Abstract
ISCOM is an efficient mucosal delivery system for RSV envelope proteins as measured by antibody responses in respiratory tract secretions and in sera of mice following two intranasal (i.n.) administrations. Intranasally administered RSV ISCOMs induced high levels of IgA antibodies both in the upper respiratory tract and in the lungs. In the lungs, a prominent and long-lasting IgA response was recorded, which still persisted 22 weeks after the second i.n. immunization when the experiment ended. Subcutaneous (s.c.) immunization only induced low IgA titres in the upper respiratory tract and no measurable response to RSV was found in the lungs. Differences were also noticed in serum between the i.n. and s.c. modes of immunization. ISCOMs given intranasally induced earlier, higher and longer lasting IgM and IgG1 serum anti-RSV antibody responses than those induced by the s.c. mode of administration. A low serum IgE response was only detectable at 2 weeks after i.n. immunization with ISCOMs and after s.c. immunization with an inactivated virus, but no IgE response was detectable after s.c. injection of ISCOMs. The serum IgA response was more pronounced following s.c. injection of inactivated virus than after i.n. application of ISCOMs, and a clear-cut booster effect was obtained with a second immunization. Virtually no serum IgA response was detected after the s.c. administration of ISCOMs. In conclusion, the high immune responses induced by RSV ISCOMs in the respiratory tract and serum after i.n. administration indicate prominent mucosal delivery and adjuvant properties of the ISCOMs, warranting further studies.
Keywords: ISCOMs, vaccine, respiratory syncytial virus, mucosal immunity
INTRODUCTION
RSV is one of the most important causative agents of viral lower respiratory tract infection in infants and young children worldwide [1], with an estimated 91 000 hospitalizations and 4500 deaths annually in the USA alone [2]. A closely related virus, bovine respiratory syncytial virus infecting young calves, is likewise an important pathogen [3]. Both national and international health organizations view the development of a vaccine to control RSV as a priority in the reduction of mortality and morbidity [4]. So far, attempts to develop an RSV vaccine have been unsuccessful. Great caution must be used because of the earlier failure in the 1960s in infants with a formalin-inactivated human respiratory syncytial virus (HRSV) vaccine. This vaccine not only failed to protect against infection and disease, but also drastically exacerbated the disease in a number of cases [5].
Another common problem for parenterally administered vaccines, including the formalin-inactivated HRSV vaccine, is the inability to induce a mucosal immune response in contrast to responses after natural infection. In general, non-replicating antigens delivered through mucosal routes do not induce an acceptable level of mucosal IgA immune response with acceptable low doses of antigens. However, experimental vaccines adjuvanted with cholera toxin (CT) have shown that a potent mucosal adjuvant can facilitate a non-replicating delivery system. Admixed or chemically linked with another antigen, CT was shown to induce prominent mucosal as well as systemic immune responses to the coadministered or linked non-replicating antigen [6]. In the present study, the capacity of ISCOMs was tested as a mucosal delivery system for RSV and the read-out of the effect was the antibody responses evoked in serum and in respiratory tract secretions.
The ISCOM allows selective incorporation of viral envelope proteins by hydrophobic interaction into a defined supra-molecular structure of Quillaja saponins. In this 40-nm particle, the antigens are arranged in a multimeric form and the in-built Quillaja saponin has strong inherent adjuvant activity [7,8]. Recent studies have also shown that the immunogenicities of the envelope proteins of influenza virus and the B subunit of CT when incorporated in ISCOMs are greatly enhanced after mucosal administration, leading to potent mucosal IgA and systemic immune responses. Further, it was reported that ISCOMs containing antigens from protoscoles of Echinococcus granulosus efficiently induced serum antibody responses in mice, in particular, the antibody response to carbohydrate antigens was enhanced by the intranasal (i.n.) mode of immunization [9].
An RSV ISCOM experimental vaccine was first introduced by Trudel et al., demonstrating its effectiveness in inducing serum neutralizing antibodies [10] and cytotoxic T cell responses [4] in mice, but the potential of ISCOMs as a mucosal delivery system for RSV antigens has not been explored.
Here we analyse the capacity of RSV ISCOMs to induce antibody responses in serum and in the respiratory tract after i.n. and subcutaneous (s.c.) administrations of mice with RSV ISCOMs, including studies of the distribution of the responses in immunoglobulin isotypes, IgG subclasses and determination of neutralizing antibodies.
MATERIALS AND METHODS
Virus and cells
The Long strain of RSV (ATCC VR-26), kindly supplied by Dr C. Örvell (Huddinge University Hospital, Stockholm, Sweden), was propagated on MA 104 cells (ECACC no. 85102918). Cells were grown in full Dulbecco's modified Eagle's medium (FDMEM; National Veterinary Institute, Uppsala, Sweden), supplemented with 100 μg/ml kanamycin, 2 mm glutamine, and 10% fetal calf serum (FCS; Gibco BRL, Life Technologies AB, Täby, Sweden).
Preparation of RSV ISCOMs and inactivated RSV
The preparation and biochemical characterization of ISCOMs were carried out as previously described [11–13]. Briefly, 2 ml (1.6 mg/ml) sucrose gradient purified RSV were solubilized with OG (1-O-n-octyl-β-d-glucopyranosid, C14H28O6; Boehringer, Mannheim, Germany) at a final concentration of 2% (w/v) for 1 h at 37°C under constant agitation. The solubilized virus was applied onto a discontinuous sucrose gradient of 2 ml 20% sucrose layer containing 0.5% OG, over a cushion of 50% sucrose. After centrifugation at 210 000 g in a Kontron TST-41 rotor (Kontron Ltd, Zurich, Switzerland) for 1 h at 4°C, the sample volume plus the 20% sucrose layer containing viral proteins were collected, and extra lipids, i.e. cholesterol and phosphatidylcholine, and Quillaja saponin (‘Spicoside’; Iscotec AB, Luleå, Sweden) were added in proportions of protein:cholesterol:phosphatidylcholine:Quillaja saponin = 1:1:1:5 calculated by weight, and the mixture was homogenized. After extensive dialysis against 0.15 m ammonium acetate for 72 h at 4°C, the ISCOMs were purified by centrifugation through 10% sucrose at 210 000 g in a Kontron TST-41 rotor for 18 h at 10°C. The pellet containing the RSV ISCOMs was resuspended in 200 μl PBS. Protein concentration was determined by the Bradford method [14]. The Quillaja saponin concentration was measured by reverse-phase high performance liquid chromatography (HPLC) [15], and the weight ratio of protein to Quillaja saponin was 1:10.
The inactivated RSV was prepared by adding 0.5% (w/v) of β-propiolactone to the virus solution, and the reaction was kept at 4°C for 7 days. Inactivation was verified by virus isolation attempts in MA 104 cell culture.
Mice
Female BALB/c mice, 8–12 weeks of age, were obtained from the National Veterinary Institute (Uppsala, Sweden). The mice were screened for viral, bacterial and mycoplasma infections, and kept in accordance with the national guidelines.
Immunizations
Three groups (1–3) of mice, each consisting of 10 BALB/c mice, were immunized twice 6 weeks apart. Mice in different groups were immunized as follows: group 1, 1 μg/mouse of RSV ISCOMs subcutaneously; group 2, 5 μg/mouse of RSV ISCOMs intranasally; group 3, 500 μl/mouse of inactivated RSV subcutaneously. The preparation contained the same amount of the fusion (F) protein as in the ISCOM preparation used for s.c. administration, i.e. 1 μg/mouse measured by a quantitative immunodot assay, in which serial two-fold diluted RSV ISCOMs or the inactivated RSV were immobilized on a cellulosenitrate paper. Anti-F MoAb, rabbit anti-mouse conjugate and its substrate were used for the blot assay. The intensity of the colour was visually compared and the difference between F protein content in the ISCOMs and in the inactivated RSV was calculated accordingly; group 4, non-immunized control group.
For i.n. immunization, 5 μg of ISCOMs were suspended in a volume of 20 μl PBS while the subcutaneous dose was suspended in a volume of 200 μl PBS.
I.n. immunizations were carried out under anaesthesia with methoxyflurane administrated by inhalation.
Collection of samples
Blood samples were collected from the retroorbital plexus in weeks 2 and 5 after the first immunization, and weeks 1, 3, 15 and 22 after the second immunization. Lungs for antibody extraction were taken 2, 15 and 22 weeks after the second immunization. The upper respiratory tract secretion was taken at one occasion 2 weeks after the second immunization. Two to five mice from each group were killed, and the lungs or upper respiratory tracts were removed. Secretory antibodies were extracted as described by Bergquist et al. [16]. Briefly, the mice were injected intraperitoneally with 0.1 ml 1% heparin–PBS under anaesthesia. The mice were exsanguinated, and perfused with 20 ml 0.1% heparin–PBS into the right chamber of the heart through the circulation system. The lungs were removed and trimmed. Each pair of lungs was kept in 3 ml 0.1% heparin–PBS, and the weights were recorded. The organs were washed with PBS and stored at −20°C. Before testing, 2% saponin in PBS solution was added at a ratio of 1 μl/mg of organ weight. After overnight extraction at 4°C, the lungs were sedimented at 12 000 g for 10 min in a Eppendorf 5415C centrifuge, and the supernatant was collected for antibody analysis. The upper respiratory tract was excised from the skull by removing skin, muscles, lower jaw, brain and surrounding bones. The tissues remaining were mainly the sinuses and the nose, which were treated with 2% saponin as described for lungs.
For samples to be assayed for virus-neutralizing antibody, the saponin treatment was exchanged with distilled water for extraction of antibodies to avoid the lytic and cell toxicity activity (Hu KF et al. Using distilled water for the extraction of mucosal antibodies and the subsequent application in RSV neutralization test. J Immunoassay, in press).
Enzyme immunoassays
ELISAs to determine serum IgM, IgG and organ extract IgG antibodies against RSV were carried out essentially using the method described by Jennings et al. [17,18]. Briefly, microtitre plates (Nunc, Roskilde, Denmark) were coated with 200 ng purified HRSV per well in 100 μl 50 mm carbonate-bicarbonate buffer pH 9.6, and kept at 4°C overnight. All washings were carried out with PBS containing 0.2% Tween 20 (PBS–T). All incubations were done at room temperature for 60 min under constant shaking. The mouse sera were titrated in two-fold dilutions. ELISA employed to determine the antibody responses in the IgG1, IgG2a, IgG2b, IgG3 subclasses, and the IgE and IgA isotypes were carried out with non-labelled goat antibodies specific for mouse IgG subclasses (Nordic Immunology, Tilburg, The Netherlands), biotinylated goat anti-mouse IgE (The Binding Site, Birmingham, UK) or biotinylated goat anti-mouse IgA (Southern Biotechnology Associates, Birmingham, AL), and a rabbit anti-goat horseradish peroxidase (HRP) conjugate or an HRP streptavidin (Dakopatts, Glostrup, Denmark), respectively. IgA levels to HRSV in mouse organ extracts were measured by an ELISA [19] with slight modifications. Two major differences compared with serum immunoglobulin ELISAs for the organ extract were: (i) microtitre plates were coated with 400 ng/well of the purified RSV instead of 200 ng/well for the serum; (ii) overnight incubation at room temperature for the extracts instead of 1 h incubation on shaker at room temperature for the serum. The enzyme reactions were visualized by adding 100 μl/well of tetramethylbenzidine (TMB) substrate buffer (TMB, H2O2; SVANOVA, Uppsala, Sweden) for 10 min. The reactions were terminated by the addition of 50 μl/well of 2 m H2SO4. The optical density (OD) was measured at 450 nm with a Titertek Multiscan Spectrophotometer (Flow Labs, Irvine, UK). Serum IgM, IgG and secretory IgG, IgA were tested on individual samples. Due to the limited amount of sample which could be collected from each mouse, IgG subclasses, IgE and IgA in serum were run on pooled samples. The titres were expressed as reciprocals of endpoint dilution.
Western blots
Western blots were done according to Towbin et al. [20] using nitrocellulose from Schleicher and Schüll (Dassel, Germany).
Virus neutralization assay
The neutralizing activity was measured using pooled sera or pooled organ secretions by a microneutralization test, similar to the method used by Trudel et al. [10] with some modifications: 25 μl of two-fold dilutions of heat-inactivated (56°C, 30 min) sera or 100 μl of two-fold dilutions of heat-inactivated (56°C, 30 min) organ extracts were mixed with 25 μl or 100 μl viral suspension containing 100 TCID50 of RSV, Long strain calculated according to Reed & Muench's method [21]. The virus serum or virus organ extract mixtures were added to 96-well flat-bottomed microtitre plates (Nunclon, Roskilde, Denmark) incubated for 4 h at 37°C. Then 100 μl of 2-day-old MA 104 cells in FDMEM medium containing 2% FCS were added to each well. The plates were finally incubated for 5–7 days at 37°C in a humidified atmosphere containing 5% CO2. All tests were performed in quadruplicate. Controls were included and the test serum or secretion was replaced with negative control serum or secretion. Titres for virus neutralization test were expressed as the reciprocal of last dilution exhibiting 50% residual infectivity (ND50/25 μl or ND50/100 μl: 50% neutralizing dose in 25 μl or 100 μl for serum and secretion, respectively).
Statistical analysis
ELISA titres for serum IgM, IgG and mucosal IgG, IgA are expressed as geometric means with 95% Wilcoxon signed rank sum confidence interval, and were compared with respect to the levels of antibody by Mann–Whitney two-tailed U-test. All calculations were run on a computer using Minitab release 10 Xtra software (Minitab, Coventry, UK).
RESULTS
Characterization of RSV ISCOMs
A sample of the ISCOMs was fractionated by analytical 10–50% (w/w) sucrose density gradient centrifugation. The fractions were analysed for specific protein content and lipid 14C-PE by liquid scintillation, and both were recovered in the same 19S fraction. Presence of ISCOMs was confirmed by electron microscopy (EM). The protein content of the ISCOMs was determined by electrophoresis and Western blotting, which showed the presence of both F and G glycoproteins of RSV (Fig. 1a). The immune response to the ISCOMs was mainly against the F protein as revealed by Western blotting (Fig. 1b).
Fig. 1.
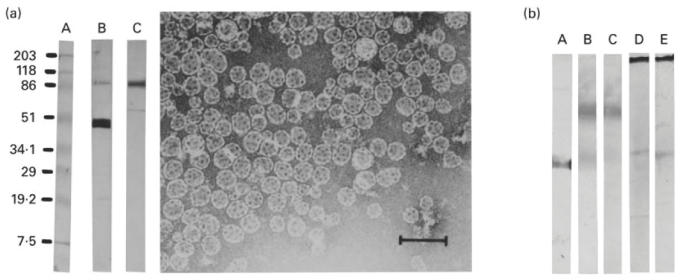
(a) Western blotting of RSV ISCOMs (on the left). A, Molecular weight standards; B, developed with anti-RSV fusion (F) protein (MoAb); C, developed with anti-RSV G protein (MoAb). Electron micrograph showing the RSV ISCOM particles (on the right), bar = 100 nm. (b) Western blotting of sera and organ extracts against RSV. A, Developed with anti-RSV F protein (MoAb); B,C, developed with pooled sera and pooled lung extracts from mice subcutaneously immunized with RSV ISCOMs, respectively; D,E, developed with pooled sera and pooled lung extracts from mice intranasally immunized with RSV ISCOMs, respectively.
ISCOMs administered intranasally induce a long-lasting serum IgM response
The IgM response was measured in mice after the first and second immunizations as described in Materials and Methods, and the results are shown in Fig. 2. Significantly (P < 0.01) higher IgM serum levels were detected in mice immunized subcutaneously or intranasally with ISCOMs (groups 1 and 2) than in mice immunized with inactivated RSV (group 3). After i.n. immunization (group 2), long-lasting serum IgM levels to RSV were recorded, which lasted beyond the second immunization. When comparing the IgM responses within the groups vaccinated with ISCOMs, significantly (P < 0.01) higher anti-RSV levels were recorded in animals immunized intranasally (group 2) than in those immunized subcutaneously (group 1).
Fig. 2.
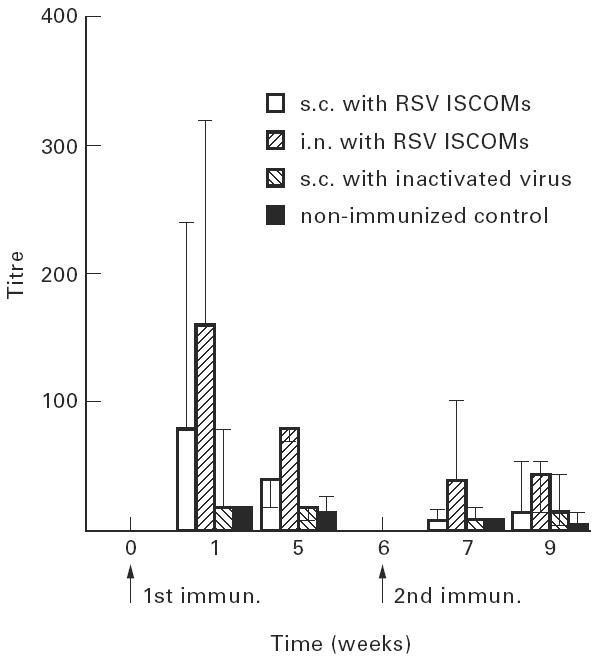
IgM serum antibody titres (geometric mean titres and 95% confidence limits) of mice to RSV measured by ELISA following two subcutaneous (s.c.) or two intranasal (i.n.) immunizations with RSV ISCOMs or two s.c. immunizations with inactivated virus, and non-immunized control.
Intranasal administration of ISCOMs induces early IgG responses in serum
The serum IgG to RSV was measured twice after the first immunization and four times after the second immunization (Fig. 3). The serum IgG response followed the IgM response in a classical manner. The ISCOM-immunized animals (groups 1 and 2) developed significantly (P < 0.01) higher anti-RSV IgG responses than the mice immunized with inactivated RSV (group 3). The IgG response lasted throughout the experimental period, i.e. 22 weeks after the second dose, although eventually declining. Mice immunized intranasally with ISCOMs (group 2) responded faster and developed significantly (P < 0.01) higher serum IgG titres 2 weeks after the first immunization than subcutaneously immunized animals (group 1). The highest titre was about 1:100 000 recorded 3 weeks after the second i.n. immunization with ISCOMs (group 2). In the subsequent bleeds, similar magnitudes (P > 0.05) of serum IgG responses were measured in the two groups immunized with ISCOMs.
Fig. 3.
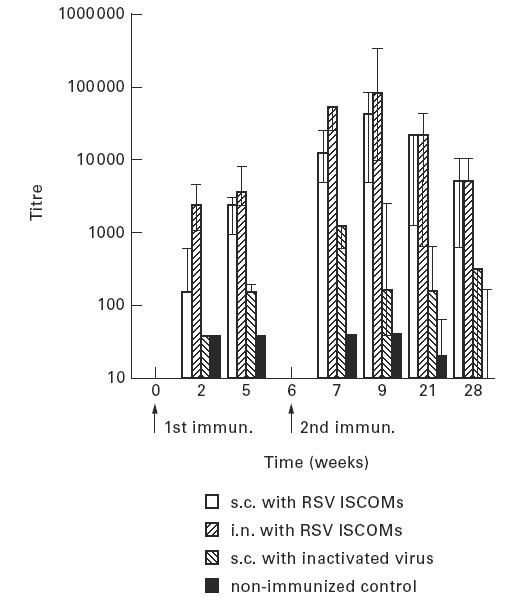
The development of IgG serum antibody titres (geometric mean titres and 95% confidence limits) of mice to RSV measured by ELISA following two subcutaneous (s.c.) or two intranasal (i.n.) immunizations with RSV ISCOMs or two s.c. immunizations with inactivated virus, and non-immunized control.
IgG subclasses in the serum response
Serum samples from the mice described above were pooled group-wise and tested in ELISA for the antibody response to RSV in the various IgG subclasses. The i.n. administration of ISCOMs induced a fast IgG1 anti-RSV response after both the first and the second immunizations measured at the first and third bleeds. At the subsequent bleeds, i.e. 5 weeks after the first immunization and 3 weeks after the second immunization, the levels of IgG1 to RSV were similar in the mice exposed to i.n. and s.c. administrations (Fig. 4).
Fig. 4.
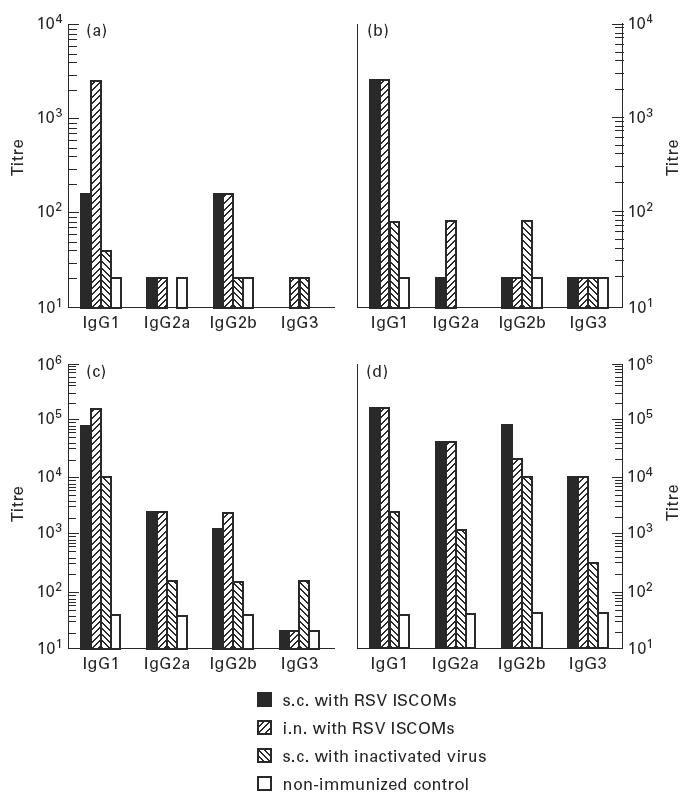
The development of IgG subclass responses to RSV measured by ELISA on pooled serum samples following two subcutaneous (s.c.) or two intranasal (i.n.) immunizations 6 weeks apart with RSV ISCOMs or two s.c. immunizations with inactivated virus, and non-immunized control. (a) First bleed, 2 weeks after the first immunization. (b) Second bleed, 5 weeks after the first immunization. (c) Third bleed, 1 week after the second immunization. (d) Fourth bleed, 3 weeks after the second immunization.
The IgG2a response became prominent in the fourth bleeding, i.e. 3 weeks after the second immunization, reaching serum titres of about 40 000 after both i.n. and s.c. immunizations. In the same bleed, apart from the relatively higher level of IgG2b in s.c. ISCOM-immunized animals, only low levels of IgG2b and IgG3 antibodies to RSV were detected. Inactivated RSV induced very low IgG subclass responses. Fifteen and 22 weeks after the second immunization, no IgG2a, IgG2b and IgG3 antibodies to RSV could be detected in any of the vaccinated groups, but high RSV-specific IgG1 antibody titres were detected (not shown).
Intranasal immunization with ISCOMs and subcutaneous immunization with inactivated whole virus induce weak, transient serum IgE and IgA responses
One week after the second immunization, IgE responses were recorded in serum after i.n. immunization with ISCOMs and s.c. immunization with inactivated RSV (Fig. 5a). No IgE response was observed with s.c. administered ISCOMs and in the other bleeds, including samples collected 15 and 22 weeks after the second immunization (not shown).
Fig. 5.
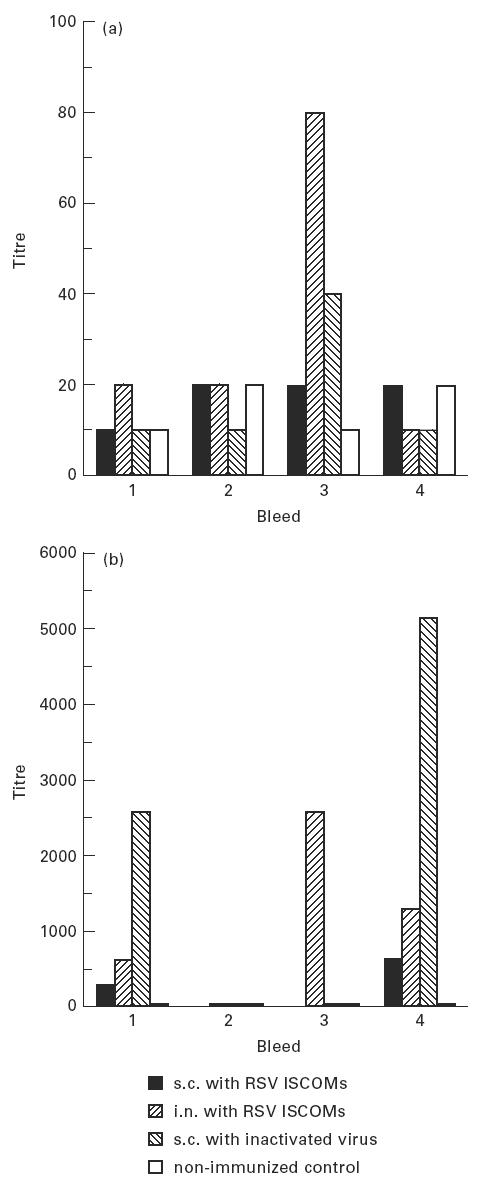
The IgE (a) and IgA (b) antibody titres of mice to RSV measured by ELISA on pooled sera following two subcutaneous (s.c.) or two intranasal (i.n.) immunizations 6 weeks apart with RSV ISCOMs or two s.c. immunizations with inactivated virus, and non-immunized control. First bleed: 2 weeks after the first immunization. Second bleed: 5 weeks after the first immunization. Third bleed: 1 week after the second immunization. Fourth bleed: 3 weeks after the second immunization.
IgA responses to RSV were detected in serum after both the first and the second immunizations in mice immunized intranasally with ISCOMs and subcutaneously with inactivated RSV, and only a marginal IgA response was observed in mice immunized subcutaneously with ISCOMs. The highest serum IgA titre was recorded in pooled sera from animals immunized with inactivated RSV (group 3) 3 weeks after the second immunization (Fig. 5b). Fifteen and 22 weeks after the second immunization, no serum IgA was detected from any group of mice (not shown).
Intranasal and subcutaneous administrations of ISCOMs induce similar levels of IgG anti-RSV antibody in the upper respiratory tract and lungs
Two weeks after the second immunization, five mice from each group were killed, and secretions were extracted as described in Materials and Methods from the upper respiratory tract and the lungs. No significant difference (P > 0.05) in the IgG ELISA titres to RSV was detected in secretions collected from these two sites between s.c. and i.n. administrations. Nor was there a significant (P > 0.05) difference between IgG anti-RSV titres in secretions from the upper respiratory tract and those from the lungs either after s.c. or i.n. administrations (Fig. 6).
Fig. 6.
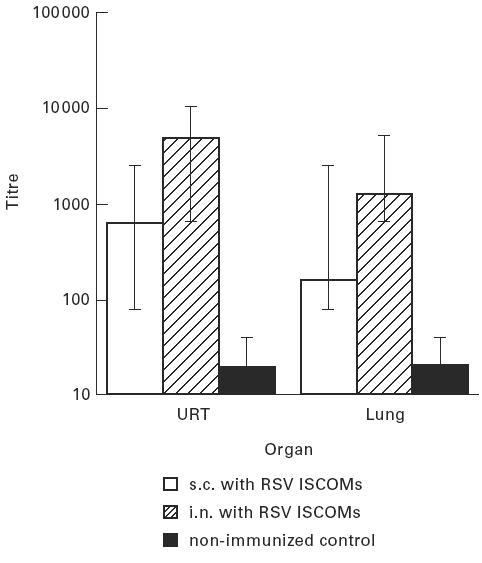
IgG antibody responses (geometric mean titres and 95% confidence limits) to RSV in upper respiratory tract (URT) and lung secretions, 2 weeks after the second immunization, as measured by ELISA, following two subcutaneous (s.c.) or two intranasal (i.n.) immunizations 6 weeks apart with RSV ISCOMs, and non-immunized control.
Intranasal administration of ISCOMs induces high and long-lasting IgA antibody responses in the respiratory tract
The IgA response to RSV was followed in lung secretions 2, 15 and 22 weeks after the second immunization. Two to five mice from each group were killed, and the lung secretions extracted as described in Materials and Methods. High IgA responses were induced by i.n. administration of ISCOMs (group 2), which declined from about 6000, 2 weeks after the second immunization, to about 1000 at 22 weeks after the second immunization. The s.c. administrations of ISCOMs (group 1) or inactivated RSV(group 3) induced little if any local IgA responses compared with the non-immunized control group (Fig. 7a).
Fig. 7.
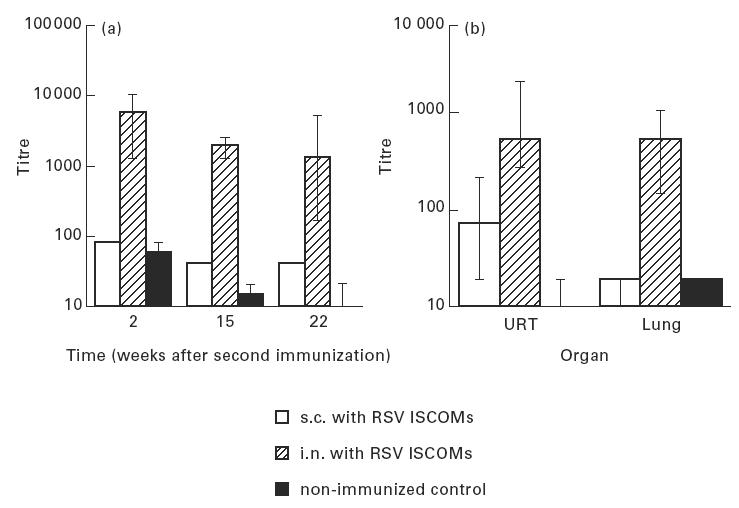
IgA responses (geometric mean titres and 95% confidence limits) to RSV as measured by ELISA, (a) in lung secretions at three occasions after the second immunization, and (b) in upper respiratory tract (URT) and lung secretions 2 weeks after the second immunization, following two subcutaneous (s.c.) or two intranasal (i.n.) immunizations 6 weeks apart with RSV ISCOMs, and non-immunized control.
Two weeks after the second immunization, secretions from both the upper respiratory tract and lungs were assayed for anti-RSV IgA. Statistical analysis revealed that the IgA titres in secretion from animals intranasally immunized with ISCOMs were all significantly higher (P = 0.0275) for upper respiratory tracts and for lungs (P = 0.0009) than those from secretions extracted from mice after s.c. immunization with ISCOMs. Low antibody titres to RSV were measured in secretions from the upper respiratory tract after s.c. administration of ISCOMs. No measurable IgA response was detected from the lungs after the s.c. immunization. No significant difference of IgA anti-RSV titres was observed in secretions from the upper respiratory tract and the lungs measured 2 weeks after the i.n. immunization (Fig. 7b).
Both i.n. and s.c. administrations of ISCOMs induce virus-neutralizing antibody in serum, in the upper respiratory tracts and lungs
Animals immunized intranasally and subcutaneously with ISCOMs were tested for virus-neutralizing antibodies with sera pooled from each group. Low virus neutralizing (VN) antibody titres were recorded after the first immunization (ND50/25 μl < 10) in both groups (i.n. and s.c.). Two weeks after the second immunization (third bleed), five to seven-fold increases of VN titres were observed in both groups, with the highest reciprocal serum titre being around 70 (ND50/25 μl) after the s.c. immunization, which is only slightly higher than that detected after i.n. immunization (Fig. 8a). No VN antibody was detected in serum from inactivated RSV-immunized mice.
Fig. 8.
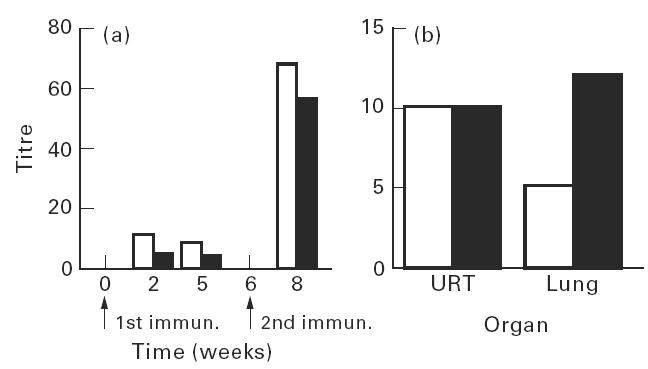
Virus-neutralizing antibody to RSV, (a) in serum after the first and the second immunization, expressed as ND50/25 μl, and (b) in upper respiratory tract (URT) and lung secretions 2 weeks after the second immunization expressed as ND50/100 μl, following two subcutaneous (□) or two intranasal (▪) immunizations with RSV ISCOMs. Controls were all negative (not shown).
Secretions assayed for VN were extracted with water instead of 2% saponin, as described in Materials and Methods, due to the cell toxicity of saponin. The neutralization assay was carried out on pooled samples. In secretions from both the upper respiratory tracts and the lungs, VN antibodies were observed after i.n. as well as s.c. modes of immunization, ranging from 5 to 13 ND50/100 μl. I.n. immunization induced a higher VN response in secretion from lungs, but these data are not suitable for statistical analysis since they are pooled samples (Fig. 8b). No VN antibody was detected in secretions from inactivated RSV-immunized mice.
DISCUSSION
The natural infectious route for RSV is the respiratory tract, where the mucosal IgA response plays an important role in the first line of defence against the infection. As a general feature, parenterally administered vaccines fail to induce mucosal IgA responses [22]. That is also the case with RSV killed whole virus vaccines, which after parenteral immunization not only failed to induce protection, but even exacerbated the disease [5].
Three major lines of evidence clearly indicate that IgA antibodies are mediators of mucosal resistance to a number of viral infections, namely, the consistent association of the level of IgA antibodies with the extent of resistance to virus infection, the mediation of mucosal resistance to virus infection by passive transfer of IgA antibodies, and the abrogation of infection-induced resistance to virus challenge by anti-IgA antibody treatment of the upper respiratory tract. Cytotoxic T lymphocytes and Th cells are likely to make important contributions in the elimination of virus-infected cells, while IgA antibodies may play the major role as a first barrier in resistance to reinfection [23].
This study shows that the ISCOM has the ability, as a non-replicating antigen delivery system, to replace live RSV by inducing an effective local IgA response as well as a systemic immune response by i.n. administration. Initially we also included live RSV in the experiment, but the antibody responses were so low in secretions and serum, probably due to the limited virus replication, that the comparisons become of no value. The i.n. immunization induced surprisingly high IgA titres to RSV in both the lungs and the upper respiratory tract. Moreover, these titres were long lasting, and persistently high titres were recorded encompassing the whole experimental period of 22 weeks. The second i.n. administration also induced a prominent booster effect recorded both in serum and in lung extract. These results suggest that the ISCOM is an efficient delivery system for mucosal administration, which has been indicated by other studies too [24]. In view of the capacity of ISCOMs to overcome the inhibitory effect of passively transferred antibodies, as shown in macaques in a measles virus model [25], it would be of interest to explore i.n. administered RSV ISCOMs for the use in infants below the age of 1 year after more extensive animal experiments, including testing in monkeys using clinical disease scores for evaluation. Besides, it is also likely that a mucosal administration would even better evade the inhibitory effect of maternal antibodies than the parenteral mode. The shortcomings of the immature immune system [26,27] combined with the interference of maternally transferred antibodies were also overcome by ISCOMs, as shown in juvenile foals [28].
The serum antibody response achieved by i.n. administration was generally higher than that obtained by ISCOMs administered subcutaneously. The difference in doses of ISCOMs used for the two modes of administration, i.e. 1 μg subcutaneously versus 5 μg intranasally, is not large, particularly in view of the rather primitive technique used for i.n. administration with a pipette. It has to be considered that a proportion of the vaccine dose after i.n. administration may be swallowed [29], and it has been suggested that only about 20% of the inoculum reaches the lungs [30]. An appropriate administration device may make the i.n. mode of administration both practical and economical.
There were striking differences in the immune responses induced by ISCOMs between s.c. and i.n. administrations, the biggest difference being that the s.c. mode did not induce an IgA mucosal immune response. The i.n. route induced faster and higher IgM, total IgG and IgG1 serum responses. The i.n. mode of immunization induced transient IgA and IgE serum responses in contrast to the s.c. administration of the ISCOMs. However, unlike the ISCOMs, the killed whole RSV induced both IgA and IgE serum responses by s.c. administration. In this respect, the serum antibody response showed similarities to responses after i.n. administration of ISCOMs. In general, however, the whole killed virus induced low serum antibody and no IgA antibody responses in secretions. While the transient serum IgA response was recorded after both the first and the second immunizations, a detectable IgE response required two immunizations. The results of this study suggest that ISCOMs, which in a number of studies have been shown to induce a prominent Th1-type of response by the parenteral route, and to a lesser extent the Th2-type response [31], exhibit a more pronounced Th2-type of response after i.n. administration. This might be beneficial for a potent mucosal IgA response. However, we have seen that ISCOMs by s.c. administration induce IL-5 production, and studies by others [32] carried out with ISCOM-supported antigens have shown that the mucosal route of immunization induced IL-5, a cytokine connected with the Th2-type response and IgA production. Whether transforming growth factor-beta (TGF-β)-mediated IgA switching is induced by ISCOMs needs to be explored. Both i.n. and s.c. immunizations with ISCOMs induced similar levels of IgG2a and IgG2b in serum, which are considered markers for the Th1 type of response being promoted by interferon-gamma (IFN-γ).
No correlation could be established between serum IgA and secretory IgA responses in i.n. or s.c. immunized animals, suggesting that the systemic IgA is not a major component of the mucosal IgA detected. Other antigens tested in ISCOMs, i.e. influenza virus envelope proteins and B-subunit of CT (Ekström J et al. Iscom and iscom matrix enhance by intranasal route the IgA responses to OVA and B-subunit of cholera toxin in local and remote mucosal secretions, (vaccine, submitted), induced considerably lower IgA antibody responses in the upper respiratory tract than in the lungs. The high IgA responses induced by RSV ISCOMs in the upper respiratory tract might indicate a unique targeting property for that locality of the incorporated RSV antigens promoted by the adjuvant antigen formulation in this complex [30]. It should be borne in mind that the upper respiratory tract is a different immunological compartment from the lungs [33]. In this context, it should be realized that the method used to extract IgA facilitated the extraction of antibodies from the upper respiratory tract, also encompassing the sinuses, which are not readily obtained by a washing procedure.
Similar levels of IgG titres to RSV were detected both in the upper respiratory tract and the lungs after s.c. and i.n. modes of administration of ISCOMs, indicating, as suggested before, that IgG reaches the secretions merely by passive diffusion, although induced by different antigen-presenting and lymphoid systems [34]. The IgG may well, however, play a role in virus-neutralizing activity recorded in mucosal secretions in this study.
Neutralizing antibodies were induced by ISCOMs, after both s.c. and i.n. modes of administration, suggesting that the conformational protective epitopes were preserved in the ISCOMs, as shown before with other ISCOMs [31]. The VN titres in the lung secretion were lower than in serum, but this should be considered in the content of the immunoglobulin concentration being 300-fold higher in the serum than in the lung or upper respiratory tract secretion, and in view of the different mechanisms involved in IgA neutralizing activity [35]. Therefore, the VN titres between organ extracts and sera are not directly comparable. Since only antibody against F glycoprotein was detected by Western blot in serum and organ extract from mice both subcutaneously and intranasally immunized with RSV ISCOMs (Fig. 1b), the VN antibody responses were likely to be induced by F protein.
This study makes it clear that ISCOMs with the envelope RSV antigens induced extraordinarily strong IgA responses by i.n. administration. This is probably partly due to functional properties of both F and G proteins, which provides for the efficient infectivity of the native virus particle. Further studies exploring the properties of the RSV ISCOMs by i.n. administration in inducing cell-mediated immunity are underway.
Acknowledgments
We thank Dr Karin Lövgren for her valuable suggestions during the ISCOMs preparation and Dr Bernard Meignier for providing anti-RSV F and G monoclonal antibodies. Thanks are also due to Dr Sergey Gennadyevich and Miss Maria Persson for their assistance. This study was supported by TFR (Swedish Research Council for Engineering Sciences).
References
- 1.McIntosh K, Chanock RM. Respiratory syncytial virus. In: Fields BN, editor. Virology. New York: Raven Press; 1990. pp. 1045–72. [Google Scholar]
- 2.Heilman CA. Respiratory syncytial and parainfluenza viruses. J Infect Dis. 1990;161:402–6. doi: 10.1093/infdis/161.3.402. [DOI] [PubMed] [Google Scholar]
- 3.Heilman CA. Respiratory syncytial and parainfluenza viruses. J Infect Dis. 1990;161:402–6. doi: 10.1093/infdis/161.3.402. [DOI] [PubMed] [Google Scholar]
- 4.Trudel M, Nadon F, Seguin C, Brault S, Lusignan Y, Lemieus S. Induction of cytotoxic T-cell response and protection of BALB/c mice by vaccination with an experimental ISCOMs respiratory syncytial virus subunit vaccine. Vaccine. 1992;10:107–12. doi: 10.1016/0264-410x(92)90026-g. [DOI] [PubMed] [Google Scholar]
- 5.Murphy BR, Sotnikov A, Paradiso PR, et al. Immunization of cotton rats with the fusion (F) and large (G) glycoproteins of respiratory syncytial virus (RSV) protects against RSV challenge without potentiating RSV disease. Vaccine. 1989;7:533–40. doi: 10.1016/0264-410x(89)90278-8. [DOI] [PubMed] [Google Scholar]
- 6.Holmgren J, Czerkinsky C, Lycke N, Svennerholm AM. Strategies for the induction of immune responses at mucosal surfaces making use of cholera toxin B subunit as immunogen, carries and adjuvant. Am J Trop Med Hyg. 1994;50(Suppl.):42–54. [PubMed] [Google Scholar]
- 7.Morein B, Sundquist B, Dalsgaard K, Osterhaus A. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature. 1984;308:457–69. doi: 10.1038/308457a0. [DOI] [PubMed] [Google Scholar]
- 8.Morein B, Lövgren K, Sundquist B. Iscom—an immunostimulating complex. Immunol Today. 1987;11:333–8. doi: 10.1016/0167-5699(87)90008-9. [DOI] [PubMed] [Google Scholar]
- 9.Morein B, Lövgren K, Rönnberg B, Sjölander A, Villacres-Eriksson M. Immunostimulating complexes, clinical potential in vaccine development. Clin Immunother. 1995;3:461–75. [Google Scholar]
- 10.Trudel M, Nadon F, Seguin C, Simard C, Lussier G. Experimental polyvalent ISCOMs subunit vaccine induces antibodies that neutralize human and bovine respiratory syncytial virus. Vaccine. 1989;7:12–16. doi: 10.1016/0264-410x(89)90004-2. [DOI] [PubMed] [Google Scholar]
- 11.Lövgren K, Lindmark J, Pipkorn R, Morein B. Antigenic presentation of small molecules and peptides conjugated to a preformed iscom as carrier. J Immunol Methods. 1989;98:137–43. doi: 10.1016/0022-1759(87)90447-9. [DOI] [PubMed] [Google Scholar]
- 12.Morein B, Sharp M, Sundquist B, Simons K. Protein subunit vaccines of parainfluenza type 3 virus: immunogenic effect in lambs and mice. J G Virol. 1983;4:1557–69. doi: 10.1099/0022-1317-64-7-1557. [DOI] [PubMed] [Google Scholar]
- 13.Sundquist B, Lovgren K, Hoglund S, Morein B. Influenza virus iscoms: biochemical characterization. Vaccine. 1988;6:44–58. doi: 10.1016/0264-410x(88)90013-8. [DOI] [PubMed] [Google Scholar]
- 14.Bradford MM. A rapid and sensitive method for the quantificaton of microgram quantities of protein untilizing the principle of protein-dye binding. Anal Biochem. 1976;72:249–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 15.Rönnberg B, Fekadu M, Morein B. Adjuvant activity of non-toxic quillaja saponaria Molina components for use in ISCOM matrix. Vaccine. 1995;13:1375–82. doi: 10.1016/0264-410x(95)00105-a. [DOI] [PubMed] [Google Scholar]
- 16.Bergquist C, Lagerg×rd T, Lindblad M, Holmgren J. Local and systemic antibody responses to dextran–cholera toxin B subunit conjugates. Infect Immun. 1995;63:2021–5. doi: 10.1128/iai.63.5.2021-2025.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jennings R, Auasim T, Sharrard TM, Hockley D, Potter CW. Zwitterionic detergent solubilisation of HSV-1-specific surface antigens. Arch Virol. 1988;98:137–53. doi: 10.1007/BF01322164. [DOI] [PubMed] [Google Scholar]
- 18.Jennings R, Smith T, Potter CW. Use of enzyme-linked immunosorbent assay (ELISA) for the estimation of serum antibodies in an influenza virus vaccine study. Med Microbiol Immunol. 1981;169:247–58. doi: 10.1007/BF02125524. [DOI] [PubMed] [Google Scholar]
- 19.Hocart MJ, Mackenzie JS, Stewart GA. The IgG subclass responses induced by wild-type, cold-adapted and purified haemagglutinin from influenza virus A/Queensland/6/72 in CBA/CaH mice. J Gen Virol. 1988;69:1873–82. doi: 10.1099/0022-1317-69-8-1873. [DOI] [PubMed] [Google Scholar]
- 20.Towbin H, Staehelin T, Gordin J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]
- 22.Walker RI. New strategies for using mucosal vaccination to achieve more effective immunization. Vaccine. 1994;12:387–400. doi: 10.1016/0264-410x(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 23.Ogra PL, Strober W, Mestecky J, McGhee JR, Lamm ME, Bienenstock J. Handbook of mucosal immunology. San Diego: Academic Press; 1994. p. 339. [Google Scholar]
- 24.Höglund S, Dalegaard K, Lögren K, Sundquist B, Osterhaus A, Morein B. ISCOMs and immunostimulation with viral antigens. Subcellular Biochem. 1989;15:39–67. doi: 10.1007/978-1-4899-1675-4_2. [DOI] [PubMed] [Google Scholar]
- 25.van Binnendijk RS, Poelen MC, van Amerongen G, de Vries P, Osterhaus AD. Protective immunity in macaques vaccinated with live attenuated recombinant and subunit measles vaccine in the presence of passively acquired antibodies. J Infect Dis. 1997;175:524–32. doi: 10.1093/infdis/175.3.524. [DOI] [PubMed] [Google Scholar]
- 26.Forsthuber T, Yip HC, Lehnmann PV. Induction of Th1 and Th2 immunity in neonatal mice. Science. 1996;271:1728–30. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 27.Adkins B, Chun K, Hamilton K, Nassire M. Naive murine neonatal T cells undergo apoptosis in response to primary stimulation. J Immunol. 1996;157:1343–9. [PubMed] [Google Scholar]
- 28.Nordengrahn A, Rusvai M, Merza M, Ekström J, Morein B, Belák S. Equine herpesvirus type 2 (EHV-2) as a predisposing factor for Rhodococcus equi pneumonia in foals: prevention of the bifactorial disease with EHV-2 immunostimulating complexes. Vet Microbil. 1996;51:55–68. doi: 10.1016/0378-1135(96)00032-6. [DOI] [PubMed] [Google Scholar]
- 29.Lövgren K. The serum antibody response distributed in subclasses and isotypes after intranasal and subcutaneous immunization with influenza virus immunostimulating complexes. Scand J Immunol. 1988;27:241–5. doi: 10.1111/j.1365-3083.1988.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 30.Yetter RA, Lehrer S, Ramphal R, Small JPA. Outcome of influenza infection: effect of site of initial infection and heterotypic immunity. Infect Immun. 1980;29:654–62. doi: 10.1128/iai.29.2.654-662.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morein B, Lövgren K, Rönnberg B, Sjölander A, Villacrés-Eriksson M. Immunostimulating complexes: clinical potential in vaccine development. Clin Immunother. 1995;3:461–75. [Google Scholar]
- 32.Maloy KJ, Donachie AM, Mowat AM. Induction of Th1 and Th2 CD4+ T cell responses by oral or parenteral immunization with ISCOMs. Eur J Immunol. 1995;25:2835–41. doi: 10.1002/eji.1830251019. [DOI] [PubMed] [Google Scholar]
- 33.Ogra PL, Strober W, Mestecky J, McGhee JR, Lamm ME, Bienestock J. Handbook of mucosal immunology. San Diego: Academic Press; 1994. pp. 625–40. [Google Scholar]
- 34.Brandtzaeg P. The role of humoral mucosal immunity in the induction and maintenance of chronic airway infections. Am J Respir Crit Care Med. 1995;151:2081–7. doi: 10.1164/ajrccm.151.6.7767561. [DOI] [PubMed] [Google Scholar]
- 35.Rosenthal KL, Gallichan WS. Challenges for vaccination against sexually transmitted diseases: induction and long-term maintenance of mucosal immune responses in the female genital tract. Sem Immunol. 1997;9:303–14. doi: 10.1006/smim.1997.0086. [DOI] [PubMed] [Google Scholar]


