied [39–41]. C8/119S, a mutant of rDer f 2, was recently shown to possess complete activity to stimulate T cell proliferation and markedly reduced binding capacity to Der f 2-specific IgE in humans [28]. These features were shared with mice. Since C8/119S has an entire sequence of Der f 2 with 129 amino acid residues, unlike a synthetic peptide with 10–20 residues, C8/119S can contain each of the T cell epitopes known to vary considerably from patient to patient [42,43]. In the present study, we examined the hyposensitizing activity of C8/119S in A/J mice sensitized with wild-type rDer f 2
In previous studies, we showed that the oral administration of 1 mg/day of rDer f 2 to mice immunized with rDer f 2 for 4 weeks significantly suppressed the immediate airway constriction and neutrophil influx into airways [26,27]. In these experiments, each mouse was orally given 20 mg of rDer f 2, but preparing such an amount of C8/119S is currently impossible. We thus chose the i.n. administration of wild-type and mutant rDer f 2 in the present study. Mice immunized with wild-type rDer f 2 were intranasally given 0.2–20 μg of either wild-type or C8/119S for 4 weeks, and then examined for their allergic reaction. The immediate reaction provoked by the i.v. injection of wild-type was suppressed in all groups that received wild-type rDer f 2 or C8/119S compared with the PBS group, and the decrease in the allergic reaction was most prominent in the C2.0 group. The VO increase and plasma histamine concentration after challenge with 10 μg of rDer f 2 in the C2.0 group were almost equivalent to the values after i.v. challenge with 2–3 μg of rDer f 2 in sensitized mice without hyposensitizing treatment (data not shown). These results indicate that the provocative concentration for the allergic reaction of the C2.0 group was increased at least several-fold by the administration of C8/119S. This decrease in the sensitivity to allergen after immunotherapy was comparable to that in several clinical studies [44,45].
It seemed that i.n. hyposensitization with C8/119S and wild-type had an optimum dose showing maximal effects. Higher doses did not always produce better results. In a range within 0.02–0.2 μg/administration, the hyposensitizing activity of C8/119S was higher than that of wild-type in our murine allergic model. The increase in plasma IgE levels in the W2.0 and C20 groups, which received the largest amount of antigens, may reflect the local ‘sensitization’. In a previous study, we showed that i.n. injection of 0.1 mg of rDer f 2 once a week for 12 weeks could induce systemic IgE production and airway hyperresponsiveness [27]. The outcome of i.n. immunotherapy may depend on the balance of local ‘sensitization’ and ‘hyposensitization’ in A/J mice immunized with wild-type rDer f 2. A clear difference in the immediate reaction between the W2.0 and C2.0 groups, both of which received 2 μg/administration of antigen, suggested roles for mast cells and basophils in the local sensitization. These cells have high-affinity IgE receptors on the cellular surface, and can be activated by the interaction between antigens and IgE molecules on the receptors, followed by the secretion of both chemical mediators and Th2-type cytokines [18,46–48]. Wild-type rDer f 2 may react to IgE and induce Th2-type cytokines/mediators more strongly than does C8/119S. These cytokines could shift the balance from ‘hyposensitization’ to ‘sensitization’, which may account for the unsuccessful immunotherapy in the W2.0 group. To confirm this hypothesis, a comparison of cytokine-inducing activity from mast cells and basophils of wild-type and mutant rDer f 2 should be performed. The suppression of the airway response was maintained for over 10 weeks after stopping the i.n. administration of C8/119S, suggesting that the effect of hyposensitization was long-lasting.
Concerning the mechanisms underlying the hyposensitization in immunotherapy, the decrease in the T cell response to allergen and the correction of the Thl/Th2 bias in allergic patients have been highlighted, instead of the drop in reaginic antibodies and the increase of blocking antibodies. The Th2-type cytokines IL-4, IL-5, IL-6 and IL-13, considered to play a crucial role in type 1 allergy, were down-regulated [49–51], while Th1 cytokines IFN-γ and IL-12 were changed or unchanged after successful immunotherapy [52, 53]. In the present study, we also observed the down-regulation of IL-6 and IFN-γ production by splenocytes in the C2.0 group, which indicated the suppression of T cell reactivity to the sensitizing antigen, wild-type rDer f 2. Although we could not determine any changes in IL-4, we found that the expression of CD23, one of the activation markers on B cells, was down-regulated in the C2.0 group, as in several clinical studies on immunotherapy [54, 55]. CD23 expression on B cells has been reported to be higher in allergic patients compared with healthy people, and can be up-regulated by stimulation with IgE and IL-4 in vitro [56–58]. Since several studies showed that the rise of CD23 expression during the pollen season in patients with pollinosis and the drop in the expression after immunotherapy were independent of serum IgE levels [55, 59], changes in CD23 may reflect the balance of IL-4 and its antagonists when IgE levels are essentially constant. Thus, the suppression of CD23 expression without decrease in plasma IgE in the C2.0 group may reflect the suppression of the activity of IL-4, though the direct measurement of cytokine production from T cells would be necessary to confirm this.
Immediate reactions are known to be triggered by the release of mediators from mast cells or basophils, and thus functional changes in these cells as well as T cells must accompany successful immunotherapy. It has been shown that the expression of high-affinity IgE receptors or several function-molecules on basophils is up-regulated in allergic patients or after stimulation in vitro in human and mouse studies [60–63]. The histamine release from basophils was sometimes decreased after immunotherapy [64, 65]. We also found in the C2.0 group that the plasma histamine concentration after antigen challenge was suppressed. In addition, the numbers of IgE and CD11b molecules on peripheral blood basophils were increased after immunization, and tended to decrease after the i.n. treatments with C8/119S in the A/J mice. Although the histamine detected in plasma was considered to be derived from both basophils and mast cells in the circulatory system [66], our findings suggest the improvement of allergic phenotypes of basophils after i.n. administration of C8/119S. We could not determine the histamine release from isolated basophils in vitro, because of the insufficient numbers and/or histamine content of the basophils. An assay must be performed to obtain direct evidence of the functional changes in basophils.
Several studies showed that Th2 cytokine-producing cells and IgE-secreting cells were increased in the airway tissues of allergic patients or intranasally sensitized animals [67–72]. Although the influence of Th1 cytokines on the phenotypes of mast cells/basophils is still unclear, Th2-type cytokines, including IL-4 and IL-6, are known to up-regulate the growth and functions of mast cells/basophils in vitro, and IgE itself enhances the expression of high-affinity IgE receptor on these cells in vitro [73–78]. It has recently been found that the density of IgE receptor on mast cells and Th2 cytokine deposition in the patients’ nasal mucosa were significantly elevated compared with that of healthy people, suggesting a close relationship between the activity of mast cells and Th2 cytokines in the airway in vivo [79, 80]. Thus, Th2 cytokines can contribute to the enhancement of the immediate airway constriction as well as to the promotion of the influx of eosinophils and other cells into the airway [81, 82]. In contrast, it is expected that suppression of Th2 cytokines after immunotherapy will give rise to the down-regulation of the expression of mast cells’ IgE receptors, as seen in basophils in our study, or other phenotypic changes in mast cells.
In the present study, we challenged sensitized mice with wild-type rDer f 2 by i.v. injection. Although the down-regulation of CD23 expression on B cells and the IL-6 production by splenocytes suggest the suppression of Th2 cytokines in the C8/119S-treated mice, we do not have any information on the immunological status of the airway tissue. For a confirmation of the hyposensitization in airway tissue after the administration of C8/119S, further studies to induce airway constriction by local allergen challenge and to examine changes in the cytokine production and phenotypes of mast cells after hyposensitization are needed. Although we have developed murine allergic models which react to wild-type rDer f 2 [26, 27], a high concentration of rDer f 2 (> 10–20 mg/ml) is needed to provoke an allergic reaction by the antigen inhalation challenge in these models. A hyposensitization study with an airway challenge using a large number of mice requires a significant amount of wild-type rDer f 2, which is currently rather expensive. We are attempting to develop an experimental model showing allergic reactions in the airway, including an eosinophilic inflammation, after the inhalation or i.n. injection of a lower concentration of wild-type rDer f 2.
In conclusion, the findings obtained in the present study give rise to optimism that i.n. immunotherapy with C8/119S in house dust mite allergy is of benefit in a murine allergic model.
Acknowledgments
We are grateful to Dr Ichiro Shibuya (Institute for Production Research and Development, The Nikka Whisky Distilling Co., Ltd) and Dr Masateru Kurumi (Research Laboratories, Torii & Co., Ltd) for providing mite allergens.
REFERENCES
- 1.Noon L. Prophylactic inoculation against hay fever. Lancet. 1991;i:1572–3. [Google Scholar]
- 2.Hejjaoui A, Dhivert H, Michel FB, Bousquet J. Immunotheraoy with a standardized Dermatophagoides pteronyssinus extract. J Allergy Clin Immunol. 1990;85:473–9. doi: 10.1016/0091-6749(90)90157-y. [DOI] [PubMed] [Google Scholar]
- 3.Walker SM, Varney VA, Gaga M, Jacobson MR, Durham SR. Grass pollen immunotherapy: efficacy and safety during a 4-year follow-up study. Allergy. 1995;50:405–13. doi: 10.1111/j.1398-9995.1995.tb01170.x. [DOI] [PubMed] [Google Scholar]
- 4.Golden DB, Kwiterovich KA, Kagey-Sobotka A, Valentine MD, Lichtenstein LM. Discontinuing venom immunotherapy: outcome after five years. J Allergy Clin Immunol. 1996;97:579–87. doi: 10.1016/s0091-6749(96)70302-0. [DOI] [PubMed] [Google Scholar]
- 5.Maasch HJ, Wahl R, Fuchs T. Application of the first international standard of Dermatophagoides pteronyssinus (house dust mite) in the evaluation of allergen extracts produced from two different source materials. Int Arch Allergy Appl Immunol. 1987;84:363–72. doi: 10.1159/000234451. [DOI] [PubMed] [Google Scholar]
- 6.Matthiesen F. Standardization of allergen extracts. Z Hautkr. 1988;63(Suppl. 4):52–54. [PubMed] [Google Scholar]
- 7.Lehrer SB, Salvaggio JE. Allergens: standardization and impact of biotechnology—a review. Allergy Proc. 1990;11:197–208. doi: 10.2500/108854190778879846. [DOI] [PubMed] [Google Scholar]
- 8.Chapman MD, Platts-Mills TAE. Purification and characterization of the major allergen from Dermatophagoides pteronyssinus P 1. J Immunol. 1980;125:587–92. [PubMed] [Google Scholar]
- 9.Leitermann K, Ohman JL. Cat allergen 1: biochemical, antigenic, and allergenic properties. J Allergy Clin Immunol. 1984;74:147–53. doi: 10.1016/0091-6749(84)90278-1. [DOI] [PubMed] [Google Scholar]
- 10.Kemeny DM, Dalton N, Lawrence AJ, Pearce FL, Vernon CA. The purification and characterization of hyaluronidase from the venom of the honey bee, Apis mellifera. Eur J Biochem. 1984;139:217–23. doi: 10.1111/j.1432-1033.1984.tb07997.x. [DOI] [PubMed] [Google Scholar]
- 11.Valenta R, Kraft D. Recombinant allergens for diagnosis and therapy of allergic disease. Curr Opin Immunol. 1995;7:751–6. doi: 10.1016/0952-7915(95)80043-3. [DOI] [PubMed] [Google Scholar]
- 12.Dreborg S. Standardization of mPEG-modified allergens. Arb Paul Ehrlich Inst Bundesamt Sera Impfstolle Frankf A M. 1992;85:147–57. [PubMed] [Google Scholar]
- 13.Kohno K, Obtsuki T, Suemoto Y, Inoue T, Taniguchi Y, Usui M, Ikeda M, Kurimoto M. Regulation of cytokine production by sugi allergen–pullulan conjugate. Cell Immunol. 1996;168:211–9. doi: 10.1006/cimm.1996.0068. [DOI] [PubMed] [Google Scholar]
- 14.Bungy Poor Fard GA, Latchman Y, Rodda S, Geysen M, Roitt I, Brostoff J. T cell epitopes of the major fraction of ryegrass Lolium perenne(Lol P I) defined using overlapping peptides in vitro and in vivo. I. Isoallergen clone 1A. Clin Exp Immunol. 1993;94:111–6. doi: 10.1111/j.1365-2249.1993.tb05986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briner TJ, Kuo M-C, Keating ICA, Rogers BL, Greenstein JL. Peripheral T cell tolerance induced in naive and primed mice by subcutaneous injection of peptides from the major cat allergen, Fel d I. Proc Natl Acad Sci USA. 1993;90:7608–12. doi: 10.1073/pnas.90.16.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishiyama C, Yuuki T, Usui Y, Iwamoto N, Okumura Y, Okudaira H. Effects of amino acid variations in recombinant Der f II on its human IgE and mouse IgG recognition. Int Arch Allergy Immunol. 1994;105:62–69. doi: 10.1159/000236804. [DOI] [PubMed] [Google Scholar]
- 17.Noguchi E, Shibasald M, Nishiyama C, Okumura Y, Takita H. IgE responsiveness to Dermatophagoides farinae in young asthmatic children: IgE binding study using recombinant allergens of Der f1, Der f2 and mutant proteins of Der f2. Int Arch Allergy Immunol. 1996;110:380–7. doi: 10.1159/000237331. [DOI] [PubMed] [Google Scholar]
- 18.Ishizaka T, Ishizaka K. Activation of mast cells for mediator release through lgE. Prog Allergy. 1984;34:188–235. [PubMed] [Google Scholar]
- 19.Norman PS, Ohman JL Jr, Long AA, et al. Treatment of cat allergy with T-cell reactive peptides. Am J Respir Crit Care Med. 1996;154:1623–8. doi: 10.1164/ajrccm.154.6.8970345. [DOI] [PubMed] [Google Scholar]
- 20.Norman PS, Nicodemus CF, Creticos PS, Wood RA, Eggleston PA, Lichtenstein LM, Kagey-Sobotka A, Proud D. Clinical and immunologic effects of component peptides in allervax® cat. Int Arch Allergy Immunol. 1997;113:224–6. doi: 10.1159/000237553. [DOI] [PubMed] [Google Scholar]
- 21.Chua KY, Greene WK, Kehal P, Thomas WR. IgE binding studies with large peptides expressed from Der p 2 cDNA constructs. Clin Exp Allergy. 1991;21:161–6. doi: 10.1111/j.1365-2222.1991.tb00825.x. [DOI] [PubMed] [Google Scholar]
- 22.Shoji H, Hanawa M, Shibuya I, Hirai M, Yasubara T, Okumura Y, Yamakawa H. Production of recombinant mite allergen Der fI in insect cells and characterization of products—removal of pro-sequence is essential to IgE-binding activity. Biosci Biotechnol Biochem. 1996;60:621–5. doi: 10.1271/bbb.60.621. [DOI] [PubMed] [Google Scholar]
- 23.Thomas WR, Smith W, Hales BJ, Carter MD. Functional effects of polymorphisms of house dust mite allergens. Int Arch Allergy Immunol. 1997;113:96–98. doi: 10.1159/000237516. [DOI] [PubMed] [Google Scholar]
- 24.Yuuki T, Okumura Y, Ando T, Yamakawa H, Suko M, Haida M, Okudaira H. Cloning and expression of cDNA coding for the major house dust mite allergen Der f2 in Escherichia coli. Agric Biol Chem. 1991;55:1233–8. [PubMed] [Google Scholar]
- 25.Iwamoto N, Nishiyama C, Yasubara T, Saito A, Yuuki T, Okumura Y, Okudaira H. Direct expression of Der f2, one of the major house dust mite allergens, in Escherichia coli. Int Arch Allergy Appl Immunol. 1996;109:356–61. doi: 10.1159/000237263. [DOI] [PubMed] [Google Scholar]
- 26.Yasue M, Yokota T, Kajiwara Y, Suko M, Okudaira H. Inhibition of airway inflammation in rDer f 2-sensitized mice by oral administration of recombinant Der f 2. Cell Immunol. 1997;11:30–37. doi: 10.1006/cimm.1997.1184. [DOI] [PubMed] [Google Scholar]
- 27.Yasue M, Yokota T, Yuasa M, Kajiwara Y, Suko M, Okudaira H. Effects of oral hyposensitization with recombinant Der f 2 on immediate airway constriction in a murine allergic model. Eur Respir J. doi: 10.1183/09031936.98.11010144. in press. [DOI] [PubMed] [Google Scholar]
- 28.Takai T, Yokota T, Yasue M, Nishiyama C, Yuuki T, Mori A, Ikudaita H, Okumura Y. Engineering of the major house dust mite allergen Der f 2 for allergen-specific immunotherapy. Nature Biotechnol. 1997;15:754–8. doi: 10.1038/nbt0897-754. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe N, Ovary Z. Antigen and antibody detection by in vivo methods; a reevaluation of passive cutaneous anaphylactic reactions. J Immunol Methods. 1977;14:381–90. doi: 10.1016/0022-1759(77)90149-1. [DOI] [PubMed] [Google Scholar]
- 30.Konzett H, Rössler R. Versuchsanordnung zu Untersuchungen an der Bronchialmuskulatur. Arch Exp Pathol Pharmakol. 1940;195:71–74. [Google Scholar]
- 31.Misawa M, Takenouchi K, Abiru T, Yoshino Y, Yanaura S. Strain difference in an allergic asthma model in rats. Jpn J Pharmacol. 1987;45:63–68. doi: 10.1254/jjp.45.63. [DOI] [PubMed] [Google Scholar]
- 32.Berrens L. Inhalant allergens in human atopic disease: their chemistry and modes of action. Ann NY Acad Sci. 1974;221:183–99. doi: 10.1111/j.1749-6632.1974.tb28216.x. [DOI] [PubMed] [Google Scholar]
- 33.Castro FFM, Schumitz-Schmann M, Tother U, Kirschfink M. Complement activation by house dust: reduced reactivity of serum complement in patients with bronchial asthma. Int Arch Allergy Appl Immunol. 1991;96:305–10. doi: 10.1159/000235513. [DOI] [PubMed] [Google Scholar]
- 34.Cook PR, Bryant JL, 2nd, Davis WE, Benke TT, Rapoport AS. Systemic reactions to immunotherapy: the American Academy of Otolaryngic allergy morbidity and mortality. Otolaryngol Head Neck Surg. 1994;110:487–93. doi: 10.1177/019459989411000603. [DOI] [PubMed] [Google Scholar]
- 35.Businco L, Zannino L, Cantani A, Corrias A, Fiocchi A, La Rosa M. Systemic reactions to specific immunotherapy in children with respiratory allergy: a prospective study. Pediatr Allergy Immunol. 1995;6:44–47. doi: 10.1111/j.1399-3038.1995.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 36.Wilner BP, Gefter NI. Immunotherapy with T-cell-reactive peptides derived from allergens. Allergy. 1994;49:302–8. doi: 10.1111/j.1398-9995.1994.tb02272.x. [DOI] [PubMed] [Google Scholar]
- 37.Heymann PW, Chapman MD, Aalberse RC, Fox JW, Platts-Mills TA. Antigenic and structural analysis of group II allergens (Der f II and Der p II) from house dust mites (Dermatophagoides spp) J Allergy Clin Immunol. 1989;83:1055–67. doi: 10.1016/0091-6749(89)90447-8. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien RM, Thomas WR. Immune reactivity to Der p I and Der p II in house dust mite sensitive patients attending paediatric and adult allergy clinics. Clin Exp Allergy. 1994;24:737–42. doi: 10.1111/j.1365-2222.1994.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 39.O’Brien RM, Thomas WR, Tait BD. An immunogenetic analysis of T-cell reactive regions on the major allergen from the house dust mite, Der p I, with recombinant truncated fragments. J Allergy Clin Immunol. 1994;93:628–34. doi: 10.1016/s0091-6749(94)70074-5. [DOI] [PubMed] [Google Scholar]
- 40.Ovsyannikova IG, Vailes LD, Li Y, Heymann PW, Chapman MD. Monoclonal antibodies to group II Dermatophagoides spp. allergens: murine immune response, epitope analysis, and development of a two-site ELISA. J Allergy Clin Immunol. 1994;94:537–46. doi: 10.1016/0091-6749(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 41.Hoyne GF, Callow MG, Kuo MC, Thomas WR. Characterization of T-cell responses to the house dust mite allergen Der p II in mice. Evidence for major and cryptic epitopes. Immunology. 1993;78:65–73. [PMC free article] [PubMed] [Google Scholar]
- 42.Davis MM. T cell receptor gene diversity and selection. Ann Rev Biochem. 1990;59:475–96. doi: 10.1146/annurev.bi.59.070190.002355. [DOI] [PubMed] [Google Scholar]
- 43.WHO nomenclature committee for factors of the BLA system. Nomenclature for factors of the HLA system 1995. Vox Sang. 1995;69:359–72. doi: 10.1111/j.1423-0410.1995.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 44.Bousquet J, Calvayrac P, Guérin B, Hejjaoui A, Dhivert H, Hewitt B, Michel FB. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. I. In vivo and in vitro parameters after a short course of treatment. J Allergy Clin Immunol. 1985;76:734–44. doi: 10.1016/0091-6749(85)90680-3. [DOI] [PubMed] [Google Scholar]
- 45.Hedlin G, Heilborn H, Lilja G, Norrlind K, Pegelow KO, Schou C, Lowenstein H. Long-term follow-up of patients treated with a three-year course of cat or dog immunotherapy. J Allergy Clin Immunol. 1995;96:879–85. doi: 10.1016/s0091-6749(95)70223-7. [DOI] [PubMed] [Google Scholar]
- 46.Schroeder JT, MacGlashan DW Jr, Kagey-Sobotka A, White JM, Lichtenstein LM. IgE-dependent IL-4 secretion by human basophils. The relationship between cytokine production and histamine release in mixed leukocyte cultures. J Immunol. 1994;153:1808–17. [PubMed] [Google Scholar]
- 47.Li H, Sim TC, Alam R. IL-13 released by and localized in human basophils. J Immunol. 1996;156:4833–8. [PubMed] [Google Scholar]
- 48.Huang SK, Suttles J, Smith JK, Stout R. Multifunctional cytokine expression by human mast cells: regulation by T cell membrane contact and glucocorticoids. J Interferon Cytokine Res. 1997;17:167–76. doi: 10.1089/jir.1997.17.167. [DOI] [PubMed] [Google Scholar]
- 49.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 50.de las Marinas MD, Sanz ML, Dièguez I, Oehling A. IgE synthesis-enhancing factor: modification of its production in the course of long-term immunotherapy. J Invest Allergol Clin Immunol. 1993;3:40–44. [PubMed] [Google Scholar]
- 51.McHugh SM, Deighton J, Stewart AG, Lachmann PJ, Ewan PW. Bee venom immunotherapy induces a shift in cytokine responses from a TH-2 to a TH-1 dominant pattern: comparison of rush and conventional immunotherapy. Clin Exp Allergy. 1995;25:828–38. doi: 10.1111/j.1365-2222.1995.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 52.O’Brien RM, Byron KA, Varigos GA, Thomas WR. House dust mite immunotherapy results in a decrease in Der p 2-specific IFN-gamma and IL-4 expression by circulating T lymphocytes. Clin Exp Allergy. 1997;27:46–51. [PubMed] [Google Scholar]
- 53.Jutel M, Pichler WJ, Skrbic D, Urwyler A, Dahinden C, Müller UR. Bee venom immunotherapy results in decrease of IL-4 and IL-5 and increase of IFN-gamma secretion in specific allergen-stimulated T cell cultures. J Immunol. 1995;154:4187–94. [PubMed] [Google Scholar]
- 54.Kljaic-Turkalj M, Cvoriscec B, Tudoric N, Stipic-Markovic A, Rabatic S, Trescec A, Gagro A, Dekaris D. Decrease in CD23+ B lymphocytes and clinical outcome in asthmatic patients receiving specific rush immunotherapy. Int Arch Allergy Immunol. 1996;111:188–94. doi: 10.1159/000237367. [DOI] [PubMed] [Google Scholar]
- 55.Ito H, Suzuki M, Mamiya S, Takagi I, Baba S. Study on changes in the level of serum IL-4 and soluble CD23 (s-CD23) with immunotherapy in nasal allergy patients. Acta Otolaryngol Suppl. 1996;525:98–104. [PubMed] [Google Scholar]
- 56.Fischer A, Pfeil Th, König W. Cytokine control of peripheral-blood CD23 expression and sCD23 release: differential regulation by IL2 and IL4. Int Arch Allergy Immunol. 1990;92:334–42. doi: 10.1159/000235161. [DOI] [PubMed] [Google Scholar]
- 57.Corominas M, Mestre M, Bas J, Verdaguer J, Valls A, Romeu A, Buendia E. CD23 expression on B-lymphocytes and its modulation by cytokines in allergic patients. Clin Exp Allergy. 1993;23:612–7. doi: 10.1111/j.1365-2222.1993.tb00902.x. [DOI] [PubMed] [Google Scholar]
- 58.Park CS, Ra DJ, Lee SM, Jeong SW, Uh S, Kim HT, Kim YH. Interleukin-4 and low-affinity receptor for IgE on B cells in peripheral blood of patients with atopic bronchial asthma. J Allergy Clin Immunol. 1996;97:1121–8. doi: 10.1016/s0091-6749(96)70267-1. [DOI] [PubMed] [Google Scholar]
- 59.Spiegelberg HL, Simon RA. Increase of lymphocytes with Fc receptor for IgE in patients with allergic rhinitis during the grass pollen season. J Clin Invest. 1981;68:845–52. doi: 10.1172/JCI110339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lantz CS, Yamaguchi M, Oettgen HC, Katona IM, Myajima I, Kinet JP, Galli SJ. IgE regulates mouse basophil Fc epsilon RI expression in vivo. J Immunol. 1997;158:2517–21. [PubMed] [Google Scholar]
- 61.Sihra BS, Kon OM, Grant JA, Kay AB. Expression of high-affinity IgE receptors (Fc epsilon RI) on peripheral blood basophils, monocytes, and eosinophils in atopic and nonatopic subjects: relationship to total serum IgE concentrations. J Allergy Clin Immunol. 1997;99:699–706. doi: 10.1016/s0091-6749(97)70033-2. [DOI] [PubMed] [Google Scholar]
- 62.Knol EF, Mul FP, Jansen H, Calafat J, Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol. 1991;88:328–38. doi: 10.1016/0091-6749(91)90094-5. [DOI] [PubMed] [Google Scholar]
- 63.Bochner BS, Sterbinsky SA. Altered surface expression of CD11 and Leu 8 during human basophil degranulation. J Immunol. 1991;146:2367–73. [PubMed] [Google Scholar]
- 64.Kimura I, Tanizaki Y, Goda Y, Komagoe H, Kitani H. Decrease in reactivity of basophils by immunotherapy with housedust extract. Clin Allergy. 1985;15:1–7. doi: 10.1111/j.1365-2222.1985.tb02248.x. [DOI] [PubMed] [Google Scholar]
- 65.Chyrek-Borowska S, Kuezewska B, Rogalewska A, Hofman J. Immunologic histamine release from isolated human basophils during specific immunotherapy. Agents Actions. 1986;18:176–7. doi: 10.1007/BF01988014. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;316:1622–6. doi: 10.1056/NEJM198706253162603. [DOI] [PubMed] [Google Scholar]
- 67.Huggins KG, Brostoff J. Local production of specific IgE antibodies in allergic-rhinitis patients with negative skin tests. Lancet. 1975;2(7926):148–50. doi: 10.1016/s0140-6736(75)90056-2. [DOI] [PubMed] [Google Scholar]
- 68.Saloga J, Renz H, Larsen GL, Gelfand EW. Increased airways responsiveness in mice depends on local challenge with antigen. Am J Respir Crit Care Med. 1994;149:65–70. doi: 10.1164/ajrccm.149.1.8111600. [DOI] [PubMed] [Google Scholar]
- 69.Chvatchko Y, Kosco-Vilbois Nffi Herren S, Lefort J, Bonnefoy JY. Germinal center formation and local immunoglobulin E (IgE) production in the lung after an airway antigenic challenge. J Exp Med. 1996;184:2353–60. doi: 10.1084/jem.184.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garlisi CG, Falcone A, Billah MM, Egan RW, Umland SP. T cells are the predominant source of interleulin-5 but not interleukin-4 mRNA expression in the lungs of antigen-challenged allergic mice. Am J Respir Cell Mol Biol. 1996;15:420–8. doi: 10.1165/ajrcmb.15.3.8810648. [DOI] [PubMed] [Google Scholar]
- 71.Humbert M, Durham SR, Ying S, et al. IL-4 and IL-5 mRNA and protein in bronchial biopsies from patients with atopic and nonatopic asthma: evidence against ‘intrinsic’ asthma being a distinct immunopathologic entity. Am J Respir Crit Care Med. 1996;154:1497–504. doi: 10.1164/ajrccm.154.5.8912771. [DOI] [PubMed] [Google Scholar]
- 72.Krzesicki RF, Winterrowd GE, Brashler JR, et al. Identification of cytokine and adhesion molecule mRNA in murine lung tissue and isolated T cells and eosinophils by semi-quantitative reverse transcriptase-polymerase chain reaction. Am J Respir Cell Mol Biol. 1997;16:693–701. doi: 10.1165/ajrcmb.16.6.9191471. [DOI] [PubMed] [Google Scholar]
- 73.Miadonna A, Roncarolo MG, Lorini M, Tedeschi A. Inducing and enhancing effects of IL-3, -5, and -6 and GM-CSF on histamine release from human basophils. Clin Immunol Immunopathol. 1993;67:210–5. doi: 10.1006/clin.1993.1067. [DOI] [PubMed] [Google Scholar]
- 74.Nilsson G, Carlsson M, Jones I, Ahlstedt S, Matsson P, Nilsson K. TNF-alpha and IL-6 induce differentiation in the human basophilic leukaemia cell line KU812. Immunology. 1994;81:73–78. [PMC free article] [PubMed] [Google Scholar]
- 75.Hsu Q, MacGlashan D. IgE antibody up-regulates high affinity IgE binding on murine bone marrow-derived mast cells. Immunol Letters. 1996;52:129–34. doi: 10.1016/0165-2478(96)02599-0. [DOI] [PubMed] [Google Scholar]
- 76.Xia HZ, Du Z, Craig S, Klisch G, Noben-Trauth N, Kochan JP, Huff TH, Irani AM, Schwartz LB. Effect of recombinant human IL-4 on tryptase, chymase, and Fc epsilon receptor type 1 expression in recombinant human stem cell factor-dependent fetal liver-derived human mast cells. J Immunol. 1997;159:2911–21. [PubMed] [Google Scholar]
- 77.Sihra BS, Kon OM, Grant JA, Kay AB. Expression of high-affinity IgE receptors (Fc epsilon RI) on peripheral blood basophils, monocytes, and eosinophils in atopic and nonatopic subjects: relationship to total serum IgE concentrations. J Allergy Clin Immunol. 1997;99:699–706. doi: 10.1016/s0091-6749(97)70033-2. [DOI] [PubMed] [Google Scholar]
- 78.Hu ZQ, Kobayashi K, Zenda N, Shimamura T. Tumor necrosis factor-alpha- and interleukin-6-triggered mast cell development from mouse spleen cells. Blood. 1997;89:526–33. [PubMed] [Google Scholar]
- 79.Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the Fc epsilon RI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J Clin Invest. 1997;99:1492–9. doi: 10.1172/JCI119311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rajakulasingam K, Durham SR, O’Brien F, Humbert M, Barata LT, Reece L, Kay AB, Grant JA. Enhanced expression of high-affinity IgE receptor (Fc epsilon RI) alpha chain in human allergen-induced rhinitis with co-localization to mast cells, macrophages, eosinophils, and dendritic cells. J Allergy Clin Immunol. 1997;100:78–86. doi: 10.1016/s0091-6749(97)70198-2. [DOI] [PubMed] [Google Scholar]
- 81.Garhsi CG, Falcone A, Kung TT, et al. T cells are necessary for Th2 cytokine production and eosinophil accumulation in airways of antigen-challenged allergic mice. Clin Immunol Immunopathol. 1995;75:75–83. doi: 10.1006/clin.1995.1055. [DOI] [PubMed] [Google Scholar]
- 82.Lee JJ, McGarry MT, Farmer SC, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–56. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]


