Abstract
We examined the effects of IL-10 on tumour necrosis factor-alpha (TNF-α) and NO production by LPS-activated macrophages and on the ability of these cells to control Trypanosoma cruzi infection. We first observed that the addition of rIL-10 to macrophages of the J774 cell line decreased their synthesis of TNF-α but increased their release of NO in a dose-dependent manner. In parallel, treatment of J774 cells with rIL-10 resulted in a better control of T. cruzi infection involving up-regulation of NO synthesis, as it was not observed in presence of N-nitro-l-arginine methyl ester (l-NAME), a competitive inhibitor of NO synthase. The enhancing effect of rIL-10 on NO production was not observed on peritoneal macrophages from wild-type C57Bl/6 mice, but well on macrophages from IL-10 knock-out mice. The control of NO production by endogenous IL-10 was confirmed by the demonstration that neutralization of IL-10 secreted by LPS-activated macrophages from wild-type mice inhibited their production of NO and, in parallel, their ability to control T. cruzi infection. Taken together, these data demonstrate that both exogenous and endogenous IL-10 up-regulate the production of NO by LPS-activated macrophages and improve thereby their ability to clear T. cruzi infection.
Keywords: IL-10, Trypanosoma cruzi, nitric oxide, lipopolysaccharide, macrophages
INTRODUCTION
When suitably activated, murine macrophages release NO by catabolizing l-arginine via an inducible nitric oxide synthase (iNOS; [1]). NO has a major role in macrophage defences against intracellular microorganisms, including parasites such as Trypanosoma cruzi (reviewed in [2]). Among the agents stimulating NO synthesis by macrophages, the combination of interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α) is especially active as well as bacterial LPS [3,4].
IL-10 is known as a potent macrophage-deactivating cytokine [5,6]. In this context, IL-10 has been shown to inhibit IFN-γ-dependent NO production by macrophages and thereby their ability to control parasitic infections [2]. However, the effect of IL-10 on LPS-induced NO production has not been clearly defined so far [5,7–11]. The present study was therefore undertaken to determine the effects of exogenous or endogenous IL-10 on NO production in this setting. The demonstration that IL-10 up-regulates NO production by LPS-activated macrophages led us to analyse the influence of IL-10 on the ability of these cells to control T. cruzi infection in vitro.
MATERIALS AND METHODS
Reagents
IFN-γ was kindly provided by Dr A. Billiau and Dr H. Herremans (Catholic University of Louvain, Belgium). LPS (Escherichia coli, serotype 0111:B4), and N-nitro-l-arginine methyl ester (l-NAME) were purchased from Sigma Chemical Co. (St Louis, MO). Recombinant murine IL-10 (rIL-10) was obtained as culture supernatants from Sf9 insect cells stably transfected with the corresponding complementary DNA, as previously described [12], using the baculoviral expression vector pBlue Bac2 (Invitrogen, San Diego, CA). It was semipurified by ionic separation chromatography. The IL-10 concentration of the preparation was determined by ELISA (Genzyme Co., Cambridge, MA). A neutralizing anti-IL-10 MoAb (JES5-2A5, a rat IgG1) was obtained in ascites form [13]. As isotype-matched control, we used ascites of LO-DNP-2 hybridoma cells (kindly provided by Dr H. Bazin, Catholic University of Louvain, Belgium), secreting a rat immunoglobulin G1 MoAb with anti-dinitrophenyl specificity. LPS levels of all the reagents and media were tested using the colourimetric Limulus Amoebocyte Lysate assay (detection limit 1 pg/ml; Coatest Endotoxin Chromogenix, Mölndal, Sweden) and were < 15 pg/ml.
Murine macrophage cell line
The murine macrophage cell line J774 was kindly provided by Professor G. Milon (Institut Pasteur, Paris, France). Cells were grown in RPMI 1640 supplemented with heat-inactivated fetal bovine serum (FBS; 10%), l-glutamine 2 mm, non-essential amino acids, N-2-hydroxyethylpiperazine-N′-2 ethanesulphonic acid 25 mm, penicillin 100 U/ml, and streptomycin 100 μg/ml (Gibco BRL, Gaithersburg, MD). Macrophages were irradiated before culture (30 Gy, Mark 1-68A Irradiator; J. L. Shepherd & Associates, San Fernando, CA) to inhibit further cell replication.
Mouse peritoneal macrophages
Male C57Bl/6 and C57Bl/6 IL-10 knock-out mice (IL-10 KO), 8–12 weeks old (Jackson ImmunoResearch Labs, West Grove, PA) were maintained in our animal facilities on standard laboratory chow. They were killed by ether inhalation. Mouse peritoneal macrophages (MPM) were obtained as previously described [14]. They were allowed to adhere (4 × 105 MPM/well) in 24-well microplates (Nunc, Roskilde, Denmark) on a coverslip (13 mm diameter, Thermanox; Miles Scientific, Napierville, IL) placed in the plates for 2 h at 37°C in a 5% CO2 water-saturated atmosphere. Non-adherent cells were removed by washing with prewarmed culture medium before adding reagents and parasites.
Trypanosoma cruzi infection and activation of macrophages
Trypanosoma cruzitrypomastigotes were obtained in vitro from infected fibroblasts as previously described [15]. They were centrifuged (15 min, 1800 g, 4°C), resuspended in macrophage culture medium and added to adherent macrophages in a 5/1 parasite–cell ratio. Infected macrophages were incubated at 37°C in a CO2 atmosphere. LPS (1 μg/ml) was added at the time of T. cruzi infection.
Macrophages were treated by adding either rIL-10, neutralizing anti-IL-10 MoAb (JES5-2A5) or isotype-matched control MoAb (LO-DNP-2). These reagents were added at the time of LPS activation and T. cruzi infection.
At 16 h post-infection (p.i.), culture supernatants were harvested for TNF-α, IL-10 or NO measurements. The cell cultures were washed with prewarmed medium to remove free parasites and medium was replaced with the respective reagents. At 48 h p.i., the macrophages were fixed with methanol, stained with Giemsa and examined by an optical microscope. The percentage of infected macrophages was recorded after examination of at least 200 cells per well [14].
Murine IL-10, TNF-α and NO assays
Murine IL-10 concentrations were determined by ELISA with a commercial kit (Genzyme, Cambridge, MA). The limit of detection of the test was 0.015 U/ml.
TNF-α was assayed, as described elsewhere [16], by sandwich ELISA using two rabbit anti-mouse TNF-α polyclonal antibodies kindly provided by W. A. Buurman (Department of Surgery, University of Limburg, Maastricht, The Netherlands). The detection limit of the assay was 20 pg/ml.
NO production was evaluated by measuring nitrite, its stable degradation product, by the Griess reaction [17]. Each culture supernatant (50 μl) was added to 50 μl of the Griess solution (1% sulfanilamide, 0.1% naphthylethylene diamine dihydrochloride, 2% H3PO4). The absorbance was measured at 540 nm in a microplate ELISA reader (Titertek Multiscan MCC/340, MKII EFLAB, Finland). Sodium nitrite (NaNO2) diluted in culture medium was used as a standard. The detection limit of the assay was 1.0 μm.
RESULTS
rIL-10 up-regulates NO synthesis by LPS-activated J774 macrophages
We first investigated the effect of exogenous rIL-10 on NO production by macrophages of the J774 cell line. Preliminary data showed that rIL-10 (10 U/ml) decreased basal TNF-α production by resting macrophages (0.44 ± 0.05 versus 0.25 ± 0.04 ng/ml; P < 0.01, Student's t-test), while their production of NO remained below detection levels in the absence as well as in the presence of rIL-10. J774 macrophages were then activated with LPS (1 μg/ml) in the presence of graded concentrations of rIL-10 (0–500 U/ml) and culture supernatants were collected after 16 h for determination of NO and TNF-α levels. As expected, TNF-α production induced by LPS was inhibited by rIL-10 (Fig. 1a). In contrast, rIL-10 enhanced NO production in a dose-dependent manner (Fig. 1b). The activating effect of rIL-10 on NO production was already observed with 0.1 U/ml rIL-10 and was maximal at 10 U/ml rIL-10 (80% increase compared with LPS-activated J774 macrophages in the absence of rIL-10). As control, we verified that control supernatant of untransfected Sf9 insect cells had no effect in this system (data not shown). Moreover, we found that addition of 1 μg/ml of neutralizing anti-IL-10 MoAb completely inhibited rIL-10-induced NO up-regulation, whereas an isotype-matched irrelevant MoAb had no effect.
Fig. 1.
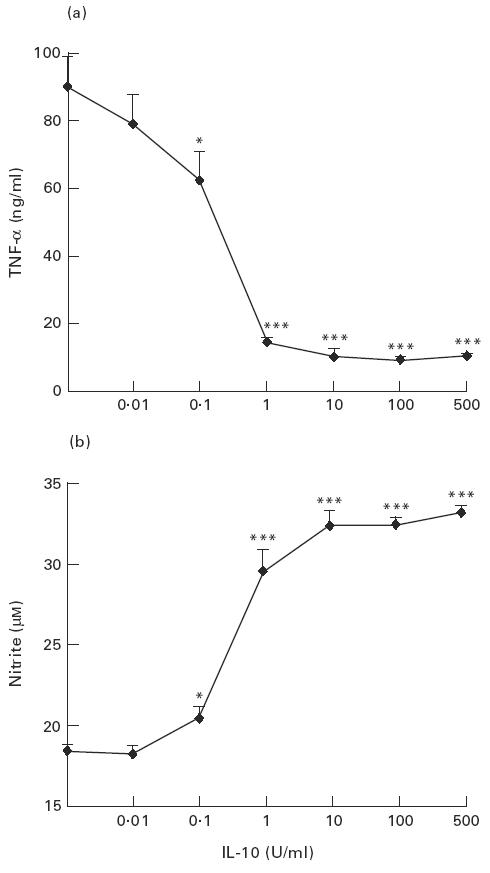
Effects of rIL-10 on the production of tumour necrosis factor-alpha (TNF-α) and NO by LPS-activated J774 macrophages. J774 cells were activated with LPS (1 μg/ml) and treated with the indicated concentrations of rIL-10. TNF-α (a) and nitrite levels (b) were determined in culture supernatants collected after 16 h. Data are expressed as mean ± s.d. of three independent experiments performed in triplicate. *P < 0.05; ***P < 0.001 compared with LPS-activated J774 cells in the absence of rIL-10 (Student's t-test).
The enhanced NO production induced by rIL-10 was observed early after LPS activation (Fig. 2). Indeed, the overproduction of NO by macrophages treated with LPS and rIL-10 compared with macrophages cultured with LPS alone was more pronounced after 16 h of culture (77% increase) than after 48 h (36% increase).
Fig. 2.

Kinetics of nitrite production by LPS-activated J774 macrophages. J774 cells were activated with LPS (1 μg/ml) in the absence or presence of rIL-10 (10 U/ml) and nitrite levels were determined in supernatants collected at the indicated time points. Data are shown as mean ± s.d. of three independent experiments performed in triplicate. P = 0.01, 0.02 and 0.04 after 16, 24 and 48 h, respectively, for macrophages treated with LPS and IL-10 compared with macrophages treated with LPS alone (Student's t-test).
rIL-10 enhanced T. cruzi control by LPS-activated J774 macrophages through an increase of NO production
To evaluate the relevance of rIL-10-induced NO overproduction in terms of macrophage defences, we investigated the ability of LPS-activated macrophages to control T. cruzi infection in the presence or absence of rIL-10. Irradiated J774 macrophages were treated with LPS (1 μg/ml) and graded concentrations of rIL-10 (0–500 U/ml) and incubated with T. cruzi trypomastigotes at a 5/1 parasite–cell ratio. Culture supernatants were collected after 16 h for determination of NO levels and macrophages were stained after 48 h to detect the presence of intracellular parasites. As shown in Fig. 3, the increased production of NO in the presence of rIL-10 was associated with a dose-dependent decrease in the percentages of infected macrophages, a maximal effect being achieved with 100 U/ml of rIL-10. These experiments were repeated on macrophages stimulated with a lower dose of LPS (10 ng/ml) and similar effects of rIL-10 were observed (data not shown).
Fig. 3.

Effects of rIL-10 on LPS-activated J774 macrophages infected with Trypanosoma cruzi. Trypanosoma cruzi-infected cells were activated with LPS (1 μg/ml) and incubated with the indicated concentrations of rIL-10. Nitrite levels were determined in culture supernatants collected after 16 h and the percentages of infected macrophages were determined after 48 h. Data are expressed as mean ± s.d. of three independent experiments performed in triplicate. *P < 0.05; ***P < 0.001 compared with cells not treated with rIL-10, for nitrite concentrations; ††P < 0.01 for percentages of infected macrophages (Student's t-test).
To evaluate the role of NO overproduction in the enhanced control of T. cruzi infection induced by rIL-10, J774 infected macrophages were cultured with LPS (1 μg) and rIL-10 (10 U/ml) in the presence or absence of l-NAME (5 mm), a competitive inhibitor of NO synthase. The major decrease in NO production induced by l-NAME was associated with a four-fold increase in the percentage of infected macrophages (Table 1), indicating that the improved control of T. cruzi by rIL-10-treated macrophages involved NO overproduction.
Table 1.
NO production and control of Trypanosoma cruzi infection by LPS-activated J774 cells
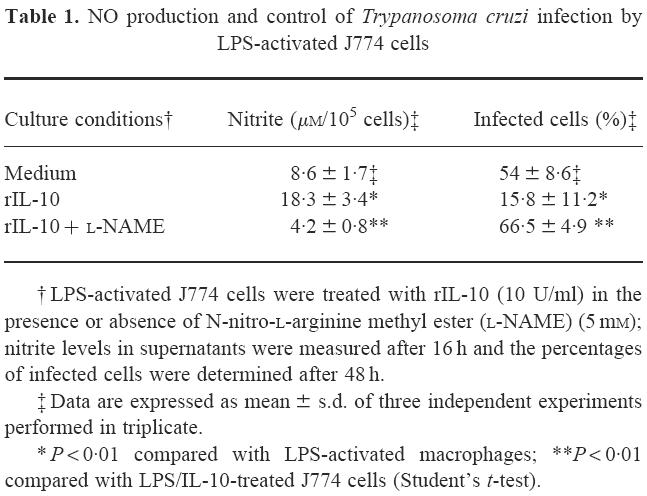
† LPS-activated J774 cells were treated with rIL-10 (10 U/ml) in the presence or absence of N-nitro-l-arginine methyl ester (l-NAME) (5 mm); nitrite levels in supernatants were measured after 16 h and the percentages of infected cells were determined after 48 h.
‡ Data are expressed as mean ± s.d. of three independent experiments performed in triplicate.
* P < 0.01 compared with LPS-activated macrophages; **P < 0.01 compared with LPS/IL-10-treated J774 cells (Student's t-test).
LPS-induced NO production by MPM was partially due to endogenous IL-10
In the next series of experiments, we analysed the effect of rIL-10 on MPM activated with LPS (1 μg/ml) and simultaneously infected with T. cruzi trypomastigotes. On these cells, rIL-10 (10 U/ml) did not significantly influence either NO production measured in culture supernatants after 16 h (11.2 ± 2.2 μmversus 13.2 ± 2.9 μm in absence of rIL-10) or the percentages of T. cruzi-infected cells determined after 48 h (36.8 ± 9.8% versus 21.1 ± 6.3% in absence of rIL-10). The difference between the responses of J774 cells and peritoneal macrophages to rIL-10 led us to compare their production of cytokines upon LPS activation. After 16 h of LPS stimulation (1 μg/ml), J774 cells did not produce detectable levels of IL-10 but produced high levels of TNF-α (90.1 ± 8.9 ng/ml), whereas significant levels of IL-10 (3.5 ± 0.2 U/ml) and low levels of TNF-α (5.2 ± 0.8 ng/ml) were detected in supernatants of peritoneal macrophages. This led us to consider that endogenous IL-10 might control NO production in peritoneal macrophages. Indeed, we found that rIL-10 induced a major increase in peritoneal cells from IL-10-deficient mice, whereas it had no significant effect on peritoneal cells from wild-type mice of the same background (C57Bl/6) (Fig. 4).
Fig. 4.
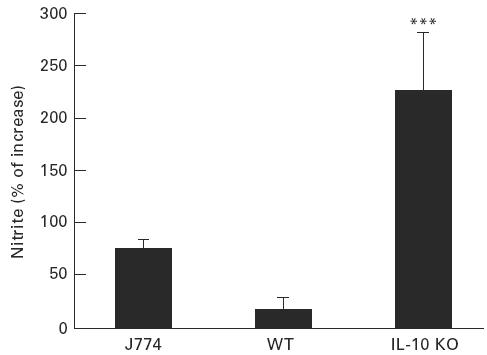
rIL-10 up-regulates NO production by peritoneal macrophages of IL-10-deficient but not wild-type mice. J774 cells and peritoneal macrophages from either IL-10-deficient mice (IL-10 KO) or wild-type control C57Bl/6 mice (WT) were treated as described in the legend of Fig. 3 in absence or presence of rIL-10 (10 U/ml); nitrite levels were measured in culture supernatants after 16 h. Results are expressed as percentages of increase compared with macrophages not treated with rIL-10 (mean ± s.d. of three independent experiments performed in triplicate). ***P < 0.001 compared with macrophages from wild-type mice (Student's t-test).
Finally, we further analysed the regulatory role of endogenous IL-10 on NO production by neutralizing endogenous IL-10 produced by normal peritoneal macrophages. As shown in Fig. 5, addition of a neutralizing anti-IL-10 MoAb (JES5-2A5; 10 μg/ml) to peritoneal macrophages incubated with LPS (1 μg/ml) and infected with T. cruzi significantly reduced their NO production and their ability to control T. cruzi infection, whereas an irrelevant isotype-matched MoAb had no effect on either parameter.
Fig. 5.
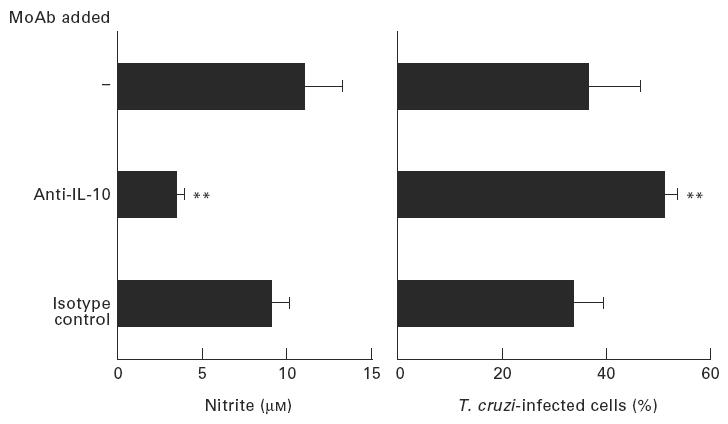
Effects of endogenous IL-10 neutralization in LPS-activated peritoneal macrophages infected with Trypanosoma cruzi. Mouse peritoneal macrophages from wild-type C57Bl/6 mice were activated with LPS (1 μg/ml), infected with T. cruzi and cultured either in medium alone or in the presence of either neutralizing anti-IL-10 MoAb (JES5-2A5; 10 μg/ml) or the same concentration of its isotype-matched control (LO-DNP-2; 10 μg/ml). Nitrite levels were determined in culture supernatants after 16 h and the percentages of infected macrophages were determined after 48 h. Data are shown as mean ± s.d. of triplicate determinations in three different experiments. **P < 0.01 compared with isotype control MoAb (Student's t-test).
DISCUSSION
Although the deactivating effects of IL-10 on monocytes/macrophages and its ability to inhibit Th1-type responses are well established, there is growing evidence that IL-10 cannot be simply considered as an anti-inflammatory and immunosuppressive cytokine. As a matter of fact, IL-10 was found to enhance cytolytic activities of CD8+ cells and natural killer (NK) cells and to favour antibody-dependent cellular cytotoxicity [18–22].
It appears that the effects of IL-10 on NO production are highly dependent on the experimental system considered, as both suppression and up-regulation of NO production by IL-10 were reported in previous studies. Indeed, although IL-10 down-regulates the production of NO by IFN-γ-activated macrophages, it increases NO production when macrophages are activated with IFN-γ and TNF-α [23], and also when macrophages are treated by IL-10 during 3 days and then activated by high doses of LPS [24]. In the present study, we found that exogenously added as well as endogenously produced IL-10 up-regulated NO production by LPS-activated macrophages. In both settings, the ability of macrophages to control T. cruzi infection was enhanced as a consequence of the increased NO synthesis.
Our observations that both exogenous and endogenous IL-10 are protective in an in vitro model of T. cruzi infection of LPS-activated macrophages are in contrast with other in vitro studies showing that IL-10 impairs the macrophage defences against intracellular microorganisms in the case of IFN-γ activation. As for in vitro studies, the role of IL-10 during in vivo infections is not clear. Although IL-10 was shown to promote the outgrowth of Streptococcus pneumoniae [25], group B Streptococcus [26], Chlamydia trachomatis [27], Klebsiella pneumoniae [28], Salmonella [29], Candida [30,31], Legionella pneumophila [32], Mycobacterium avium [33,34], a protective effect of IL-10 was recently described in Pseudomonas aeruginosa pneumonia [35]. The role of endogenous IL-10 in the course of experimental T. cruzi infections was investigated in IL-10 knock-out mice: IL-10-deficient mice had lower parasite burden than their wild-type counterparts but their mortality rate was higher, most likely because of the toxicity associated with the overproduction of IL-12, IFN-γ and TNF-α [36,37]. Similar results have been observed in Toxoplasma [38,39] and P. chabaudi [40] infections. Moreover, in vivo injection of rIL-10 in T. cruzi-infected mice resulted in higher levels of parasitaemia, but did not affect either the mortality rate or the histopathology [40].
In parallel with its stimulating effect on the production of inflammatory mediators, administration of LPS during T. cruzi infection resulted in early mortality [41,42]. In this setting, rIL-10 administration might not only protect against the acute cytokine-mediated pathology as indicated by previous studies in experimental endotoxaemia [12,43], but also promote parasite clearance by enhancing NO synthesis, as suggested by the in vitro observations reported in the present study.
In conclusion, we demonstrated that both exogenous and endogenous IL-10 up-regulate the production of NO by LPS-activated macrophages, which results in a better control of T. cruzi infection. These data confirm that IL-10 can up-regulate or down-regulate NO production by macrophages according to the experimental model.
Acknowledgments
This work was supported by the Communauté Française de Belgique through an Action de Recherche Concertée and by an Interuniversity Poles of Attraction Programme, Belgian State, Prime Minister's Office, Federal Office for Scientific, Technical and Cultural Affairs.
REFERENCES
- 1.MacMicking J, Xie Q-W, Nathan C. Nitric oxide and macrophage function. Ann Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 2.James SL. Role of nitric oxide in parasitic infections. Microbiol Rev. 1995;59:533–47. doi: 10.1128/mr.59.4.533-547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton TA, Adams DO. Molecular mechanisms of signal transduction in macrophages. Immunol Today. 1987;8:151–8. doi: 10.1016/0167-5699(87)90145-9. [DOI] [PubMed] [Google Scholar]
- 4.Jorens PG, Matthys KE, Bult H. Modulation of nitric oxide synthase activity in macrophages. Med Inflammation. 1995;4:75–89. doi: 10.1155/S0962935195000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–55. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Waal Malefyt R, Abrams J, Bennett B, et al. Interleukin 10 inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1992;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesrown SE, Monnier J, Visner G, et al. Regulation of inductible nitric oxide synthase mRNA levels by LPS, IFN-γ, TGF-β, and IL-10 in murine macrophage cell lines and rat peritoneal macrophages. Biochem Biophys Res Commun. 1994;200:126–34. doi: 10.1006/bbrc.1994.1424. [DOI] [PubMed] [Google Scholar]
- 8.Loftis LL, Meals EA, English BK. Differential effects of pentoxifylline and interleukin-10 on production of tumor necrosis factor and inductible nitric oxide synthase by murine macrophages. J Infect Dis. 1997;175:1008–11. doi: 10.1086/513960. [DOI] [PubMed] [Google Scholar]
- 9.Perretti M, Szabo C, Thiemermann C. Effect of interleukin-4 and interleukin-10 on leucocyte migration and nitric oxide production in the mouse. Br J Pharmacol. 1995;116:2251–7. doi: 10.1111/j.1476-5381.1995.tb15061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg DJ, Kuhn R, Rajewsky K, et al. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96:2339–47. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nemoto Y, Otsuka T, Niiro H, et al. Inhibitory effects of interleukin-10 and viral IL-10 on the functions of monocytes/macrophages. Nihon Rinsho Meneki Gakkai-Kaishi. 1995;18:152–9. doi: 10.2177/jsci.18.152. [DOI] [PubMed] [Google Scholar]
- 12.Gérard C, Bruyns C, Marchant A, et al. Interleukin-10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–50. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosmann TR, Scumacher JH, Fiorentino DF, et al. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2 specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990;145:38–45. [PubMed] [Google Scholar]
- 14.Olivares FE, Heirman C, Thielemans K, et al. Granulocyte-macrophage colony-stimulating factor: involvement in control of Trypanosoma cruzi infection in mice. Infect Immun. 1996;64:3429–34. doi: 10.1128/iai.64.8.3429-3434.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Bouhdidi A, Truyens C, Rivera M-T, et al. Trypanosoma cruzi infection in mice induces a polyisotypic hypergammaglobulinaemia and parasite-specific response involving high IgG2a concentrations and highly avid IgG1 antibodies. Parasite Immunol. 1994;16:69–76. doi: 10.1111/j.1365-3024.1994.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 16.Dentener MA, Greve JW, Maessen JG, et al. Role of tumor necrosis factor in the phenomenon of the enhanced sensitivity of animals to endotoxin after exposition to lead. Immunopharmacol Immunotoxicol. 1989;11:321–4. doi: 10.3109/08923978909005373. [DOI] [PubMed] [Google Scholar]
- 17.Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 18.Chen WF, Zlotnik A. IL-10: a novel cytotoxic T cell differentiation factor. J Immunol. 1991;147:528–34. [PubMed] [Google Scholar]
- 19.Carson WE, Lindemann MJ, Baiocchi R, et al. The functional characterization of interleukin-10 receptor expression on human natural killer cells. Blood. 1995;85:3577–85. [PubMed] [Google Scholar]
- 20.Yang G, Hellström KE, Mizuno MT, et al. In vitro priming of tumor-reactive cytolytic T lymphocytes by combining IL-10 with B7-CD28 costimulation. J Immunol. 1995;155:3897–903. [PubMed] [Google Scholar]
- 21.Zheng LM, Ojcius DM, Garaud F, et al. Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism. J Exp Med. 1996;184:579–84. doi: 10.1084/jem.184.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.te Velde AA, de Waal Malefijt R, Huijbens RJF, et al. IL-10 stimulates monocytes FcγR surface expression and cytotoxic activity. J Immunol. 1992;149:4048–52. [PubMed] [Google Scholar]
- 23.Corradin SB, Fasel N, Buchmüller-Rouiller Y, et al. Induction of macrophage nitric oxide production by interferon-γ and tumor necrosis factor-α is enhanced by interleukin-10. Eur J Immunol. 1993;23:2045–8. doi: 10.1002/eji.1830230851. [DOI] [PubMed] [Google Scholar]
- 24.Appelberg R. Opposing effects of interleukin-10 on mouse macrophage functions. Scand J Immunol. 1995;41:539–44. doi: 10.1111/j.1365-3083.1995.tb03605.x. [DOI] [PubMed] [Google Scholar]
- 25.van der Poll T, Marchand A, Keogh CV, et al. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J Infect Dis. 1996;174:994–1000. doi: 10.1093/infdis/174.5.994. [DOI] [PubMed] [Google Scholar]
- 26.Cusumano V, Genovese F, Mancuso G, et al. Interleukin-10 protects neonatal mice from lethal group B streptococcal infection. Infect Immun. 1996;64:2850–2. doi: 10.1128/iai.64.7.2850-2852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, HayGlass KT, Brunham RC, et al. Genetically determined differences in IL-10 and IFN-γ responses correlate with clearance of Chlamydia trachomatis mouse peritonitis infection. J Immunol. 1996;156:4338–44. [PubMed] [Google Scholar]
- 28.Greenberger MJ, Strieter RM, Kunkel SL, et al. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995;155:722–9. [PubMed] [Google Scholar]
- 29.Arai T, Hiromatsu K, Nishimura H, et al. Effects of in vivo administration of anti-IL-10 monoclonal antibody on the host defence mechanism against murine Salmonella infection. Immunology. 1995;85:381–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Tonetti L, Spaccapelo R, Cenci E, et al. Interleukin-4 and -10 exacerbate candidiasis in mice. Eur J Immunol. 1995;25:1559–65. doi: 10.1002/eji.1830250614. [DOI] [PubMed] [Google Scholar]
- 31.Romani L, Pucetti P, Mencacci A, et al. Neutralization of IL-10 up-regulates nitric oxide production and protects susceptible mice from challenge with Candida albicans. J Immunol. 1994;152:3514–21. [PubMed] [Google Scholar]
- 32.Park DR, Skerrett SJ. IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-γ: differential responses of blood monocytes and alveolar macrophages. J Immunol. 1996;157:2528–38. [PubMed] [Google Scholar]
- 33.Denis M, Ghadirian E. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J Immunol. 1993;151:5425–30. [PubMed] [Google Scholar]
- 34.Bermudez LE, Champsi J. Infection with Mycobacterium avium induces production of interleukin-10, and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993;61:3093–7. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawa T, Corry DB, Gropper MA, et al. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J Immunol. 1997;159:2858–66. [PubMed] [Google Scholar]
- 36.Hunter CA, Ellis-Neyes LA, Slifer T, et al. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol. 1997;158:3311–6. [PubMed] [Google Scholar]
- 37.Abrahamsohn IA, Coffman RL. Trypanosoma cruzi: IL-10, TNF, IFN-γ, and IL-12 regulate innate and acquired immunity to infection. Exp Parasitol. 1996;84:231–44. doi: 10.1006/expr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 38.Neyer LE, Grunig G, Fort M, et al. Role of interleukin-10 in regulation of T-cell-dependent and T-cell-independent mechanisms of resistance to Toxoplasma gondii. Infect Immun. 1997;65:1675–82. doi: 10.1128/iai.65.5.1675-1682.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gazzinelli RT, Wysocka M, Hieny S, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, and TNF-α. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 40.Linke A, Kuhn R, Muller W, et al. Plasmodium chabaudi chabaudi: differential susceptibility of gene-targeted mice deficient in IL-10 to an erythrocytic-stage infection. Exp Parasitol. 1996;84:253–63. doi: 10.1006/expr.1996.0111. [DOI] [PubMed] [Google Scholar]
- 41.Bambirra EA, da Cruz MQ, Campos DS, et al. Some characteristics of the hyperreactivity to bacterial lipopolysaccharide induced in mice by Trypanosoma cruzi infection. Mem Inst Oswaldo Cruz. 1994;79:433–7. doi: 10.1590/s0074-02761984000400006. [DOI] [PubMed] [Google Scholar]
- 42.Kierszenbaum F, Saavedra LE. The effects of bacterial endotoxin on the infection of mice with Trypanosoma cruzi. Protozool. 1972;19:655–7. doi: 10.1111/j.1550-7408.1972.tb03552.x. [DOI] [PubMed] [Google Scholar]
- 43.Howard M, Muchamuel T, Andrade S, et al. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993;177:1205–8. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]


