Abstract
Although systemic immune reactivity to 65-kD mycobacterial hsp65 (m-hsp65) has been shown previously in Behçet's disease (BD), local immune response was not investigated. We studied anti-m-hsp65 IgG, IgM and IgA antibodies in the serum and cerebrospinal fluid (CSF) of 25 BD patients with cerebral parenchymal involvement (p-NBD), seven BD patients with intracranial hypertension (ih-NBD), eight BD patients without central nervous system (CNS) involvement, 30 patients with multiple sclerosis (MS) and 24 patients with non-inflammatory CNS disorders (NIC). Significantly higher CSF IgG responses were detected in p-NBD patients (ELISA ratio 1.3 ± 0.9) compared with NIC (0.7 ± 0.4, P < 0.01). In p-NBD patients' IgG, IgM or IgA CSF anti-m-hsp65 positivity rate was 48% (12/25); this was significantly higher when compared with MS (3/30; P < 0.03) and NIC (3/24; P < 0.01). CSF anti-m-hsp65 IgG ratios correlated with the duration of BD (r = 0.4, P < 0.04) but not with the duration of neurological involvement. Serum IgM and IgA responses were elevated in ih-NBD, suggesting a different type of involvement than p-NBD. These results implicate an increased local humoral response to m-hsp65 in the CSF of p-NBD patients, which might be related to the pathogenesis of neurological involvement.
Keywords: neuro-Behçet's disease, cerebrospinal fluid, heat shock protein 65
INTRODUCTION
The heat shock or the stress response, as it is commonly referred to, is found in all eukaryotic cells and protects the cell against stress factors such as hypoxia, hypoglycaemia and hormonal changes by initiating the synthesis of hsp [1]. Among the most important stressor factors leading to synthesis of hsp are bacterial and viral infections [1]. Hsp are subgrouped according to their molecular weight. The 65-kD hsp of Mycobacterium tuberculosis (m-hsp65) was shown to have 47% amino acid homology with the human hsp60 (h-hsp60) [2]. It is postulated that this homology may initiate autoimmune reactions by the mechanism of ‘molecular mimicry’ [2,3].
The aetiology of Behçet's disease (BD) is unknown. Infectious agents such as herpes simplex type 1 [4] and several streptococci [5] are implicated in the pathogenesis. With rabbit anti-m-hsp65 serum, Lehner et al. observed a 65-kD band against six Streptococcus sanguis strains and S. pyogenes, and the sera from BD patients revealed similar bands of 65–70 kD of the IgA and IgG isotypes against all streptococcal strains [6]. A significant IgA and IgG antibody response and a proliferative T cell response were shown against the synthetic peptides derived from m-hsp65 and homologous h-hsp60 [7–9]. The T cell response was recently shown to be mainly of the γδ T cell subset [10,11]. An increase of γδ T cells in the peripheral blood [12–14] and cerebrospinal fluid (CSF) [14] was also shown in patients with BD.
Involvement of the central nervous system (CNS) is a serious manifestation of BD and has an important impact on the quality of life [15,16]. In this study we aimed to investigate the local humoral response against m-hsp65 in the CSF of neuro-Behçet patients and to compare the findings with the responses of other inflammatory and non-inflammatory diseases of the CNS.
PATIENTS AND METHODS
Patients and controls
Thirty-two patients (27 males, five females) followed in the neuro-BD clinic of the University of Istanbul Medical Faculty Neurology Department, diagnosed according to the classification criteria of the International Study Group for BD [17], were studied. The demographic and clinical findings of the patients are given in Table 1. Twenty-five patients (21 males, four females) had parenchymal involvement (p-NBD). Parenchymal involvement was defined as focal or diffuse neurological signs, pleocytosis on lumbar puncture, and basal ganglia and/or medullary involvement on cranial or spinal magnetic resonance imaging [15,16]. Seven other patients with NBD (six males, one female) were being followed with the diagnosis of intracranial hypertension (ih-NBD). These patients presented to the clinic with elevated intracranial pressure; dural venous thrombosis (without parenchymal involvement) was found in five of them and an elevated pressure without pleocytosis was found in all lumbar punctures [15,16]. The lumbar punctures of 12 patients in the p-NBD group were performed during an acute attack (within 1 week after the onset of symptoms) and within 1 month following onset in two other patients. The patients who did not give a history of acute deterioration were regarded as having chronic or primary progression and their lumbar punctures were performed at various stages of the disease (n = 11). Pleocytosis was observed in 56% (14/25) of the p-NBD patients and 14 of them were on immunosuppressive therapy. In the ih-NBD group lumbar punctures were done during an acute attack in five patients, and during the remission period in two. Three patients were on immunosuppressive therapy.
Table 1.
Demographic and clinical features of the Behçet disease (BD) patients
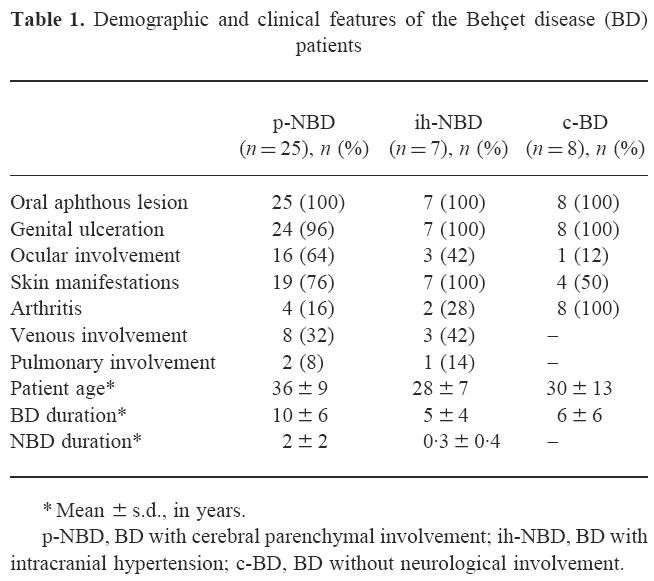
* Mean ± s.d., in years.
p-NBD, BD with cerebral parenchymal involvement; ih-NBD, BD with intracranial hypertension; c-BD, BD without neurological involvement.
Three control groups were investigated. The first control group, c-BD, consisted of eight patients (four females, four males) with BD being followed for recurrent headaches. They had normal neurological examinations, CSF findings and cranial magnetic resonance investigations, and were accepted as not having CNS involvement.
The second control group consisted of 24 patients (14 females, 10 males) who had a non-inflammatory central nervous system disease (NIC). All patients had lumbar punctures and CSF pressure determinations, myelography, oligoclonal band examination and serologies for herpes simplex virus for the differential diagnosis of their diseases. Their diagnoses were as follows: lumbar disc disease (n = 7), acute psychotic reaction (n = 4), headache (n = 4), idiopathic epilepsy (n = 3), benign intracranial hypertension (n = 2), hereditary spastic paraparesis (n = 1), rickets (n = 1), senile dementia (n = 1), restless legs syndrome (n = 1). All the patients in this group had normal CSF findings on routine examinations.
Thirty patients (17 females, 13 males) with multiple sclerosis (MS) were investigated as the inflammatory control group. Twenty-four of the patients had clinically definite MS, five probable MS, and one laboratory-supported definite MS, according to Poser et al. criteria [18]. Their clinical courses were as follows: relapsing-remitting (n = 12), secondary progressive (n = 11), and primary progressive (n = 7).
ELISA
The CSF and serum samples were aliquoted and stored at −80°C until the antibody determinations were performed. IgG, IgM and IgA antibodies against m-hsp65 were investigated by ELISA in paired CSF and serum samples. Plates (Maxisorp; Nunc, Roskilde, Denmark) were coated with 100 μl of 1 μg/ml m-hsp65 (kindly provided by Dr M. Singh, World Health Organization) in PBS overnight at 4°C. After washing three times with 1% Tween 20 in PBS (PBS–20), the plates were blocked with 5% dried milk in PBS–20 (5% PBS–20) at 37°C for 1 h. Then diluted serum samples (1:200 for IgG and 1:100 for IgM and IgA in 5% PBS–20) and undiluted CSF samples were added in duplicate. The samples were incubated at 37°C for 2 h. After extensive washing, peroxidase-conjugated specific anti-human IgG, IgM or IgA (Sigma, St Louis, MO) were added and the plates were incubated at room temperature for 1 h. After the last wash, peroxidase substrate (ortho-phenylenediamine (OPD)) was added and following an incubation of 15 min at room temperature, absorbances were read at 492 nm as optical density (OD). The samples were investigated blindly. A standard control pool of four sera, and CSF from a patient with congenital hydrocephalus known to be anti m-hsp65-negative, was run at each assay. The results were calculated by dividing the sample OD by the standard control OD and given as ELISA ratio [19]. Positivity was defined as 2 s.d. above the mean of NIC.
Statistical analysis
Since data were not distributed normally, non-parametric statistical tests were used. Differences between groups were tested by anova. Differences between pairs of groups were tested by Mann–Whitney's U-test. Differences in the incidence of positive antibodies between groups were tested by χ2 test. Correlations between CSF and serum ELISA ratios and between disease duration and CSF ELISA ratios were tested by Spearman's rank order test.
RESULTS
CSF ELISA ratios
The mean CSF IgG ELISA ratio for p-NBD group was 1.3 ± 0.9. This was significantly higher compared with NIC (0.7 ± 0.4, P < 0.01). No statistically significant differences were observed between the responses of ih-NBD, c-BD and MS, and NIC (Fig. 1,Table 2). The mean CSF IgM ratio was also high in p-NBD compared with all other groups, without statistical significance (Fig. 2,Table 2). Mean CSF IgA ratio was not increased in p-NBD when compared with the controls mentioned above (Fig. 3,Table 2).
Fig. 1.
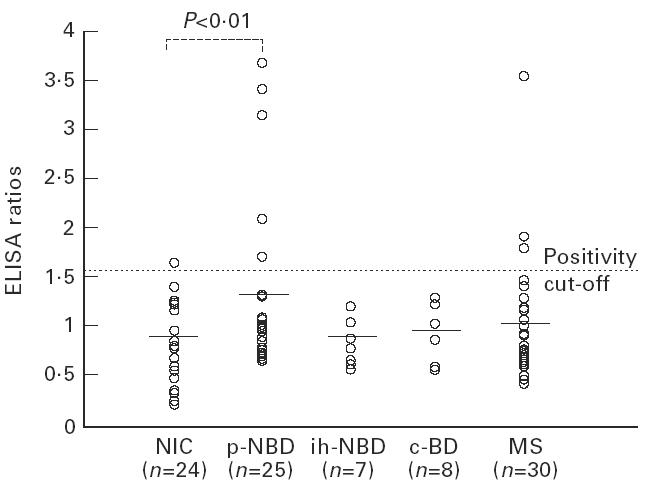
Anti-mycobacterial hsp65 (m-hsp65) IgG responses detected in the cerebrospinal fluid (CSF) of the patient groups. NIC, Non-inflammatory controls; p-NBD, neuro-Behçet's disease with parenchymal involvement; ih-NBD, neuro-Behçet's disease with intracranial hypertension; c-BD, Behçet's disease without neurological involvement; MS, multiple sclerosis. Horizontal lines indicate the mean value of each group.
Table 2.
Cerebrospinal fluid (CSF) and serum results of the groups as ELISA ratios
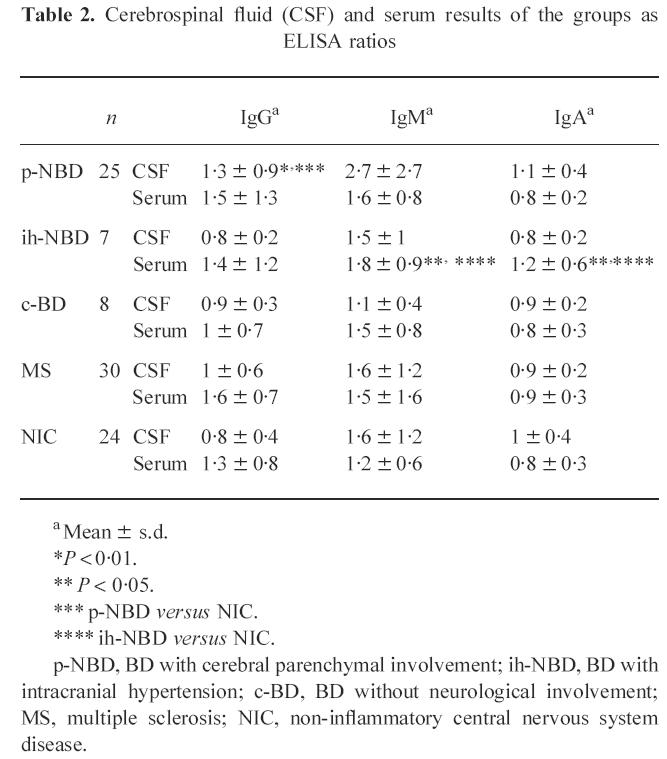
a Mean ± s.d.
*P < 0.01.
** P < 0.05.
*** p-NBD versus NIC.
**** ih-NBD versus NIC.
p-NBD, BD with cerebral parenchymal involvement; ih-NBD, BD with intracranial hypertension; c-BD, BD without neurological involvement; MS, multiple sclerosis; NIC, non-inflammatory central nervous system disease.
Fig. 2.
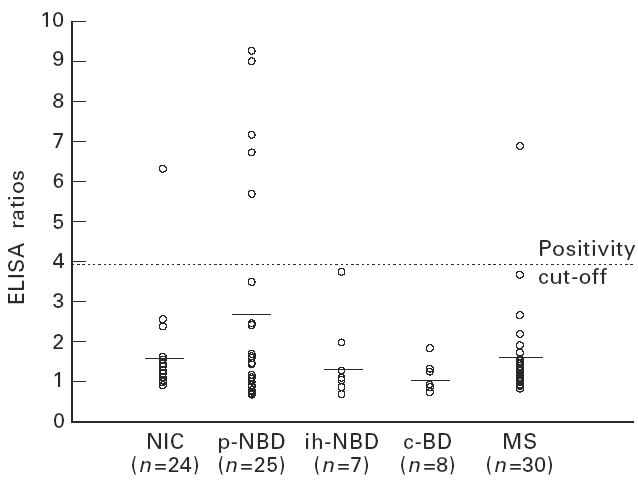
Anti-mycobacterial hsp65 (m-hsp65) IgM responses detected in the cerebrospinal fluid (CSF) of the patient groups. NIC, Non-inflammatory controls; p-NBD, neuro-Behçet's disease with parenchymal involvement; ih-NBD, neuro-Behçet's disease with intracranial hypertension; c-BD, Behçet's disease without neurological involvement; MS, multiple sclerosis. Horizontal lines indicate the mean value of each group.
Fig. 3.
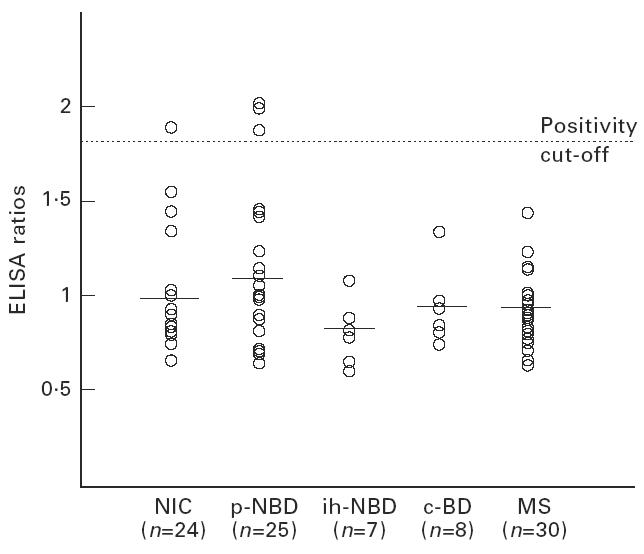
Anti-mycobacterial hsp65 (m-hsp65) IgA responses detected in the cerebrospinal fluid (CSF) of the patient groups. NIC, non-inflammatory controls; p-NBD, neuro-Behçet's disease with parenchymal involvement; ih-NBD, neuro-Behçet's disease with intracranial hypertension; c-BD, Behçet's disease without neurological involvement; MS, multiple sclerosis. Horizontal lines indicate the mean value of each group.
In the p-NBD group, four patients were positive for IgG, five for IgM and two for IgA antibodies, and one patient was positive both for IgG and IgA; altogether 12 patients were positive in the p-NBD group (48%) (Figs 1, 2,3) and this was significantly higher compared with NIC (12%, 3/24) and MS (10%, 3/30, one patient both IgG- and IgM-positive). No positivity was detected in ih-NBD and c-BD.
Among the patients who had positive antibody results, seven were in an acute attack and five of them showed a progressive course. Six of the patients in an acute attack had pleocytosis (86%). In the NIC group, the IgG-positive patient had a psychotic reaction, the IgM-positive patient had lumbar disc disease, and the IgA-positive patient had senile dementia.
Mean ELISA CSF ratios of the ih-NBD and c-BD did not differ from NIC.
Serum ELISA ratios
As shown in Table 2, the responses of p-NBD patients did not differ significantly from the inflammatory and non-inflammatory control groups. In ih-NBD, IgM and IgA ratios were significantly higher compared with NIC (IgM, 1.8 ± 0.9 versus 1.2 ± 0.6; and IgA, 1.2 ± 0.6 and 0.8 ± 0.3, both P < 0.05) (Table 2). Forty-three percent (3/7) of the patients were positive (IgG, IgM and IgA) in the ih-NBD group, while positivity was 20% (5/25) in p-NBD.
CSF and serum correlations
Significant correlations were found between CSF and serum ELISA ratios for IgG responses (r = 0.4 for p-NBD, 0.38 for ih-NBD, and 0.24 for c-BD, P < 0.03). No correlation was found in IgM and IgA responses.
Clinical course and antibody response correlations
The presence of acute attacks or progressive course or CSF pleocytosis did not correlate with the ELISA ratios. On sequential antibody studies on seven patients with two to four samples, the CSF responses in all antibody groups did not show statistically significant changes between the corresponding samples (data not shown).
BD and NBD duration and antibody response correlations
An increase of the IgG CSF ratios was observed with an increase in BD duration in p-NBD (r = 0.4, P < 0.04). In the same group this relation could not be shown with IgG serum, IgM and IgA serum, and CSF responses. The duration of neurological disease did not correlate with the ratios in p-NBD and ih-NBD.
Clinical features, therapy and antibody response correlations
The serum IgA ratios were significantly high in patients with arthritis (arthritic patients, 1.1 ± 0.3 versus non-arthritic, 0.77 ± 0.4; P < 0.01). Uveitis, venous involvement, and mucocutaneous features (pseudofolliculitis and erythema nodosum) as well as immunosuppressive therapy had no effect on the ELISA ratios (analysed only in p-NBD group).
Multiple sclerosis
When the MS patients were analysed, no statistically significant difference was observed compared with NIC and with all BD patients for any antibody group in CSF responses. No correlation was found between serum and CSF values. No difference was observed between MS subgroups except statistically insignificant high CSF IgM responses of the secondary progressive MS (ELISA ratios mean for all MS, 1.57; secondary progressive MS, 2.23).
DISCUSSION
In this study we investigated the presence of local humoral response to m-hsp65 in the CSF of neuro-Behçet patients. In p-NBD the CSF anti-hsp65 antibodies were positive in 48% of the patients (four IgG, five IgM, two IgA and one both IgG and IgA). This was significantly higher than the positivity rate found in ih-NBD, c-BD, MS and NIC. In all patients with BD, a correlation was observed between CSF and serum ratios for IgG, but not for IgM and IgA. IgG CSF ratio also had a correlation with the BD duration, while the duration of neurological involvement did not show any effect.
The stress responses of the cells are implicated in the aetiopathogenesis of autoimmune disorders, and stress proteins are considered as candidate (auto)antigens. Molecular mimicry or interspecies cross-reactivity is suggested as the mechanism of pathology [3]. T and B cell responses to hsp65 in different inflammatory and autoimmune diseases have been demonstrated [1,3]. Although anti-m-hsp65 responses did not seem to be disease-specific, specific responses to certain epitopes of m-hsp65, such as residues 2–13 in reactive arthritis [20], 180–88 in juvenile rheumatoid arthritis [21] and 473–83 in Kawasaki disease [22], were observed. IgA and IgG anti-m-hsp65 antibodies were also found to be increased in the sera of BD patients, but also in rheumatoid arthritis and recurrent oral ulcers [6]. Specifying this response with epitope mapping showed that certain m-hsp65- and h-hsp60-derived peptides were preferentially recognized in BD (residues 111–25, 219–33, 154–72, 311–326 and their human homologues) [7,8]. The demonstration of the specific γδ T cell responses against the same epitopes of hsp has also strengthened the importance of hsp in BD [9,10].
As BD is considered a systemic disorder, most immune responses were evaluated in the peripheral blood. However, different organ involvements during the course of BD suggest the role of local immune responses. These responses in a relatively well protected compartment of the human body, namely CSF, provide a unique opportunity to test the specific responses related to CNS involvement in NBD. Confirming this, a proinflammatory cytokine IL-6 and anticardiolipin antibodies were found to be increased in the CSF of NBD patients [23]. T cells in the CSF, especially of the γδ subset [11], are also activated showing increased expression of IL-2 receptor [14] and adhesion molecules CD11a/CD18 [24]. Likewise, the elevated CSF IgM and IgA antibody responses not accompanied by a parallel increase in the sera in our study suggested intrathecal humoral response to m-hsp65 in p-NBD patients. On the other hand, the correlation between serum and CSF IgG levels between all patients with BD (with or without neurological involvement) and the effect of BD duration on the CSF responses also suggests the possibility of the leakage of serum immunoglobulins into the CSF through a damaged blood–brain barrier.
Previous studies have shown that ih-NBD is a part of the spectrum of systemic vascular involvement and that it differs from p-NBD [25]. Our results were in favour of this observation, since the CSF IgG and IgM responses were high in p-NBD, while serum IgM and IgA responses were found to be raised in ih-NBD.
Anti-m-hsp65 CSF antibody responses were investigated previously in MS, with controversial results [26, 27]. Our results are in accordance with the study of Gao et al. showing no significant difference between MS and other neurological diseases [27].
Although the anti-m-hsp65 antibody response we have shown may be helpful in the differentiation of parenchymal and non-parenchymal neurological involvement in BD, usefulness as a diagnostic test will be limited due to the low sensitivity.
Diffuse perivascular lymphocytic infiltration, small necrotic foci, and diffuse glial reaction were shown previously by others in post-mortem cerebral specimens of neuro-Behçet patients. Glial proliferation was thought to be due to microglia nodule formation and accompanying oligodendrocyte and macroglial reactivity [28]. On the other hand, pathological studies on brain specimens from MS patients showed co-localization of γδ T cells with oligodendrocytes, and it was proposed that the observed myelin damage was due to γδ T cell cytotoxicity [29]. Hsp65 expression of oligodendrocytes supported this hypothesis in MS [30]. The anti-hsp65 antibody response we detected in the CSF might be against hsp65 expressed in the tissues of neuro-Behçet patients. As recognition of hsp65 by peripheral γδ T cells was previously demonstrated in BD, the findings above may suggest that oligodendrocyte damage in p-NBD could also be caused by γδ T cells crossing a damaged blood–brain barrier [10]. Immune cytochemical studies performed in the future might shed further light on the pathogenesis of p-NBD.
Acknowledgments
This study was supported by the University of Istanbul Research Fund (project no. T-40) and also received financial support from the UNDP/World Bank/Who Special programme for Research and training in Tropical diseases (TDR).
REFERENCES
- 1.Kaufmann SHE. Heat shock proteins and the immune response. Immunol Today. 1990;11:129–39. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- 2.Young DB, Ivanyi J, Cox JH, Lamb JR. The 65kDa antigen of mycobacteria—a common bacterial protein? Immunol Today. 1987;8:215–9. doi: 10.1016/0167-5699(87)90168-X. [DOI] [PubMed] [Google Scholar]
- 3.Cohen IR, Young DB. Autoimmunity, microbial immunity, and the immunological homunculus. Immunol Today. 1991;12:105–10. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- 4.Eglin RP, Lehner T, Subak-Sharpe JH. Detection of RNA complementary to herpes simplex virus in mononuclear cells from patients with Behçet's syndrome and recurrent oral ulcers. Lancet. 1982;ii:1356–61. doi: 10.1016/s0140-6736(82)91268-5. [DOI] [PubMed] [Google Scholar]
- 5.The Behçet's Disease Research Committee of Japan. Skin hypersensitivity to streptococcal antigens and induction of systemic symptoms by the antigens in Behçet's disease—a multicenter study. J Rheumatol. 1989;16:506–11. [PubMed] [Google Scholar]
- 6.Lehner T, Lavery E, Smith R, van der Zee R, Mizushima Y, Shinnick T. Association between the 65-kilodalton heat shock protein, Streptococcus sanguis, and the corresponding antibodies in Behçet's syndrome. Infect Immun. 1991;59:1434–41. doi: 10.1128/iai.59.4.1434-1441.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Direskeneli H, Hasan A, Shinnick T, et al. Recognition of B cell epitopes of the 65 kDa HSP in Behçet's Disease. Scand J Immunol. 1996;43:1–8. doi: 10.1046/j.1365-3083.1996.d01-53.x. [DOI] [PubMed] [Google Scholar]
- 8.Pervin K, Childerstone A, Shinnick T, et al. T cell epitope expression of mycobacterial and homologous human 65 kilodalton heat shock protein peptides in short term lines from patients with Behçet's disease. J Immunol. 1993;151:2273–82. [PubMed] [Google Scholar]
- 9.Kaneko S, Suzuki N, Yamashita N, et al. Characterization of T cells specific for an epitope of human 60-kD heat shock protein (hsp) in patients with Behçet's disease (BD) in Japan. Clin Exp Immunol. 1997;108:204–12. doi: 10.1046/j.1365-2249.1997.3611265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasan A, Fortune F, Wilson A, et al. Role of γδ T cells in pathogenesis and diagnosis of Behçet's disease. Lancet. 1996;347:789–94. doi: 10.1016/s0140-6736(96)90868-5. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita N, Kaneoka H, Kaneko S, et al. Role of γδ T lymphocytes in the development of Behçet's disease. Clin Exp Immunol. 1997;107:241–7. doi: 10.1111/j.1365-2249.1997.274-ce1159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki Y, Hoshi K, Matsuda T, Mizushima T. Increased peripheral blood γδ T cells and natural killer cells in Behçet's disease. J Rheumatol. 1992;19:588–92. [PubMed] [Google Scholar]
- 13.Fortune F, Walker J, Lehner T. The expression of γδ T cell receptor and the prevalence of primed, activated and IgA-bound T cells in Behçet's syndrome. Clin Exp Immunol. 1990;82:326–32. doi: 10.1111/j.1365-2249.1990.tb05447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamzaoui K, Hamzaoui A, Hentati F, et al. Phenotype and functional profile of T cells expressing γδ receptor from patients with active Behçet's disease. J Rheumatol. 1994;21:2301–6. [PubMed] [Google Scholar]
- 15.Serdaroğlu P, Yazici H, Özdemir C, Yurdakul S, Bahar S, Aktin E. Neurological involvement in Behçet's syndrome, a prospective study. Arch Neurol. 1989;46:265–9. doi: 10.1001/archneur.1989.00520390031011. [DOI] [PubMed] [Google Scholar]
- 16.Akman-Demir G, Baykan-Kurt B, Serdaroğlu P, et al. Seven-year follow-up of neurological involvement in Behçet syndrome. Arch Neurol. 1996;53:691–4. doi: 10.1001/archneur.1996.00550070133022. [DOI] [PubMed] [Google Scholar]
- 17.International Study Group for Behçet's Disease. Criteria for diagnosis of Behçet's disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 18.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 19.Danieli MG, Candela M, Ricciatti AM, et al. Antibodies to mycobacterial 65 kDa heat shock protein in systemic sclerosis (scleroderma) J Autoimmun. 1992;5:443–52. doi: 10.1016/0896-8411(92)90004-a. [DOI] [PubMed] [Google Scholar]
- 20.Gaston JSH, Life MS, Jenner PJ, Colston MJ, Bacon PA. Recognition of a mycobacteria specific epitope in the 65 kD heat shock protein by synovial fluid derived T cell clones. J Exp Med. 1990;171:831–41. doi: 10.1084/jem.171.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danieli MG, Markovits D, Gabrielli A, et al. Juvenile rheumatoid arthritis patients manifest immune reactivity to the mycobacterial 65 kDa heat shock protein, to its 180–88 peptide, and to a partially homologous peptide of the proteoglycan link protein. Clin Immunol Immunopathol. 1992;64:121–8. doi: 10.1016/0090-1229(92)90189-u. [DOI] [PubMed] [Google Scholar]
- 22.Yokota S, Tsubaki K, Kuriyama T, et al. Presence in Kawasaki disease of antibodies to mycobacterial heat shock protein HSP65 and autoantibodies to epitopes of human HSP65 cognate antigen. Clin Immunol Immunopathol. 1993;67:163–70. doi: 10.1006/clin.1993.1060. [DOI] [PubMed] [Google Scholar]
- 23.Wang CR, Chuang CY, Chen CY. Anti-cardiolipin antibodies and IL-6 in CSF and blood of Chinese patients with neuro-Behçet syndrome. Clin Exp Rheumatol. 1992;10:599–602. [PubMed] [Google Scholar]
- 24.Hamzaoui K, Hentati F, Hamzaoui A, et al. CD11/CD18 bearing lymphocytes in cerebrospinal fluid from patients with Behçet's Disease. Clin Exp Rheumatol. 1994;12:S75–S76. [PubMed] [Google Scholar]
- 25.Akman-Demir G, Bahar S, Baykan-Kurt B, Gürvit H, Serdaroğlu P. Intracranial hypertension in Behçet's disease. Eur J Neurol. 1996;3:66–70. [Google Scholar]
- 26.Prabhakar S, Kurien E, Gupta RS, Zielinski S, Freedman MS. Heat shock protein immunoreactivity in CSF. Correlation with oligoclonal banding and demyelinating disease. Neurol. 1994;44:1644–8. doi: 10.1212/wnl.44.9.1644. [DOI] [PubMed] [Google Scholar]
- 27.Gao YL, Raine CS, Bronan CF. Humoral response to hsp 65 in multiple sclerosis and other neurologic conditions. Neurol. 1994;44:941–6. doi: 10.1212/wnl.44.5.941. [DOI] [PubMed] [Google Scholar]
- 28.Rubinstein LJ, Urich H. Meningoencephalopathy of Behçet's disease. Case report with pathological findings. Brain. 1963;86:151–66. doi: 10.1093/brain/86.1.151. [DOI] [PubMed] [Google Scholar]
- 29.Selmaj K, Brosnan CF, Raine CS. Expression of heat shock protein 65 by oligodendrocytes in vivo and in vitro: implications for multiple sclerosis. Neurology. 1992;42:795–800. doi: 10.1212/wnl.42.4.795. [DOI] [PubMed] [Google Scholar]
- 30.Freedman MS, Buu NN, Ruijs TCJ, Williams K, Antel JP. Differential expression of heat shock proteins by human glial cells. J Neuroimmunol. 1992;41:231–8. doi: 10.1016/0165-5728(92)90074-u. [DOI] [PubMed] [Google Scholar]


