Abstract
Over-expression of iNOS is implicated in the pathogenesis of glomerulonephritis in animal models of systemic lupus erythematosus. The aim of this study was to evaluate the effect of aminoguanidine, a selective inhibitor of iNOS, for the protection from glomerulosclerosis in NZB/W F1 mice. Female NZB/W F1 mice (n = 8) were treated with aminoguanidine (1 g/l) in drinking water for 4 months starting at age 2 months before the onset of glomerulonephritis. Controls were age- and sex-matched mice (n = 10) without aminoguanidine treatment. By glomerular microdissection and reverse-transcription competitive polymerase chain reaction, we found that glomerular iNOS/β-actin and TGF-β1/β-actin mRNA ratios were reduced 15.1% (P < 0.05) and 61.3% (P < 0.01), respectively, in aminoguanidine-treated mice. Aminoguanidine significantly reduced the glomerular iNOS staining, urinary nitrite production and degree of glomerulosclerosis. In addition, the glomerular volume and mean glomerular cell number were reduced 33.2% (P < 0.01) and 32.8% (P < 0.01), respectively. Likewise, the urinary proteinuria was also significantly reduced by aminoguanidine. These results indicate that administration of aminoguanidine may reduce the progression of glomerulosclerosis in NZB/W F1 mice, possibly through inhibition of glomerular nitric oxide production.
Keywords: aminoguanidine, inducible nitric oxide synthase, transforming growth factor-beta, glomerulosclerosis
INTRODUCTION
Nitric oxide, synthesized enzymatically by a family of nitric oxide synthases (NOS) from the amino acid l-arginine, is implicated as a messenger molecule in a large number of physiologic and pathologic responses. Over-production of nitric oxide plays an important role in mediating various autoimmune diseases, inflammatory diseases, septic shock, transplant rejection and stroke [1–3]. In kidney, a constitutive nitric oxide release within the glomerular vasculature may be protective by decreasing glomerular capillary pressure and enables autoregulation of renal blood flow [4]. However, in pathological conditions, local generation of high nitric oxide output induced by various cytokines through the expression of the calcium-independent, cytokine-inducible form of nitric oxide synthase (iNOS) may result in damaging effects. Generated by activated monocyte/macrophages, vascular smooth muscle cells and glomerular mesangial cells, increased levels of nitric oxide may cause injury to the glomerular mesangium [5–8] through the production of peroxynitrite and reactive oxygen species [9,10]. Accumulating evidence suggests that glomerular induction of iNOS mediates glomerular cell injury in experimental glomerulonephritis [11] and in immune complex glomerulonephritis in human [12].
The development of drugs to suppress iNOS expression or inhibit its activity provides a novel therapeutic approach for a diverse set of dysfunctions, including asthma, inflammatory processes, and autoimmune diseases. Administration of NOS inhibitors, NG-monomethyl-l-arginine (NMMA) and aminoguanidine, delayed the onset of diabetes in the spontaneously diabetic BB rat [13]. Inhibition of nitric oxide provides protection against immune complex-induced vascular injury [14]. Selective iNOS blockade by infusing antisense oligonucleotides inhibited the cytotoxic effects of nitric oxide in ischaemic acute renal failure [15]. Aminoguanidine, a selective inducible NOS inhibitor, ameliorates experimental autoimmune encephalomyelitis in SJL mice [16] and reduces glomerular damage in experimental anti-glomerular basement membrane glomerulonephritis [17]. These studies suggest that nitric oxide is an important mediator in autoimmune diseases and modification of nitric oxide production by NOS inhibition may influence the autoimmune disease process.
NZB/W and MRL-lpr mice are murine models for systemic lupus erythematosus (SLE) and lupus nephritis. Nitric oxide production and iNOS expression were increased in MRL-lpr mice and administration of NMMA, a non-selective inhibitor of NOS, could reduce the severity of spontaneous immune complex glomerulonephritis [18]. A recent study further showed that administration of NMMA at a later stage following onset of proteinuria in NZB/W F1 mice reduced proteinuria and glomerular pathology [19]. Besides iNOS, NMMA also inhibits neuronal and endothelial constitutive NOS (cNOS), thus potentially affecting cell–cell communication, vascular tone, and neuro-transmission. Aminoguanidine provides a relatively selective inhibition of iNOS that is 10- to 100-fold more effective in inhibiting iNOS than cNOS [20]. We therefore hypothesize that selective inhibition of iNOS could affect the clinical outcome of lupus nephritis. The aim of this study was to evaluate the effect of long-term administration of aminoguanidine in the expression of glomerular iNOS and TGF-β1 mRNA and the protective effects against glomerulosclerosis in NZB/W F1 mice.
MATERIALS AND METHODS
Animals
Female NZB/W F1 mice, bred in the National Taiwan University Animal Centre, were treated with aminoguanidine (1 g/l) (n = 8) in daily changed drinking water for 4 months starting at age 2 months before the onset of glomerulonephritis. Controls were age- and sex-matched mice (n = 10) without aminoguanidine treatment. Mice were fed ad libitum. The food consumption was not different between the two groups of mice. Before sacrifice, serum and spot urine were collected for measurement of serum creatinine and urinary protein:creatinine ratio. At sacrifice, right kidneys were collected for glomerular microdissection to study glomerular gene expression. The left kidneys were saved for morphologic and morphometric studies. The care and the disposal of the animals followed the Guidelines of Animal Study by National Institutes of Health, Bethesda, MD.
Glomerular microdissection and in situ reverse transcription
After anaesthesia with sodium pentobarbital (50 mg/g body weight, intraperitoneally), mice were killed by heart puncture. A piece of kidney was digested with saline containing collagenase and RNase inhibitors at 37°C for 30 min. Glomeruli were isolated by microdissection under dissecting microscope and glomerular cDNA was obtained following in situ reverse transcription of mRNA as described [21].
Primer construction and competitive polymerase chain reaction
Primers
The TGF-β1 primer pair consisted of sense 5′ ATA CAG GGC TTT CGA TTC AGC 3′, and antisense 5′ GTC CAG GCT CCA AAT ATA GG 3′, and yielded a 360-bp polymerase chain reaction (PCR) product. The iNOS primer pair was 5′ TGC ATG GAC CAG TAT AAG GCA AGC 3′ (sense) and 5′ CTC CTG CCC ACT GAG TTC GTC 3′ (antisense), yielding a 288-bp PCR product. The β-actin primer pair was 5′ TCT AGG CAC CAA GGT GTG 3′ (sense) and 5′ TCA TGA GGT AGT CCG TCA GG 3′ (antisense), yielding a 460-bp PCR product.
As described [21–24], these primers were chosen so that only specific mRNA-derived cDNA sequences, but not genomic DNA sequences, would be amplified. Restriction enzyme analysis was performed on each of the PCR products to confirm the amplification of specific mRNA sequences.
Competitive PCR assay
The mutated cDNA templates competed with test cDNA on an equimolar basis, as described [21–24]. For TGF-β1, iNOS and β-actin, deletion cDNA mutant templates were developed to create 58-, 60- and 103-bp deletions in the middle of the molecules, resulting in mutant cDNAs of 282, 228 and 357 bp, respectively.
To quantify cDNAs, competitive PCR assays were performed by adding decreasing amounts of mutant templates to glomerular cDNA (Fig. 1). The test template for all PCR reactions was an aliquot of cDNA collected from a pool of 50 glomeruli. Each aliquot corresponded to 1/10 of a glomerulus. Following agarose gel electrophoresis, amplification bands stained by ethidium bromide were quantified from the film negative by scanning densitometry. As reported, the ratio of mutant to wild type band density was calculated for each lane and plotted as a function of the amount of initial mutant template added to the reaction. The amount of glomerular cDNA was derived from linear regression analysis with duplicate or triplicate assays. The mean values for glomerular cDNA levels were expressed as a percentage of that of control mice.
Fig. 1.
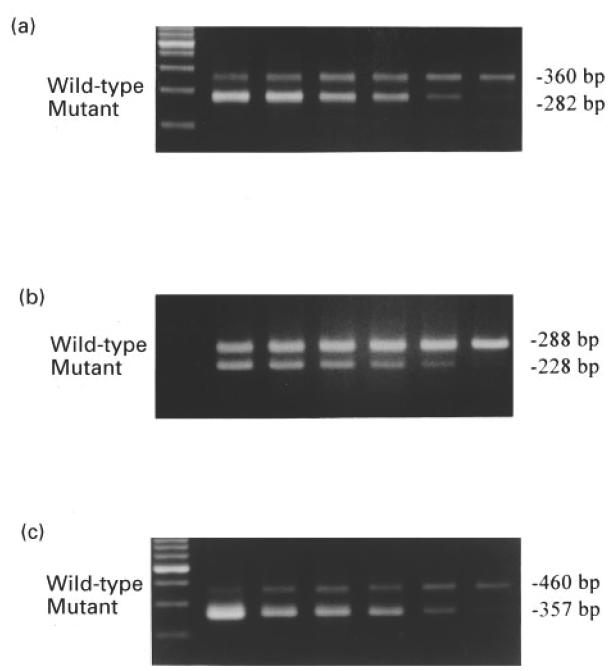
Reverse transcriptase (RT)-competitive polymerase chain reaction (PCR) of glomerular (a) TGF-β1, (b) iNOS, (c) β-actin mRNA. To quantify the cDNA derived from glomerular mRNA, competitive PCR assays were performed by adding decreasing amounts of mutant templates to glomerular wild-type test cDNA. The TGF-β1, iNOS and β-actin primers amplified a specific PCR product of 360 bp, 288 bp and 460 bp, respectively. Mutants for TGF-β1, iNOS and β-actin were generated at 282 bp, 228 bp and 357 bp, respectively.
Light microscopy
Coronal kidney sections were fixed in Carnoy's fixative and embedded in paraffin. Sections (4 μm) were stained with haematoxylin–eosin (H–E), periodic acid-Schiff (PAS) and examined without knowledge of the experimental groups. The degree of mesangial sclerosis was assessed using a scale from 0 to 4 + from 50 glomeruli in each mouse kidney, as described previously [25].
Immunohistochemistry for detecting iNOS in the glomerulus
Paraffin-embedded kidney sections (4 μm) were heated at 60°C for 35 min and deparaffinized by 100% xylene at 60°C for another 35 min. Sections were then coated with polyclonal rabbit anti-iNOS antibody (ABR Inc., Gloden, CO) followed by biotin-conjugated goat anti-rabbit IgG (Tago Immunologicals, Carmarillo, CA) and ABC reagent (Vector Labs, Burlingame, CA). Inducible NOS protein was detected by adding AEC substrate (Dako, Glostrup, Denmark), which revealed red-brownish colour, and counter-stained by methylene green. The sections were examined, coded, and the glomerular staining intensity was graded on a 0–4 + scale from 50 glomeruli in each mouse kidney as described [25].
Glomerular volume
The profile areas of 100 glomeruli were measured with a computer-assisted Windows-based image analysis software (Optimas version 6.1; Optimas Corp., Bothell, WA) for morphometric analysis and the mean glomerular volume was derived from harmonic mean of glomerular equatorial surface area as described [25].
Mean glomerular cell number
The mean glomerular cell number was determined as described [23]. Briefly, the nuclei of 100 successive glomerular profiles were counted by scanning H–E-stained tissue sections in a serpentine fashion. Taking into account the mean cell number per glomerular profile (C), the simple mean of the glomerular equatorial area (A), and glomerular volume (GV), the mean glomerular cell number (N) was derived by the following equation: N = (C/A) ×GV. The mean of these values in aminoguanidine-treated mice was expressed as a percentage of the mean values in control mice.
Serum creatinine and urine protein/creatinine ratio
Creatinine in serum and urine was determined by a quantitative colourimetric method (Sigma Diagnostic, St Louis, MO). Urine protein was determined by semiquantitative dipstick method (Uropaper, Eiken Chemical, Tokyo, Japan).
Urine nitrite/creatinine ratio
Urine nitrite/creatinine ratios were used for monitoring urinary nitric oxide excretion. Nitrite (NO2−) in the urine was determined by the Griess reagent and was taken as an index of nitric oxide production as described [26]. Briefly, Griess reagent was added to a 150-μl urine sample. The Griess reagent comprised equal amounts of 0.1% naphthylethylenediamine dihydrochloride and 1% sulfanilamide in 5% concentrated H3PO4. The reaction was performed when the Griess reagent reacted with nitrite in the urine sample which formed a pink to dark colour after incubation for 15 min, and was read by a spectrophotometer at 546 nm. NaNO2 was included as standard.
Statistical analysis
Differences between groups were analysed by unpaired Student's t-test. Differences in glomerular sclerosis and staining index were analysed by the Wilcoxan non-parametric test. All values were expressed as means ± s.e.m. Statistical significance was set at P < 0.05.
RESULTS
Glomerular mRNAs
Glomerular mRNAs for iNOS, TGF-β1 and β-actin were measured by reversed transcription-competitive PCR (Fig. 1), and the results were expressed as percentage change of ratio of iNOS/β-actin and TGF-β1/β-actin mRNA. Administration of aminoguanidine to NZB/W F1 mice for 4 months showed a 15.1% reduction (84.9 ± 3.8%) of iNOS/β-actin mRNA ratio when compared with controls (100 ± 2.8%, P < 0.05) (Fig. 2a). Furthermore, a 61.3% reduction of mean glomerular TGF-β1/β-actin mRNA ratio (38.7 ± 11.3%) was found in aminoguanidine-treated mice when compared with control mice (100 ± 14.8%, P < 0.01) (Fig. 2b).
Fig. 2.
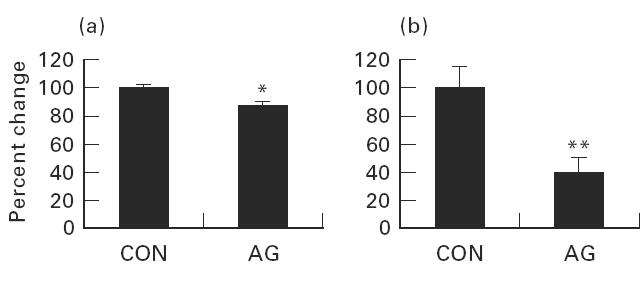
Glomerular iNOS/β-actin and TGF-β1/β-actin mRNA ratio changes. (a) A 15.1% reduction of mean glomerular iNOS:β-actin mRNA ratio in aminoguanidine-treated NZB/W F1 mice. (b) A 63.6% reduction of mean glomerular TGF-β1:β-actin mRNA ratio in aminoguanidine-treated mice compared with control mice. CON, Control NZB/W F1 mice; AG, aminoguanidine-treated mice. *P < 0.05; **P < 0.01.
Immunohistochemistry
In the control mice, iNOS protein accumulates in the glomerular mesangium and in the tubules (Fig. 3). A semiquantitative scoring system was applied for glomerular staining intensity of iNOS. Aminoguanidine treatment significantly reduced the amount of iNOS staining in the glomerular mesangium (1.9 ± 0.07 versus 0.2 ± 0.04; P < 0.01) (Fig. 3). The tubular staining of iNOS was not different between the control and aminoguanidine-treated groups. In control mice the percentage of glomeruli with staining indices of grade 4, 3, 2, 1 and 0 were 0%, 16.3%, 59.3%, 23.3% and 1.2%, respectively. In aminoguanidine-treated mice, the percentages of glomeruli for staining index were 0% for grades 4, 3 and 2. Most of the glomeruli were grade 0 (82.6%) and grade 1 (17.4%).
Fig. 3.
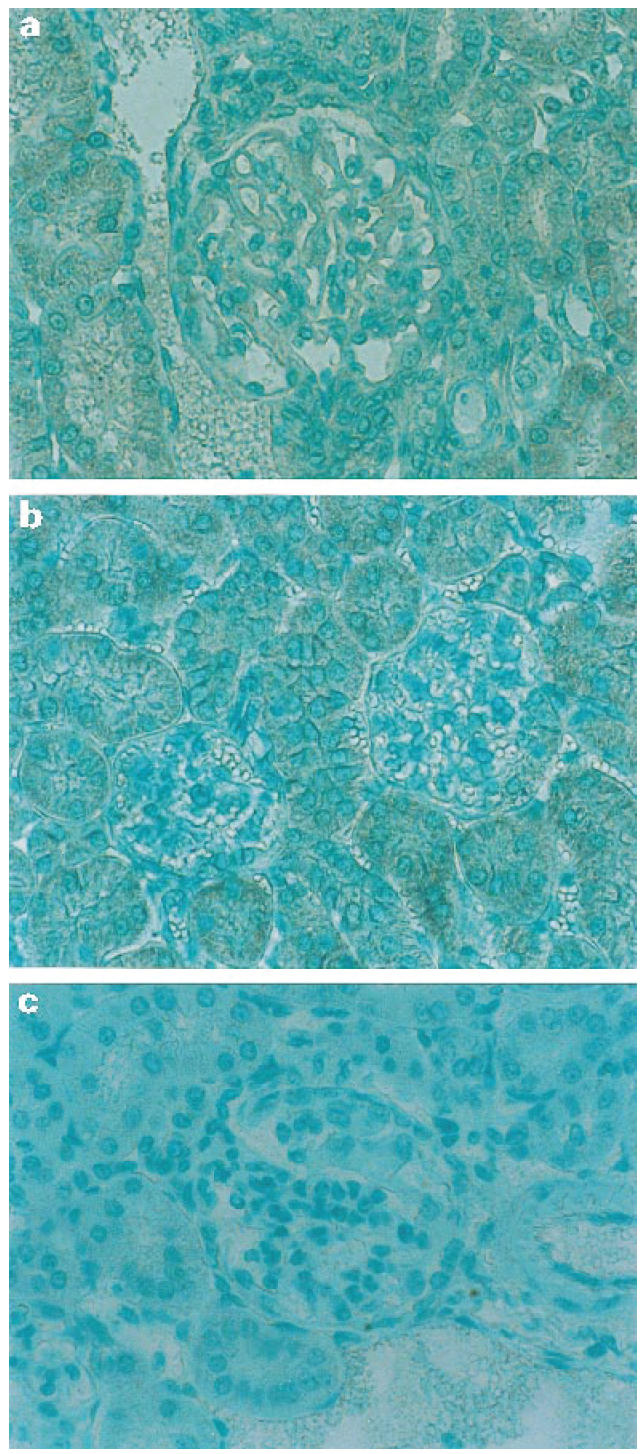
Immunohistochemistry of iNOS detection in the glomerulus. Aminoguanidine treatment significantly reduced the amount of iNOS staining in the glomerular mesangium. (a) Control NZB/W F1 mouse. (b) Aminoguanidine-treated NZB/W F1 mouse. (c) Negative control without adding anti-iNOS antibody in a control NZB/W F1 mouse.
Light microscopy
When analysed by light microscopy of the glomerular histology, mean score of glomerulosclerosis was found significantly reduced in aminoguanidine-treated mice (2.1 ± 0.06 versus 0.5 ± 0.04; P < 0.001) (Fig. 4). The percentages of sclerotic glomeruli were more evident in the control mice. In control mice the percentages of glomeruli with sclerosis indices of grade 4, 3, 2, 1 and 0 were 10.3%, 23.9%, 32.6%, 27.6% and 5.3%, respectively. In aminoguanidine-treated mice the percentages of glomeruli for sclerosis index were none for grades 4 and 3. Most of the glomeruli were grade 0 (60.9%), grade 2 (8.6%) and grade 1 (30.8%).
Fig. 4.
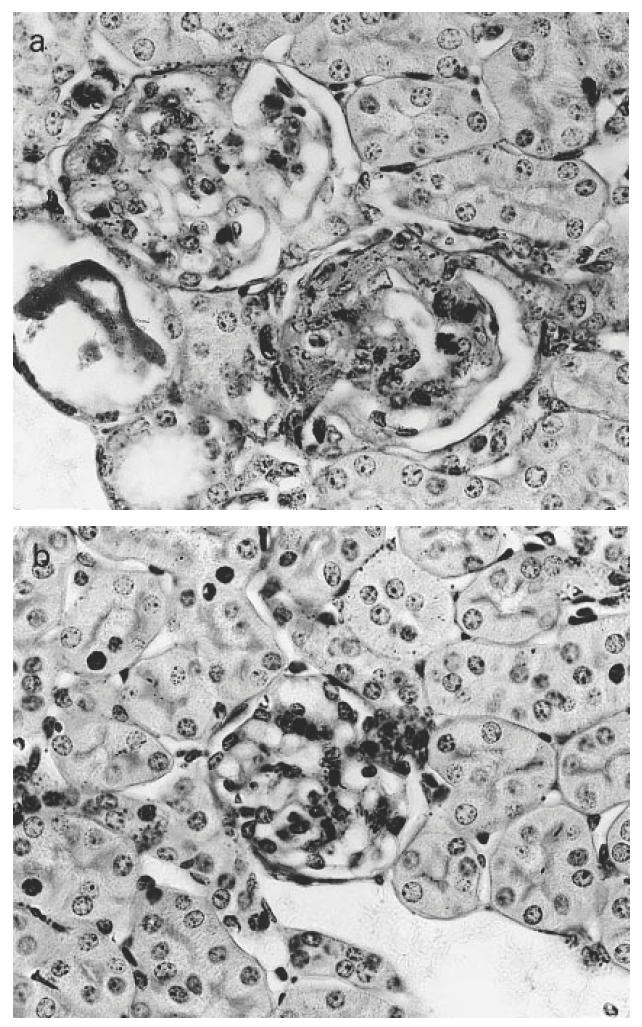
Representative photographs of periodic acid-Schiff-stained glomeruli from (a) a control NZB/W F1 mouse, (b) an aminoguanidine-treated NZB/W F1 mouse. Note that glomerulosclerosis is less marked in the aminoguanidine-treated mouse (original mag. × 200).
Mean glomerular volume
Glomerular hypertrophy was significantly reduced by aminoguanidine. The mean glomerular volume showed a 33.2% reduction in aminoguanidine-treated mice (1.47 ± 0.07 × 105 μm3) compared with controls (2.20 ± 0.18 × 105 μm3; P < 0.001) (Fig. 5a).
Fig. 5.
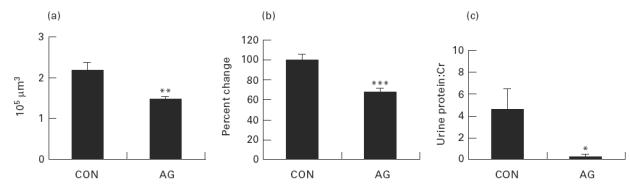
Aminoguanidine treatment in NZB/W F1 mice resulted in (a) a 34% reduction of glomerular volume, (b) a 22.8% reduction of mean glomerular cell number, and (c) significantly lower levels of urine protein:creatinine ratio compared with controls. *P < 0.05; **P < 0.001; ***P < 0.005.
Mean glomerular cell number
The mean glomerular cell number was 32.8% (P < 0.005) reduced in the aminoguanidine-treated mice (67.2 ± 3.9%) when compared with controls (100 ± 6.12%; P < 0.005) (Fig. 5b).
Serum creatinine and urine protein/creatinine ratio
The serum creatinine levels were in normal range, and similar in the two groups of mice (0.82 ± 0.07 mg/dl in control mice and 0.79 ± 0.07 mg/dl in aminoguanidine-treated mice; P = 0.513). The urine protein:creatinine ratio was 4.61 ± 1.85 mg/mg in control mice; aminoguanidine treatment significantly reduced the ratio to 0.25 ± 0.03 mg/mg (P < 0.05) (Fig. 5c).
Urine nitrite:creatinine ratio
The urine nitrite:creatinine ratio was 99.9 ± 16.5 μg/mg in control mice; aminoguanidine treatment significantly reduced the ratio to 58.4 ± 13.0 μg/mg (P < 0.05).
Kidney and body weight
No statistically significant differences of body and kidney weight changes were found in the mice studied. Body weight in controls was 37.5 ± 3.3 g and in aminoguanidine-treated mice was 33.5 ± 2.0 g (P = 0.403). Kidney weight was 0.26 ± 0.02 g in controls and 0.24 ± 0.02 g in aminoguanidine-treated mice (P = 0.541).
DISCUSSION
Selective inhibition of iNOS by aminoguanidine to reduce the production of nitric oxide alleviated the proliferative glomerular responses and diminished glomerulosclerosis in experimental lupus nephritis as shown in the present study. Non-specific inhibition of NOS by NMMA has been shown effective in reducing the severity of glomerulonephritis in MRL-lpr and NZB/W F1 mice [18,19]. Our study, for the first time, demonstrates that selective inhibition of iNOS by aminoguanidine diminishes glomerulosclerosis in NZB/W F1 mice. The reduction of glomerulosclerosis is possibly through down-regulation of iNOS. Although the reduction of glomerular iNOS mRNA was small in amount, the reduction of protein levels was significant, as demonstrated by immunohistochemistry and in urine as reflected by lower nitrite excretion. As suggested by crystal structural analysis recently [27], aminoguanidine inhibits iNOS activity via binding to its active centre, indicating that the inhibition may act at post-translational rather than at transcriptional levels. Aminoguanidine treatment did not affect iNOS mRNA synthesis or intragraft nitric oxide production, but decreased iNOS immunoreactivity in smooth muscle cells of a heterotopic rat tracheal allograft rejection model [28]. In our study, aminoguanidine treatment may have resulted in reduced iNOS activity, reduced nitric oxide production and diminished glomerular injury, which further, in turn, down-regulated glomerular TGF-β1 expression and glomerulosclerosis. The reduction of glomerular iNOS both at mRNA and protein levels suggests that the down-regulation of iNOS may possibly act through autocrine or paracrine inhibition when iNOS activity is inhibited. These observations confirm our hypothesis that nitric oxide, particularly produced by iNOS, has an important impact on the progression of immune complex nephritis in the lupus mouse model.
Immune complex increases nitric oxide production in the glomerulus [6,11,18,29]. The over-produced nitric oxide may be damaging via several mechanisms. Nitric oxide, through interaction with superoxide to form peroxynitrite, spontaneously produces hydroxyl radical, which is highly damaging to the cells [9]. Nitric oxide may also interact with iron-containing moieties in key enzymes of the mitochondrial respiratory chain and of DNA synthesis that may be cytotoxic [30,31]. In addition, nitric oxide may augment the inflammatory responses by induction of proinflammatory cytokines, tumour necrosis factor (TNF) and IL-1 [32,33], and modulate inflammatory responses by inhibiting the production of interferon-gamma (IFN-γ) and IL-2 by Th1 cells [34]. The increased nitric oxide synthesis in acute glomerulonephritis in rats resulted in increased levels of nitrite, lysis of glomerular mesangial cells, and accumulation of extracellular matrix [7]. On the other hand, inhibition of nitric oxide synthesis by NMMA reversed some of these effects, preventing cell lysis and extracellular matrix accumulation. These findings suggest that glomerular cell injury and accumulation of extracellular matrix were partly mediated by excessive nitric oxide production.
TGF-β is a cytokine or signalling molecule with potent fibrogenic properties [35]. It stimulates strongly the deposition of extracellular matrix [36] and is an important mediator of fibroproliferative changes in chronic progressive renal disease [37]. There is a strong positive correlation between the amount of TGF-β1 present in renal biopsy and the degree of glomerulosclerosis in the underlying glomerular disease [23,38,39]. For example, it was found that diffuse proliferative lupus nephritis contained more TGF-β1 mRNA than did normal glomeruli [40]. It implies that treatment modality that reduces TGF-β1 production may alleviate later tissue fibrosis. As shown in this study, the reduction of glomerular iNOS expression is accompanied by a reduction of glomerular TGF-β1 mRNA. To date, there is no direct evidence linking the reduced nitric oxide production to down-regulation of glomerular TGF-β1 mRNA. On the contrary, TGF-β1 may inhibit nitric oxide production through inhibition of iNOS in several cell types, including mesangial cells [41–43]. Given the fact that glomerular expression of TGF-β1 and accumulation of extracellular matrix were suppressed by the NMMA treatment in anti-thymocyte serum-induced glomerulonephritis [7], suppression of over-produced nitric oxide may alleviate fibrogenic activities. The present study demonstrated that NZB/W F1 mice treated with aminoguanidine showed a reduced TGF-β1 and iNOS mRNA expression and less severe glomerulosclerosis.
The reduced expression of glomerular iNOS expression by aminoguanidine is also associated with a reduction of mean glomerular cell number, indicating that a reduced proliferative or infiltrative response may be elicited by aminoguanidine. Glomerular hypertrophy, a common finding accompanied by glomerulosclerosis, was also reduced in aminoguanidine-treated mice in accordance with the diminished severity of glomerulosclerosis. In a functional approach, although the serum creatinine levels were similar in the two groups, the urine protein:creatinine ratios were significantly lower in the aminoguanidine-treated mice. All these findings strongly indicate that aminoguanidine, a selective inhibitor of iNOS expression, may reduce the glomerular responses to immune complex-induced chain reactions in lupus nephritis and protect it from glomerulosclerosis.
In the present study, NZB/W F1 mice were treated with aminoguanidine before the onset of clinical glomerulonephritis at the age of 2 months. Although less severe glomerulosclerosis was observed, aminoguanidine treatment even at this early stage did not completely prevent the progression of lupus nephritis in NZB/W F1 mice. There are several possibilities. First, the production of nitric oxide is not completely inhibited by aminoguanidine at the experimental dose. Second, multiple pathways other than those mediated by nitric oxide are involved in the pathogenesis of lupus nephritis. Third, some substances, cytokines for example, also induce nitric oxide production and are not completely blocked by aminoguanidine. Thus, once the disease process is initiated, the progression is not completely inhibited by aminoguanidine. A combination of these hypotheses seems most likely.
The staining intensity of iNOS protein levels was not different in the tubules in both groups of mice. It may thus indicate that the dose of aminoguanidine used in this study abolishes the production of iNOS in the glomeruli more than in the tubules. The tubular lesions were, however, more severe in the control group than in the aminoguanidine-treated mice (data not shown). Whether the inhibition of tubular expression of iNOS may affect the outcome remains to be elucidated.
Although the inhibition of iNOS is considered specific, aminoguanidine does not act only on iNOS. Unlike NMMA, aminoguanidine inhibits diamine oxidase, aldose reductase, and has an effect in reducing polyamine production [44]. As polyamines are important mediators of cell proliferation and growth, inhibition by aminoguanidine may be beneficial in the progression of lupus nephritis. Aminoguanidine also inhibits non-enzymatic glycation [45] and has been used to prevent diabetic complications. To our knowledge, there is no current evidence to support that polyamines or non-enzymatic glycation pathways play a major role in the progression of lupus nephritis. Therefore, the protective effect of aminoguanidine, as has been demonstrated by other NOS inhibitors, may largely act through the inhibition of excessive glomerular nitric oxide production. However, it may be possible that combined inhibitory effects on nitric oxide and other pathways make aminoguanidine treatment more effective in alleviating glomerulosclerosis in this lupus nephritis model.
In conclusion, our study confirms that aminoguanidine reduces glomerular iNOS and TGF-β1 mRNA expression and diminishes glomerulosclerosis in NZB/W F1 mice. These data suggest that selective inhibition of iNOS may ameliorate lupus glomerulonephritis in the animal model. Further studies are necessary to evaluate the effect of selective iNOS inhibition on the clinical outcome in human lupus nephritis.
Acknowledgments
This study is supported by grants from the National Science Council, Republic of China (NSC87-2314-B182A-040).
REFERENCES
- 1.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–12. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 2.Xie Q, Nathan C. The high-output nitric oxide pathway: role and regulation. J Leukoc Biol. 1994;56:576–82. doi: 10.1002/jlb.56.5.576. [DOI] [PubMed] [Google Scholar]
- 3.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–8. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 4.Romero JC, Strick DM. Nitric oxide and renal function. Curr Opin Nephrol Hypertens. 1993;2:114–21. doi: 10.1097/00041552-199301000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Cattell V, Lianos E, Largen P, Cook T. Glomerular NO synthase activity in mesangial cell immune injury. Exp Nephrol. 1993;1:36–40. [PubMed] [Google Scholar]
- 6.Cook HT, Ebrahim H, Jansen AS, Foster GR, Largen P, Cattell V. Expression of the gene for inducible nitric oxide synthase in experimental glomerulonephritis in the rat. Clin Exp Immunol. 1994;97:315–20. doi: 10.1111/j.1365-2249.1994.tb06087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narita I, Border WA, Ketteler M, Noble NA. Nitric oxide mediates immunologic injury to kidney mesangium in experimental glomerulonephritis. Lab Invest. 1995;72:17–24. [PubMed] [Google Scholar]
- 8.Hruby Z, Beck KF. Cytotoxic effect of autocrine and macrophage-derived nitric oxide on cultured rat mesangial cells. Clin Exp Immunol. 1997;107:76–82. doi: 10.1046/j.1365-2249.1997.d01-906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–4. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci USA. 1996;93:6770–4. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen A, Cook T, Taylor GM, et al. Induction of nitric oxide synthase in rat immune complex glomerulonephritis. Kidney Int. 1994;45:1215–9. doi: 10.1038/ki.1994.161. [DOI] [PubMed] [Google Scholar]
- 12.Kashem A, Endoh M, Yano N, Yamauchi F, Nomoto Y, Sakai H. Expression of inducible-NOS in human glomerulonephritis: the possible source is infiltrating monocytes/macrophages. Kidney Int. 1996;50:392–9. doi: 10.1038/ki.1996.328. [DOI] [PubMed] [Google Scholar]
- 13.Wu G. Nitric oxide synthesis and the effect of aminoguanidine and NG-monomethyl-L-arginine on the onset of diabetes in the spontaneously diabetic BB rat. Diabetes. 1995;44:360–4. doi: 10.2337/diab.44.3.360. [DOI] [PubMed] [Google Scholar]
- 14.Mulligan MS, Moncada S, Ward PA. Protective effects of inhibitors of nitric oxide synthase in immune complex-induced vasculitis. Br J Pharmacol. 1992;107:1159–62. doi: 10.1111/j.1476-5381.1992.tb13423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noiri E, Peresleni T, Miller F, Goligorsky MS. In vivo targeting of inducible NO synthase with oligodeoxynucleotides protects rat kidney against ischemia. J Clin Invest. 1996;97:2377–83. doi: 10.1172/JCI118681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cross AH, Misko TP, Lin RF, Hickey WF, Trotter JL, Tilton RG. Aminoguanidine, an inhibitor of inducible nitric oxide synthase, ameliorates experimental autoimmune encephalomyelitis in SJL mice. J Clin Invest. 1994;93:2684–90. doi: 10.1172/JCI117282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bremer V, Tojo A, Kimura K, et al. Role of nitric oxide in rat nephrotoxic nephritis: comparison between inducible and constitutive nitric oxide synthase. J Am Soc Nephrol. 1997;8:1712–21. doi: 10.1681/ASN.V8111712. [DOI] [PubMed] [Google Scholar]
- 18.Weinberg JB, Granger DL, Pisetsky DS, et al. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-L-arginine. J Exp Med. 1994;179:651–60. doi: 10.1084/jem.179.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oates JC, Ruiz P, Alexander A, Pippen AM, Gilkeson GS. Effect of late modulation of nitric oxide production on murine lupus. Clin Immunol Immunopathol. 1997;83:86–92. doi: 10.1006/clin.1997.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misko TP, Moore WM, Kasten TP, et al. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993;233:119–25. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- 21.Peten EP, Garcia-Perez A, Terada Y, et al. Age-related changes in alpha 1- and alpha 2-chain type IV collagen mRNAs in adult mouse glomeruli: competitive PCR. Am J Physiol. 1992;263:F951–7. doi: 10.1152/ajprenal.1992.263.5.F951. [DOI] [PubMed] [Google Scholar]
- 22.Yang CW, Hattori M, Vlassara H, et al. Overexpression of transforming growth factor-beta 1 mRNA is associated with up-regulation of glomerular tenascin and laminin gene expression in nonobese diabetic mice. J Am Soc Nephrol. 1995;5:1610–7. doi: 10.1681/ASN.V581610. [DOI] [PubMed] [Google Scholar]
- 23.Yang CW, Striker GE, Chen WY, Kopchick JJ, Striker LJ. Differential expression of glomerular extracellular matrix and growth factor mRNA in rapid and slowly progressive glomerulosclerosis: studies in mice transgenic for native or mutated growth hormone. Lab Invest. 1997;76:467–76. [PubMed] [Google Scholar]
- 24.Yang CW, Vlassara H, Peten EP, He CJ, Striker GE, Striker LJ. Advanced glycation end products up-regulate gene expression found in diabetic glomerular disease. Proc Natl Acad Sci USA. 1994;91:9436–40. doi: 10.1073/pnas.91.20.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang CW, Striker LJ, Pesce C, et al. Glomerulosclerosis and body growth are mediated by different portions of bovine growth hormone. Studies in transgenic mice. Lab Invest. 1993;68:62–70. [PubMed] [Google Scholar]
- 26.Yang CW, Hwang TL, Wu CH, et al. Peritoneal nitric oxide is a marker of peritonitis in patients on continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 1996;11:2466–71. doi: 10.1093/oxfordjournals.ndt.a027216. [DOI] [PubMed] [Google Scholar]
- 27.Crane BR, Arvai AS, Gachhui R, et al. The structure of nitric oxide synthase oxygenase domain and inhibitor complexes. Science. 1997;278:425–31. doi: 10.1126/science.278.5337.425. [DOI] [PubMed] [Google Scholar]
- 28.Kallio EA, Koskinen PK, Aavik E, Vaali K, Lemstom KB. Role of nitric oxide in experimental obliterative bronchiolitis (chronic rejection) in the rat. J Clin Invest. 1997;100:2984–94. doi: 10.1172/JCI119852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cook HT, Sullivan R. Glomerular nitrite synthesis in in situ immune complex glomerulonephritis in the rat. Am J Pathol. 1991;139:1047–52. [PMC free article] [PubMed] [Google Scholar]
- 30.Stadler J, Billiar TR, Curran RD, Stuehr DJ, Ochoa JB, Simmons RL. Effect of exogenous and endogenous nitric oxide on mitochondrial respiration of rat hepatocytes. Am J Physiol. 1991;260:C910–6. doi: 10.1152/ajpcell.1991.260.5.C910. [DOI] [PubMed] [Google Scholar]
- 31.Nussler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993;54:171–8. [PubMed] [Google Scholar]
- 32.Lander HM, Sehajpal P, Levine DM, Novogrodsky A. Activation of human peripheral blood mononuclear cells by nitric oxide-generating compounds. J Immunol. 1993;150:1509–16. [PubMed] [Google Scholar]
- 33.Magrinat G, Mason SN, Shami PJ, Weinberg JB. Nitric oxide modulation of human leukemia cell differentiation and gene expression. Blood. 1992;80:1880–4. [PubMed] [Google Scholar]
- 34.van der Meide PH, de Labie MC, Botman CA, Aten J, Weening JJ. Nitric oxide suppresses IFN-gamma production in the spleen of mercuric chloride-exposed brown Norway rats. Cell Immunol. 1995;161:195–206. doi: 10.1006/cimm.1995.1027. [DOI] [PubMed] [Google Scholar]
- 35.Sporn MB, Roberts AB. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992;119:1017–21. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–92. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 37.Border WA, Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992;90:1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto T, Noble NA, Cohen AH, et al. Expression of transforming growth factor-beta isoforms in human glomerular diseases. Kidney Int. 1996;49:461–9. doi: 10.1038/ki.1996.65. [DOI] [PubMed] [Google Scholar]
- 39.Yang CW, Hsueh S, Wu MS, et al. Glomerular transforming growth factor-beta1 mRNA as a marker of glomerulosclerosis—application in renal biopsies. Nephron. 1997;77:290–7. doi: 10.1159/000190290. [DOI] [PubMed] [Google Scholar]
- 40.Iwano M, Akai Y, Fujii Y, Dohi Y, Matsumura N, Dohi K. Intraglomerular expression of transforming growth factor-beta 1 (TGF-beta 1) mRNA in patients with glomerulonephritis: quantitative analysis by competitive polymerase chain reaction. Clin Exp Immunol. 1994;97:309–14. doi: 10.1111/j.1365-2249.1994.tb06086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kunz D, Walker G, Pfeilschifter J. Transforming growth factor-beta 2 inhibits interleukin 1 beta-induced expression of inducible nitric oxide synthase in rat renal mesangial cells. Inflamm Res. 1997;46:327–31. doi: 10.1007/s000110050196. [DOI] [PubMed] [Google Scholar]
- 42.Owens MW, Milligan SA, Grisham MB. Inhibition of rat pleural mesothelial cell nitric oxide synthesis by transforming growth factor-beta 1. Inflammation. 1996;20:637–46. doi: 10.1007/BF01488801. [DOI] [PubMed] [Google Scholar]
- 43.Lopez Farre A, Mosquera JR, Sanchez de Miguel L, et al. Endothelial cells inhibit NO generation by vascular smooth muscle cells. Role of transforming growth factor-beta. Arterioscler Thromb Vasc Biol. 1996;16:1263–8. doi: 10.1161/01.atv.16.10.1263. [DOI] [PubMed] [Google Scholar]
- 44.Moulinoux JP, Quemener V, Chambon Y. Effect of aminoguanidine sulfate on the hepatic level of polyamines after partial hepatectomy in rats. C R Seances Soc Biol Fil. 1981;175:828–34. [PubMed] [Google Scholar]
- 45.Edelstein D, Brownlee M. Mechanistic studies of advanced glycosylation end product inhibition by aminoguanidine. Diabetes. 1992;41:26–29. doi: 10.2337/diab.41.1.26. [DOI] [PubMed] [Google Scholar]


