Abstract
Rheumatoid arthritis (RA) is an autoimmune disease characterized by inflammation of the synovial membrane of multiple joints. This inflammatory microenvironment allows fibroblast-like synoviocytes (FLS) to express or enhance several adhesion or costimulatory molecules. This phenotypic shift, under proinflammatory cytokines, seems to be related to functional consequences for antigen presentation to T cells. The sensory neuropeptide substance P (SP), present at high levels, is able to act on FLS proliferation and enzyme secretion. These data led us to investigate whether SP could also provoke a phenotypic change of FLS. Using flow cytometry and a three-step cellular ELISA method, we determined whether SP has an influence on the expression of MHC class II, intercellular adhesion molecule-1 (ICAM-1), VCAM-1, LFA-3, CD40, B7.1 or B7.2 molecules on RA FLS incubated with interferon-gamma (IFN-γ) or IL-1β or tumour necrosis factor-alpha (TNF-α) with or without SP. Our results indicate that SP potentiates the effect of proinflammatory cytokines on the expression of VCAM-1 on RA FLS. We verified the presence of specific SP (NK1) receptor mRNA. Using reverse transcription-polymerase chain reaction, we showed that RA FLS of patients express NK1 receptor mRNA. These results suggest that SP increase of cytokine-induced VCAM-1 expression acts via this specific SP receptor. Thus, during chronic inflammation RA FLS are at the interface between the immune and the nervous systems.
Keywords: rheumatoid arthritis, tachykinins, NK1 receptor, type B synoviocyte
INTRODUCTION
In inflamed joints of rheumatoid arthritis (RA) patients, the synovial membrane consists of a rich lymphocyte infiltration of the subintima layer and hyperplasia of the synovial lining cell layer. There are multiple interactions, either direct or indirect, developed between the majority of cells in these two zones [1]. The synovial lining cell layer contains at least two distinct populations of cells. These are synoviocyte type A cells, which are derived from the monocyte-macrophage lineage, and synoviocyte type B, which predominate in in vitro cell culture and have fibroblastic morphology and phenotype [2]. These fibroblast-like synoviocytes express MHC class II, adhesion or costimulatory molecules that presumably help anchor them to the extracellular matrix, regulate the flux of cells that pass into the synovial fluid space and play a role as potential antigen-presenting cells (APC) [3,4]. The enhancement of the surface molecules implicated in such a function is known to be linked to the cytokine-rich microenvironment of the synovial membrane.
In addition to the action of cytokines, numerous works have shown that neuropeptides, and particularly substance P (SP), present at high levels in synovial fluid, are also involved in inflammatory mechanisms of RA [5]. SP is a neuropeptide composed of 11 amino acids, belonging to the family of mammalian tachykinins, which include neurokinin A (NKA) and neurokinin B (NKB). The effects elicited by these neuropeptides are mediated by at least three membrane receptors structurally and functionally related to adrenergic receptors. These receptors belong to the family of protein G-coupled receptors and are designated NK1, NK2 and NK3 receptors. Pharmacological techniques demonstrated that SP could act on the various tachykinin receptors, but it has greatest action and affinity with the NK1 receptor [6]. SP has proinflammatory properties and various groups have shown its action on monocytes, lymphocytes and synoviocytes [7–9].
These data led us to investigate whether SP, alone or in addition to the proinflammatory cytokines IL-1β, tumour necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ), has an effect on RA fibroblast-like synoviocyte (FLS) adhesion or costimulatory molecules. Using flow cytometry and a three-step cellular ELISA method, we analysed the expression of HLA-DR, CD40, LFA-3, intercellular adhesion molecule-1 (ICAM-1), VCAM-1, B7.1 and B7.2. This analysis showed that SP alone did not modify the expression of these molecules, but it potentialized the action of proinflammatory cytokines on the VCAM-1 FLS surface.
Using reverse transcription-polymerase chain reaction (RT-PCR), cDNA cloning, and sequence analysis, we checked whether synoviocytes possess the substance P receptor (NK1) mRNA. Our results showed that FLS from RA synovial membranes of several patients express this authentic substance P (NK1) receptor mRNA, whereas T lymphocytes, even under phytohaemagglutinin (PHA) stimulation, expressed only NK3 mRNA.
This study showed the ability of SP to potentialize the action of proinflammatory cytokines on VCAM-1 expression of RA FLS. Furthermore, the presence of the substance P (NK1) receptor mRNA in RA synoviocytes makes them one of the preferential target cells for the neuropeptide SP.
MATERIALS AND METHODS
Cellular cultures
Synoviocyte cultures
Synovial tissues from patients diagnosed as suffering from RA according to the American Rheumatism Association 1987 criteria [10] and synovial tissues from two patients suffering from no inflammatory joint disease were minced and digested with collagenase 3 mg/ml (Sigma Chemical Co., St Louis, MO) for 4 h at 37°C. Cells were washed and seeded in Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% fetal calf serum (FCS), penicillin 100 μg/ml and streptomycin 100 U/ml. Cells were used from passages 3–9 in these experiments. At this point, cells had a morphology and phenotype similar to type B synovial fibroblasts (positive intracytoplasmic vimentin expression). For cytokine treatment, we used IFN-γ at 100 U/ml (Boehringer, Mannheim, Germany), IL-1β (Genzyme S.A., Cambridge, MA), generally used at 10 ng/ml unless otherwise stated, TNF-α (Genzyme) used at 100 ng/ml unless otherwise stated, and substance P (Sigma) from 10−7 m to 10−9 m.
Lymphocyte cultures
Synovial tissue fragments were placed in 24-well tissue culture plates in 2 ml of RPMI medium supplemented with 10% human serum and 10 U/ml recombinant IL-2 (a gift from Sanofi, Toulouse, France). T lymphocytes were grown out of each synovial sample for 4–6 days in the absence of any additional antigen or mitogen. T cells were stimulated with PHA (1 mg/ml; Sigma) and irradiated feeder cells. After two or three stimulations, cell populations were again stimulated for 8 h or 16 h before lysing for RNA preparation. Lymphocytes were tested for their CD3 expression and cell populations were > 95% positive.
Flow cytometric analysis
Two-step surface staining was performed by incubating cells with the appropriate MoAbs: anti-ICAM-1 (anti-CD54; Immunotech), anti-LFA3 (anti-CD58; Immunotech, Marseille, France), anti-B7.1 (anti-CD80; Immunotech), anti-B7.2 (anti-CD86; Pharmingen, San Diego, CA), anti-CD40 (Immunotech) or anti-HLA-DR (L243 supernatant from ATCC HB55) followed by FITC-conjugated goat anti-mouse immunoglobulin (Immunotech). A mouse monoclonal IgG1 (Immunotech) was used in each experiment as control. Flow cytometry analysis was performed on a Coulter (Hialeah, FL) EPICS (argon laser 488).
Measurement of ICAM-1 and VCAM-1 expression on fibroblastic synoviocytes using a three-step ELISA method
FLS were seeded (10 000 cells/well) in a gelatin-coated 96-well microtitre plate and stimulated for 0, 6, 12 or 18 h with or without IFN-γ, IL-1β or TNF-α and/or SP. The assays were carried out in triplicate using FLS from five patients. Culture medium was removed, the adherent cells were washed with PBS (Gibco BRL, Breda, The Netherlands) and fixed with formalin 2%. After washing, they were incubated with PBS containing 1% bovine serum albumin (BSA) at 4°C for 30 min to block non-specific binding sites. The amount of ICAM-1 and VCAM-1 was measured by a cell ELISA method using 10 μg/ml of anti-ICAM-1 MoAb (anti-CD54; Immunotech) or anti-VCAM-1 MoAb (anti-CD106; Immunotech). As a control, the cells were incubated with a mouse monoclonal IgG1 (Immunotech). After 30 min of incubation, cells were washed again and incubated with a biotinylated rabbit anti-mouse immunoglobulin for 30 min at 4°C and subsequently with streptavidin/biotinylated horseradish peroxidase (HRP) complex for 20 min. Then, the substrate was added for 15 min and plates were read at 450 nm (Dynatech Labs, Chantilly, VA).
RNA extraction and cDNA synthesis
Total RNA was extracted from synovial tissue, synoviocytes and T lymphocytes by Trizol reagent (Gibco BRL). Total RNA (5 μg) was used for the synthesis of the first strand cDNA (superscript preamplification kit; Gibco BRL). The 20 μl of reaction mixture were diluted in 100 μl of sterilized water.
PCR amplification of SP (NK1), NKA (NK2), NKB (NK3) receptors
Diluted cDNA (5 μl) was amplified using specific primers: 5′-CCACTAACACCTCGGAACCCA-3′ and 5′-GACCCAGATGACACAGATGACCA-3′ chosen, respectively, on exon 1 and exon 2 of substance P (NK1) receptor mRNA, 5′-GGGGGCAAGACGCTCCTCCT-3′ and 5′-AAACCATACCCAAACCATAGCCCT-3′ for NK2 receptor, 5′-CTGGATAGACGGGGGTGG-AGG-3′ and 5′-ACCACCACACCATACGCCAGG-3′ for NK3 receptor.
The PCR reaction was performed in a Cetus (Emeryville, CA) Thermal Cycler with 40 cycles in 50 μl reaction volume containing 1 × PCR buffer (10 mm Tris–HCl pH 8.4, 50 mm KCl and 0.01% gelatin), 1.5 mm MgCl2, 0.1 mm of each dNTP, 25 pmol of each primer and 1.25 U Taq polymerase (Promega, Madison, WI). The amplification cycle profile was: denaturation for 7 min at 95°C followed by 40 cycles consisting of denaturation for 30 s at 95°C, annealing for 30 s at 62°C for NK1 and 58°C for NK2 and NK3 and extension for 30 s at 72°C. To ensure complete synthesis, the last cycle was extended for 5 min at 72°C. Negative controls with no added cDNA and positive controls with cDNA from astrocyte cell line U373 MG (a cell line well characterized for SP receptor expression) [11], or with cDNA from chinese hamster ovary (CHO) cell line expressing human NK2 receptor (a generous gift from J.E. Krause, Washington University, St Louis, MO) or CHO cell line expressing human NK3 receptor (a generous gift from Sanofi Research, Montpellier, France) were included in each experiment.
The quality of each sample of cDNA was controlled by PCR amplification of β-actin with two specific primers: 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ and 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′.
PCR amplification of SP (NK1) receptor was purified on a Qia quick spin column (Qiagen, Chatworth, CA) and cloned into a pGEM-T plasmid (Promega). The recombinant plasmids were sequenced in an automatic sequencer and we checked that the sequences obtained were identical to the NK1 sequences previously published [12].
RESULTS
Effects of IFN-γ, IL-1β, TNF-α and SP on MHC class II, CD40, ICAM-1, LFA-3, B7.1, and B7.2 expression on cultured RA FLS
We chose to focus our analysis on MHC class II molecules and on the adhesion or costimulatory molecules most representative of an APC. After three passages in culture, FLS constitutively expressed LFA-3, ICAM-1 and CD40 (Table 1). In the absence of cytokines, FLS did not express HLA-DR, B7.1 or B7.2. Treatment with IFN-γ, IL-1β or TNF-α, three proinflammatory cytokines involved in RA pathogenesis, had no effect on LFA-3 tested after 24 h or 48 h of treatment. All these cytokines greatly increased ICAM-1 expression, especially after 48 h of treatment. CD40 was only affected by IFN-γ with a visible increase by 24 h, which persisted until 48 h. HLA-DR expression was also only induced by IFN-γ. B7.1 (CD80) and B7.2 (CD86) expression was not induced by any of these cytokines.
Table 1.
Flow cytometry analysis of the expression of HLA-DR, CD40, intercellular adhesion molecule-1 (ICAM-1) and LFA-3 on fibroblast-like synoviocytes (FLS) from five different rheumatoid arthritis (RA) patients
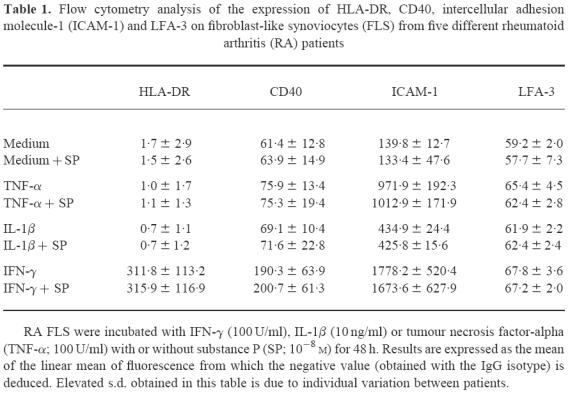
SP, used from 10−7 m to 10−9 m, modified the expression of none of these six surface molecules after either 24 h or 48 h of cell culture. Also, when added simultaneously to the culture medium, SP did not modify the expression of these molecules.
Effects of IFN-γ, IL-1β, TNF-α and SP on VCAM-1 expression on cultured FLS using a three-step ELISA method
We began by studying VCAM-1 surface expression on FLS using flow cytometry analysis and we found a low constitutive level of expression. We considered that flow cytometry was not sensitive enough to study the variations of this molecule after treatment with various cytokines. Therefore, we developed a three-step ELISA technique and we studied simultaneously VCAM-1 and ICAM-1 expression, with ICAM-1 as the internal control for the technique. Table 2 summarizes the results obtained from FLS from five different RA patients on VCAM-1 expression. Figure 1 is a representative illustration of the percentage of VCAM-1 augmentation after cytokine and neuropeptide treatment. We found spontaneous VCAM-1 surface expression on each of these cultured primary cells. Supplementing the culture medium with IFN-γ, IL-1β or TNF-α increased VCAM-1 expression. This increase was visible from 6 h for IL-1β and TNF-α and reached a peak between 6 h and 12 h of culture. The effect of TNF-α was prolonged, whereas that of IL-1β was brief and had returned to base level by 18 h. The action of TNF-α was maximal from 10 ng/ml, but 0.1 ng/ml of TNF-α did not increase VCAM-1 expression. IL-1β reached its maximum activity from concentrations as low as 1 ng/ml. The increase in VCAM-1 expression induced by IFN-γ was less significant than for the other cytokines. The effect of SP was tested at 10−7 m and 10−8 m. At both of these concentrations it was identical. SP treatment alone had no effect on VCAM-1 surface expression on RA FLS. In contrast, SP significantly increased the VCAM-1 expression induced by IL-1β (from 1 to 10 ng/ml) and TNF-α (from 10 to 100 ng/ml) (Table 2), but substance P was incapable of overcoming low TNF-α levels (0.1 ng/ml). With IFN-γ, however, SP did not provoke a significant increase compared with the results obtained with the cytokine alone. This ELISA method gave comparable results to the FACS analysis for measurement of ICAM-1 expression. Thus, the base level was increased by each of the three cytokines, whereas SP alone or combined with the cytokines did not modify ICAM-1 expression. We carried out the same studies of VCAM-1 expression on synovial fibroblasts from two patients who had had a ligament reconstruction and who suffered from no inflammatory joint disease. We found that VCAM-1 expression on these cells was also increased by IFN-γ, IL-1β and TNF-α. However, at 10−8 m SP did not potentiate the effect of these cytokines.
Table 2.
Effect of substance P (SP) on cytokine-induced VCAM-1 expression as measured by cell ELISA
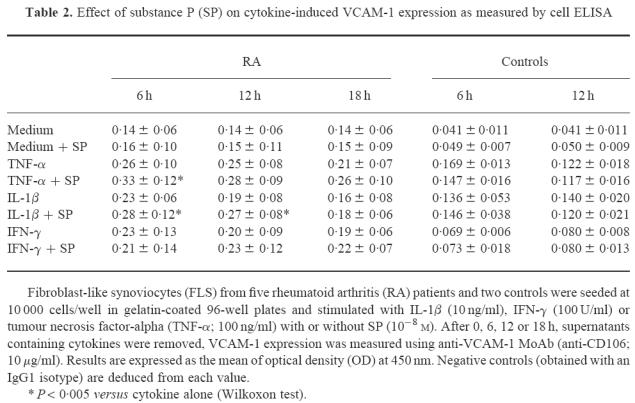
Fig. 1.
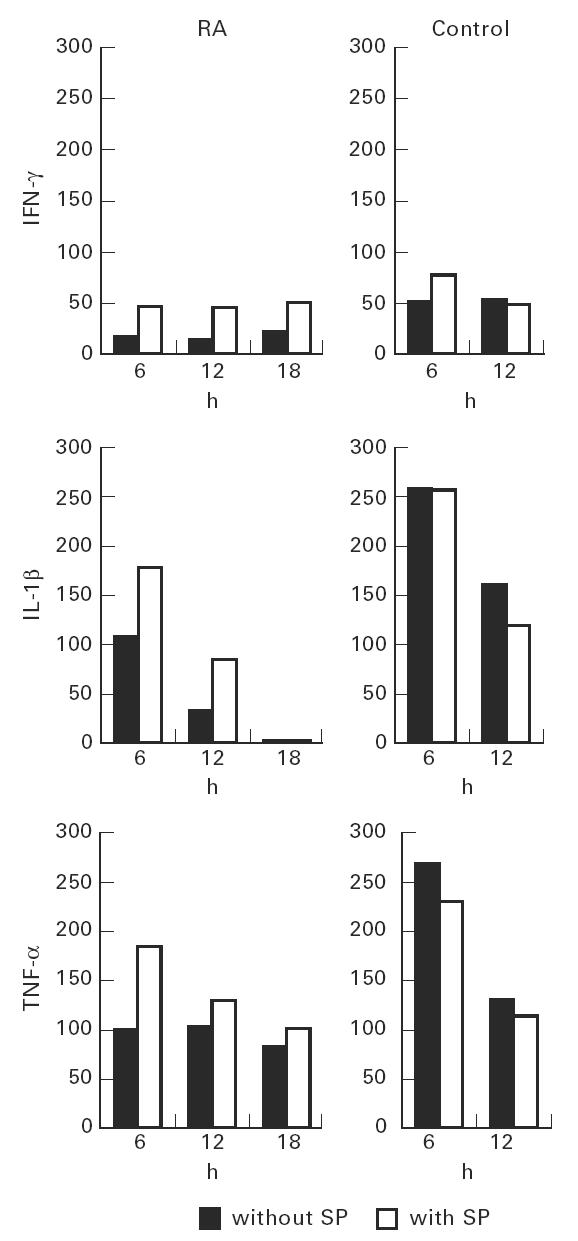
Effect of IFN-γ, IL-1β or tumour necrosis factor-alpha (TNF-α) with or without substance P (SP) on VCAM-1 expression on fibroblast-like synoviocytes (FLS). FLS were stimulated with IL-1β (10 ng/ml), IFN-γ (100 U/ml) or TNF-α (100 ng/ml) with or without SP (10−8m) for 0, 6, 12 and 18 h. Constitutive FLS VCAM-1 expression was taken as 0% on the ordinate. Results are the mean of triplicate measurements, representative of five performed on different rheumatoid arthritis (RA) patients and of two performed on controls; s.d. is < 5%.
Identification of NK1, NK2 and NK3 receptor mRNA in synovial tissue
In this study using RT-PCR, we looked for the presence of mRNA for the three types of tachykinin receptor. Amplified products were at the following sizes: 422 base pairs (bp), 596 bp and 261 bp for NK1, NK2 and NK3 receptors, respectively. The specificity of amplification was verified by sequencing the products.
Total RNA was extracted from eight synovial membranes of patients with RA. We detected NK1 and NK3 mRNA in the eight synovial tissues tested (Fig. 2). T cell lines from the same synovial membranes were established and RNA was extracted after 0, 8 and 16 h of non-specific stimulation with PHA. We subsequently determined whether activated, synovial T lymphocytes expressed one of the three receptor mRNA using the same RT-PCR technique as above. NK3 receptor mRNA was detected in all the cases and at each time. However, we found neither NK1 nor NK2 mRNA in these T cell lines. The FLS cell lines obtained from the same patients were tested after at least three passages in culture. In all the FLS lines, the specific bands for NK1 and NK3 amplification were observed at the expected sizes. The PCR profile of these FLS lines was the same as that of the synovial membranes, with no expression of NK2 mRNA.
Fig. 2.
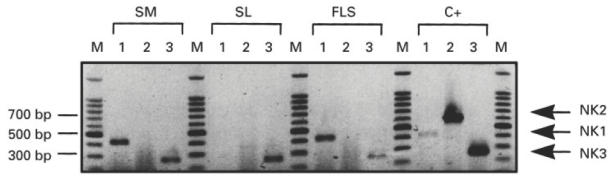
Identification of NK1, NK2 and NK3 receptor mRNA in synovial tissue. Reverse transcription-polymerase chain reaction (RT-PCR) was performed on mRNA extracted from different samples: synovial membrane (SM), synovial T lymphocytes (SL), fibroblast-like synoviocytes (FLS) and positive controls (C +) (U373MG for NK1, chinese hamster ovary (CHO) expressing either human NK2 or NK3 receptor). Lanes 1, 2 and 3 represent amplification with NK1, NK2 and NK3 probes, respectively. Expected sizes were 422 bp for NK1, 596 bp for NK2 and 261 bp for NK3. M, DNA ladder 100 bp (Promega). Results are from one patient representative of eight patients (five patients for FLS).
DISCUSSION
Several neuropeptides have been detected in synovia, including SP, neurokinin A, and neuropeptide Y [13]. The significant increase of SP in the serum and joint fluid of RA patients led some authors to investigate the ability of SP to contribute to the pathogenesis of RA. Numerous studies have been performed and demonstrate that SP, by a direct effect on the vascular system, causing vasodilatation and oedema, plays an important part in the process of inflammation [14]. This neuropeptide may also generate indirect effects mediated by effector cells, such as mast cells, via the release of histamine and other inflammatory mediators [15]. SP influences neutrophil chemotaxis [16], enhances cytokine release from monocytes [8], has an action on lymphocyte proliferation and immunoglobulin synthesis, both in vitro and in vivo [17,18]. This neuropeptide is also able to stimulate synoviocytes to proliferate and to secrete prostaglandin E2 and collagenase, and consequently enhance the formation of pannus [9,19].
The present study is the first one focused on the action of SP on adhesion or costimulatory molecule expression on rheumatoid fibroblastic synoviocytes.
We analysed the action of the neuropeptide SP and of the most important proinflammatory cytokines (IFN-γ, IL-1β, TNF-α) on the expression of HLA-DR, ICAM-1, VCAM-1, LFA-3, CD40, B7.1 or B7.2 molecules on RA FLS. We found that HLA-DR and CD40 were only responsive to IFN-γ, in agreement with other authors [20,21]. The expression of two adhesion molecules, ICAM-1 and VCAM-1, was enhanced by all three cytokines, whereas LFA-3, constitutively present, was never augmented by any cytokine. The same profile of regulation of the expression of LFA-3, ICAM-1 and VCAM-1 has been observed by other authors [22,23]. We also found that the costimulatory molecules B7.1 and B7.2 were never detected by flow cytometry analysis on the RA FLS surface [24,25].
We showed that the neuropeptide SP, added to each of the proinflammatory cytokines, significantly enhances VCAM-1 expression on rheumatoid fibroblast-like synoviocytes. VCAM-1 is an adhesion molecule, apparently involved in costimulatory mechanisms, which is recognized by VLA-4 present on T lymphocytes. Despite the absence of B7.1 and B7.2 expression, synovial fibroblasts are in fact capable of activating T lymphocytes [4,26,27], unlike dermal fibroblasts [28]. The difference in costimulatory activity of synovial versus dermal fibroblasts is related to expression of VCAM-1. Moreover, inhibition studies showed that MoAbs efficiently suppress the VCAM-1/VLA-4 pathway, one of the pathways involved in the binding of T lymphocytes to type B synovial cells [29]. Furthermore, Kriegsmann et al. [30] suggested that the strong expression of VCAM-1 in the fibroblast-like cells appears to be associated with the proliferating synovial cells prone to attach to and subsequently invade articular cartilage. The greater evidence that VCAM-1 is a determinant adhesion molecule of FLS [31] made it interesting to study its enhancement under neuromodulation. The increase of VCAM-1 expression by SP could also account for the flare up of the disease due to various neurological stimuli such as stress, and during which large amounts of SP are released by amyelinated fibres into the synovial membrane [32].
Most of the cytokines (IL-1β, TNF-α, IFN-γ) acting on the expression of adhesion molecules have a similar effect on ICAM-1 and VCAM-1 [23] on FLS, but the present data clearly demonstrate that the expression of VCAM-1, but not that of ICAM-1, was enhanced by addition of SP to cytokine-activated FLS. Thus, these results demonstrate a different process of activation for the two adhesion molecules. Although it has been shown that SP can increase TNF-α production by granulocytes [33], SP-enhanced VCAM-1 expression observed in our work was not via increased TNF-α synthesis, because we did not observe a change in ICAM-1 expression with SP treatment. Although VCAM-1 and ICAM-1 genes contain functionally identical sequences in their promoter regions that are recognized by the nuclear factor κB (NF-κB) [34], a recent study carried out on human umbilical vein endothelial cells (HUVEC) revealed that gene expression of these two adhesion molecules is regulated differently, which could explain the difference observed following SP treatment [35].
These results led us to hypothesize an SP acting directly via the specific receptor NK1 on rheumatoid FLS. We determined, by molecular analysis, that RA synovial membrane and FLS, but not synovial T lymphocytes, produced NK1 (SP) receptor mRNA. These results are in agreement with Krause et al. [36], who showed that cultured synoviocytes from a patient with RA expressed NK1 receptor mRNA, whereas synoviocytes from a normal synovial membrane did not express detectable NK1 receptor mRNA or protein. This also explains why SP did not potentiate cytokine-enhanced VCAM-1 expression in our two control joints free from RA. Because mRNAs of the high-affinity NK1 receptor exist in RA FLS, these results probably suggest that SP increases VCAM-1 expression through binding to the NK1 receptor.
In addition, only NK3 mRNA was detected in synovial T lymphocytes. SP can bind to this receptor but with less affinity than NKB, the more potent agonist for NK3 receptor. Thus, the exclusive presence of only NK3 mRNA in rheumatoid synovial T lymphocytes could explain why higher doses of SP are necessary to increase synovial T lymphocyte proliferation compared with synoviocytes in the studies of Agro et al. [19]. In the same way, Payan et al. [37] have quantified by flow cytometry and direct binding assays the interaction of SP to a specific cell surface receptor on human T lymphocytes, with a dissociation constant (Kd) 100-fold (180 nmol/l) greater than the published figures for the Kd of SP with its receptor (0.5–2.0 nmol/l).
In conclusion, our studies demonstrate that, in addition to its known properties on FLS as a collagenase and prostaglandin inducer and enhancer of proliferation, SP can also be an up-regulator of cytokine-induced VCAM-1 molecules. This is likely to occur in vivo, since SP can be released at high levels in inflammatory joints and it has been demonstrated that VCAM-1 is strongly expressed on RA FLS, whereas non-rheumatoid FLS showed a very weak or negative expression [30]. Thus, the presence of SP-producing fibres within the synovium and the VCAM-1-inducing capacity of SP could explain why VCAM-1 expression on RA fibroblast-like synoviocytes was so easily detected. These results support the concept that the nervous system modulates inflammatory events by neuropeptide-mediated RA synoviocyte VCAM-1 expression.
Acknowledgments
We wish to thank Hélène Brun and Georges Cassar for their technical help in flow cytometry analysis. This work has been supported by grants from the Fondation pour la Recherche Médicale, the regional council and by INSERM.
REFERENCES
- 1.Palmer DG. The anatomy of the rheumatoid lesion. Br Med Bull. 1995;51:286–95. doi: 10.1093/oxfordjournals.bmb.a072961. [DOI] [PubMed] [Google Scholar]
- 2.Burmester GR, Dimitriu-Bona A, Waters SJ, Winchister RJ. Identification of three major synovial lining cell populations by monoclonal antibodies directed to Ia antigens and antigens associated with monocytes/macrophages and fibroblasts. Scand J Immunol. 1983;17:69–82. doi: 10.1111/j.1365-3083.1983.tb00767.x. [DOI] [PubMed] [Google Scholar]
- 3.Amento EP, Bhan AK, McCullagh KG, Krane SM. Influences of IFNγ on synovial-like cells. Ia induction and inhibition of collagen synthesis. J Clin Invest. 1985;76:837–48. doi: 10.1172/JCI112041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boots AMH, Wimmers-Bertens Ajmh, Rijnders AWM. Antigen-presenting capacity of rheumatoid synovial fibroblasts. Immunology. 1994;82:268–74. [PMC free article] [PubMed] [Google Scholar]
- 5.Matucci-Cerini M, Marabini S, Partsch G, Cagnoni M. High levels of substance P in rheumatoid arthritis synovial fluid. Lack of substance P production by synoviocytes in vitro. Clin Exp Rheumatol. 1991;9:441–2. [PubMed] [Google Scholar]
- 6.Maggi CA. Review: the mammalian tachykinin receptors. Gen Pharmacol. 1995;26:911–44. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- 7.Eglezos A, Andrews PV, Boyd RL, Helme RD. Modulation of the immune response by tachykinins. Immunol Cell Biol. 1991;69:285–94. doi: 10.1038/icb.1991.39. [DOI] [PubMed] [Google Scholar]
- 8.Lotz M, Vaughan JH, Carson DA. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988;241:1218–21. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- 9.Lotz M, Carson DA, Vaughan JH. Substance P activation of rheumatoid synoviocytes neural pathway in pathogenesis of arthritis. Science. 1987;235:893–5. doi: 10.1126/science.2433770. [DOI] [PubMed] [Google Scholar]
- 10.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 11.Eistetter HR, Mills A, Brewster R, Alouani S, Rambosson C, Kawashima E. Functional characterization of neurokinine-1 receptors on human U373 MG astrocytoma cells. Glia. 1992;6:89–95. doi: 10.1002/glia.440060203. [DOI] [PubMed] [Google Scholar]
- 12.Takeda Y, Chou KB, Takeda J, Sachais BS, Krause JE. Molecular cloning, structural characterization and functional expression of the human substance P receptor. Biochem Biophys Res Commun. 1991;179:1232–40. doi: 10.1016/0006-291x(91)91704-g. [DOI] [PubMed] [Google Scholar]
- 13.Larsson J, Ekblom A, Henriksson K, Lundeberg T, Theodorsson E. Concentration of substance P, neurokinin A, calcitonin gene related peptide, neuropeptide Y and vasoactive intestinal polypeptide in synovial fluid from knee joints in patients suffering from rheumatoid arthritis. Scand J Rheumatol. 1991;20:326–5. doi: 10.3109/03009749109096808. [DOI] [PubMed] [Google Scholar]
- 14.Lam FY, Ferrel WR. Acute inflammation in the rat knee joint attenuates sympathetic vasoconstriction but enhances neuropeptide-mediated vasodilatation assessed by laser doppler perfusion imaging. Neurosci. 1992;52:443–9. doi: 10.1016/0306-4522(93)90170-k. [DOI] [PubMed] [Google Scholar]
- 15.Okayama Y, el-Lati SG, Leiferman KM, Church MK. Eosinophil granule proteins inhibit substance P-induced histamine release from human skin mast cells. J Allergy Clin Immunol. 1994;93:900–9. doi: 10.1016/0091-6749(94)90384-0. [DOI] [PubMed] [Google Scholar]
- 16.Wozniack A, Betts WH, McLennan G, Scicchitano R. Activation of human neutrophils by tachykinins: effect on formyl-methionyl-leucyl-phenylalanine- and platelet-activating factor-stimulated superoxide anion production and antibody-dependent cell-mediated cytotoxicity. Immunology. 1993;78:629–34. [PMC free article] [PubMed] [Google Scholar]
- 17.Payan DG, Brewster DR, Goetzl EJ. Specific stimulation of human T lymphocytes by substance P. J Immunol. 1983;131:1613–5. [PubMed] [Google Scholar]
- 18.Scicchitano R, Bienenstock J, Stanisz AM. In vivo immunomodulation by the neuropeptide substance P. Immunology. 1988;63:733–5. [PMC free article] [PubMed] [Google Scholar]
- 19.Agro A, Stanisz AM. Are lymphocytes a target for substance P modulation in arthritis? Sem Arthritis Rheum. 1992;21:252–8. doi: 10.1016/0049-0172(92)90056-j. [DOI] [PubMed] [Google Scholar]
- 20.En Chin J, Winterrowd GE, Krzesicki RF, Sanders ME. Role of cytokines in inflammatory synovitis. Arthritis Rheum. 1990;33:1776–86. doi: 10.1002/art.1780331204. [DOI] [PubMed] [Google Scholar]
- 21.Rissoan MC, Van Kooten C, Chomarat P, Galibert L, Durand I, Thivolet-Bejui F, Miossec P, Banchereau J. The functional CD40 antigen of fibroblasts may contribute to the proliferation of rheumatoid synovium. Clin Exp Immunol. 1996;106:481–90. doi: 10.1046/j.1365-2249.1996.d01-858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindsley HB, Smith DS, Davis LS, Koch AE, Lipsky P. Regulation of the expression of adhesion molecules by human synoviocytes. Sem Arthritis Rheum. 1992;21:330–4. doi: 10.1016/0049-0172(92)90026-a. [DOI] [PubMed] [Google Scholar]
- 23.Morales-Ducret J, Wayner E, Elices MJ, Alvaro-Garcia JM, Zwaifler NJ, Firestein GS. A4/β1 integrin (VLA-4) ligands in arthritis. Vascular cell adhesion molecule-1 expression in synovium and on fibroblast-like synoviocytes. J Immunol. 1992;149:1424–31. [PubMed] [Google Scholar]
- 24.Balsa A, Dixey J, Sansom DM, Maddisson PJ, Hall ND. Differential expression of the costimulatory molecule B7.1 (CD80) and B7.2 (CD86) in rheumatoid synovial tissue. Br J Rheumatol. 1996;35:33–37. doi: 10.1093/rheumatology/35.1.33. [DOI] [PubMed] [Google Scholar]
- 25.Liu MF, Kohsaka H, Sarukai H, Azuma M, Okumura K, Saito I, Miyasaka N. The presence of costimulatory molecules CD86 and CD28 in rheumatoid arthritis synovium. Arthritis Rheum. 1996;391:110–4. doi: 10.1002/art.1780390115. [DOI] [PubMed] [Google Scholar]
- 26.Looney RJ, Hooper M, Pudiak D. Costimulatory activity of human synovial fibroblasts. J Rheumatol. 1995;22:1820–4. [PubMed] [Google Scholar]
- 27.Tsai C, Diaz LA, Singer NG, et al. Responsiveness of human T lymphocytes to bacterial superantigens presented by cultured rheumatoid arthritis synoviocytes. Arthritis Rheum. 1996;39:125–36. doi: 10.1002/art.1780390117. [DOI] [PubMed] [Google Scholar]
- 28.Geppert TD, Lipsky PE. Dissection of defective antigen presentation by interferon γ-treated fibroblasts. J Immunol. 1987;138:385–92. [PubMed] [Google Scholar]
- 29.Van Dinther-Janssen Achm, Kraal G, van Soesbergen RM, Scheper RJ, Meijer Cjlm. Immunohistological and functional analysis of adhesion molecule expression in the rheumatoid synovial lining cell destruction. J Rheumatol. 1994;21:1998–2004. [PubMed] [Google Scholar]
- 30.Kriegsmann J, Keyszer GM, Geiler T, Brauer R, Gay RE, Gay S. Expression of VCAM-1 mRNA and protein in rheumatoid synovium demonstrated by in situ hybridization and immunochemistry. Lab Invest. 1995;72:209–14. [PubMed] [Google Scholar]
- 31.Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Rheum. 1996;39:1781–90. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- 32.Jorgensen C, Sany J. Modulation of the immune response by the neuro-endocrine axis in rheumatoid arthritis. Clin Exp Rheumatol. 1994;12:435–41. [PubMed] [Google Scholar]
- 33.Saban MR, Saban R, Bjorling D, Haak-Frendscho M. Involvement of leucotrienes, TNF-α, and the LFA-1/ICAM-1 interaction in substance P-induced granulocyte infiltration. J Leuk Biol. 1997;61:445–51. doi: 10.1002/jlb.61.4.445. [DOI] [PubMed] [Google Scholar]
- 34.Chen CC, Rosenbloom CL, Anderson DC, Manning AM. Selective inhibition of E-selectin, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 expression by inhibitors of IkB-a phosphorylation. J Immunol. 1995;155:3538–45. [PubMed] [Google Scholar]
- 35.Ledebur HC, Parks TP. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-kappa B site and p65 homodimers. J Biol Chem. 1995;270:933–43. doi: 10.1074/jbc.270.2.933. [DOI] [PubMed] [Google Scholar]
- 36.Krause JE, DiMaggio DA, McCarson KE. Alterations in neurokinin 1 receptor gene expression in models of pain and inflammation. Can J Physiol Pharmacol. 1995;73:854–9. doi: 10.1139/y95-117. [DOI] [PubMed] [Google Scholar]
- 37.Payan DG, Brewster DR, Missirian-Bastian A, Goetzl EJ. Substance P recognition by a subset of human T lymphocytes. J Clin Invest. 1984;74:1532–9. doi: 10.1172/JCI111567. [DOI] [PMC free article] [PubMed] [Google Scholar]


