Abstract
In a previous investigation, we found that murine MoAb 42C3, raised against human Tg, recognized Tg differently depending upon its level of iodination of Tg. A possible explanation for this finding is that iodine is directly involved with the specific epitope recognized by MoAb 42C3. In the present study, we report that the binding of MoAb 42C3 to iodinated Tg is inhibited by T4, T3, reverse T3 (rT3), triiodothyroacetic acid (triac), diiodothyronine (T2), diiodotyrosine (DIT), but not by thyronine (T0) or tyrosine. The order of inhibition of these iodinated compounds is T4 > T3 > rT3 > triac > T2 > DIT. The MoAb 42C3 does not have the same specificity as the T3, T4-receptor since the order of binding of these iodinated compounds on the receptor differed from the order of their inhibition of this MoAb. Monoclonal antibody 42C3 also recognized non-iodinated Tg that was subsequently iodinated in vitro. It failed to recognize another protein, bovine serum albumin, that was iodinated in vitro by the same method. These results suggest that iodinated tyrosines and thyronines determine the binding specificity of MoAb 42C3. The inhibitory effects of these compounds on MoAb 42C3 depend on their iodine content as well as location of iodine in the aromatic ring.
Keywords: thyroglobulin, monoclonal antibodies, iodine, iodinated thyronines
INTRODUCTION
Using MoAbs, we previously showed that many healthy individuals develop autoantibodies to conserved sites on human Tg that are related to the thyroid iodohormones, thyroxine (T4), and triiodothyronine (T3) [1]. Patients with thyroiditis exhibit these same autoantibodies, but the immunological response to Tg in patients is expanded further to include additional epitopes that are unique to human Tg. Autoantibodies to these human-specific determinants show a strong association with the disease state [1,2].
We also showed that human T cells do not respond in vitro to human Tg that has no detectable iodine [3]. These same cells reacted vigorously if that same Tg preparation was subsequently iodinated [3]. Others have found that an iodine-bearing hormonogenic site at the N-terminal portion of the molecule was the favoured site for autoantibody recognition [4,5]. Furthermore, a T4-containing peptide at the carboxyl-terminal end is involved in the induction of thyroiditis in mice [6]. Thus, the iodinated sites appear to be early targets of the immune response in thyroid autoimmunity, which may later spread to other epitopes as the autoimmune response progresses to disease. Clearly, though, iodinated sites are not the only epitopes recognized by antibodies from patients with autoimmune thyroiditis or involved with disease induction in mice [7,8]; but they may be important for initiating or enhancing the autoimmune response. In light of the importance of characterizing the initial autoimmune response to Tg, we wanted to understand the effect of iodination on the Tg molecule.
In previous communications, we showed that MoAb 42C3, produced against human Tg, reacted differently with Tg preparations depending on their level of iodination, ranging from strong reactivity to Tg with a high iodine content to no reactivity with Tg with no detectable iodine [9,10]. Here, we probe the relationship of iodine atoms on thyronines and tyrosines for their ability to inhibit the binding of MoAb 42C3 to Tg.
MATERIALS AND METHODS
T4, T3, reverse T3 (rT3), triiodothyroacetic acid (triac), diiodothyronine (T2), diiodotyrosine, and thyronine (T0) were obtained from Sigma (St Louis, MO). The chemical formulas of these compounds are shown in Fig. 1. The other chemicals used in this study and the method used for protein assay were described in the previous communication [11].
Fig. 1.
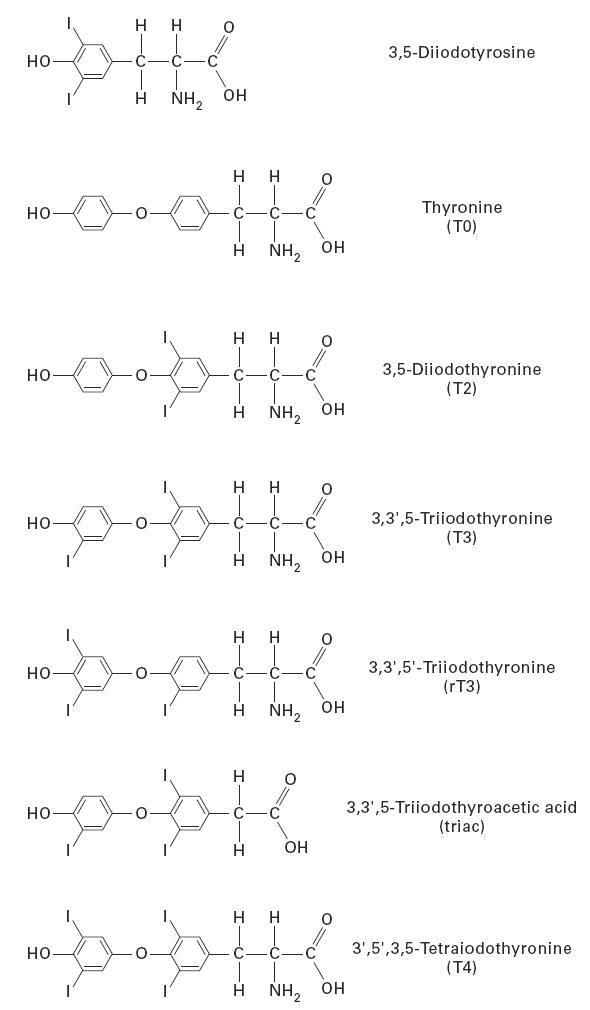
Chemical structure of iodinated tyrosines and thyronines.
Murine MoAbs to Tg
Preparation of murine MoAbs to Tg was described in a previous communication [12].
Iodination of Tg and bovine serum albumin
The method of iodination of bovine serum albumin (BSA) was the same as the one used to iodinate Tg, and this method was described in a companion paper [10].
Preparation of antibody to BSA
Antibody to BSA was prepared in a rat by subcutaneous injection of 300 μl of 0.34 mg/ml BSA, emulsified with Freund's incomplete adjuvant (FIA). The rat was bled 43 days later and the serum was used as the source of anti-BSA.
ELISAs
The details of the ELISA were presented in a previous publication [12].
Competitive inhibition ELISA of MoAbs 42C3 and 133B1 by T4, T3, rT3, triac, T2, diiodotyrosine, and T0
The ELISA plates were coated with normal Tg (N-Tg) by adding 50 μl of 1 μg/ml Tg in carbonate-bicarbonate buffer pH 9.6 to individual wells. The plates were incubated at 4°C for 14 h. On the next day, the wells were washed and were blocked for 1 h at room temperature with 100 μl of 1% BSA in PBS pH 7.2 containing 0.05% Tween 20 (PBS–T). T4, T3, rT3, triac, T2, diiodotyrosine, and T0 were dissolved in dimethylsulfoxide at a concentration of 1 mg/ml. This solution was then diluted with PBS to get the appropriate concentration of individual compound ranging from 0.0002 μg to 200 μg/ml. Monoclonal antibodies 42C3 and 133B1 were diluted with 1% BSA in PBS, so that it contained about a two-fold greater concentration than its end-point titre with Tg. The diluted MoAbs were then added to tubes containing the individual compounds. The tubes were then incubated at room temperature for 14 h. The final concentration of the inhibiting compounds ranged from 0.0001 to 100 μg/ml. Then, 100 μl of the treated antibody were added to an individual well of an ELISA plate containing Tg. Bound antibody was determined by ELISA for Tg. Alkaline phosphatase-labelled goat anti-mouse IgG (Jackson ImmunoResearch Labs, West Grove, PA), diluted 1:1000 with PBS–T, was used as a secondary antibody for this assay. Inhibition was calculated by dividing the absorption of ELISA assay of the treated MoAb by the absorption of the untreated (control) MoAb.
Competitive inhibition ELISA of anti-BSA by T4, T3, T2, and T0
For this assay, the plates were coated with 100 μl of 1 μg/ml of iodinated-BSA (I+-BSA) in carbonate-bicarbonate buffer pH 9.6, and incubated at 4°C for 14 h. On the next day, wells were blocked with 100 μl of 2% rat serum (RS) in PBS–T and incubated at room temperature for 1 h. T4, T3, T2 and T0 prepared as above were added to individual tubes containing anti-BSA (diluted in 2% RS) and incubated at room temperature for 14 h. One hundred microlitres of T4-, T3-, T2- and T0-treated anti-BSA were added to each well of an ELISA plate containing BSA and the plate was used for ELISA assay for BSA with rat anti-BSA as the detecting antibody. Phosphatase-labelled goat anti-rat IgG (Jackson), diluted 1:1000 (in 2% RS), was used as the secondary antibody for this assay. Inhibition of anti-BSA was calculated as described above.
Competitive inhibition ELISA of MoAb 42C3 by an irrelevant iodinated protein
This experiment was performed to find out if an irrelevant protein competes for binding of MoAb 42C3 to Tg. For this assay, I+-BSA was added at different concentrations, ranging from 5 ng to 50 μg, to tubes containing MoAb 42C3 and incubated at room temperature for 4 h. At the end of the incubation period, 100 μl of the I+-BSA-treated solution were added to each well of an ELISA plate, previously coated with Tg. A solution of untreated MoAb 42C3 was added to the control wells. The plate was used in an ELISA assay for Tg.
Gel electrophoresis and Western immunoblotting
The methods for gel electrophoresis and Western immunoblotting were described in detail in a previous communication [11].
RESULTS
Immunoreactivity of iodinated BSA with MoAb 42C3
Since MoAb 42C3 reacted with iodinated Tg, we conducted an experiment to determine if this MoAb also reacted with an unrelated iodinated protein, BSA. This protein was iodinated in vitro with iodobeads by the same method as Tg. The I+-BSA contained 24 atoms of iodine/molecule of BSA after iodination. The I+-BSA and Tg with different levels of iodination (INeg-Tg and I+-Tg) were analysed by SDS–PAGE and were transferred onto nitrocellulose (NC) membranes. The membranes were then treated with either MoAb 42C3 or anti-BSA. Figure 2a shows that BSA and I+-BSA reacted equally well with anti-BSA. The MoAb 42C3 reacted with I+-Tg, but failed to react with I+-BSA (Fig. 2b). These results show that MoAb 42C3 does not recognize an unrelated iodinated protein and further suggest that the recognition of I+-Tg by this MoAb is dependent upon a specific iodinated epitope of Tg.
Fig. 2.
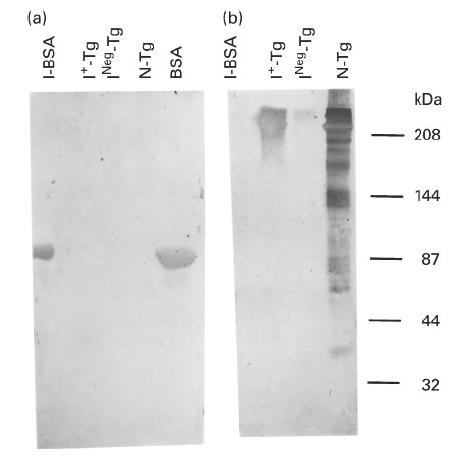
Western immunoblot pattern of binding of MoAb 42C3 and anti-bovine serum albumin (BSA) with normal Tg (N-Tg), INeg-Tg, I+-Tg, BSA and I+-BSA. Thirty micrograms of each protein were analysed on 7.5% SDS–PAGE and the protein was transferred into nitrocellulose (NC) membranes. The membrane was either treated with MoAb 42C3 or anti-BSA. (a,b) The immunoblot pattern of these proteins with anti-BSA and MoAb 42C3, respectively. The molecular weights of protein standards are shown on the right. INeg-Tg, Tg with no detectable iodine from a patient with non-toxic goitre; I+-Tg, Tg with no iodine that was iodinated in vitro; I+-BSA, BSA that was iodinated in vitro.
Inhibitory effect of iodinated thyronines and tyrosine on the binding of MoAb 42C3 to Tg
Bresler et al. [12] reported that certain MoAbs raised against Tg, including MoAb 42C3, were inhibited by different concentrations of T4 and T3. Here, we confirm the inhibition of MoAb 42C3 by T3 and T4 and extend the work to measure the inhibitory effect of different concentrations of not only T4 and T3 but also rT3, triac, T2, diiodotyrosine, and T0 on the binding of MoAb 42C3 to Tg. Thyronine or tyrosine with one added iodine was not available.
Figure 3 shows that T4, T3, rT3, triac, T2, and diiodotyrosine inhibited the binding of MoAb 42C3 to Tg to different degrees. The 50% inhibition value was used to compare the inhibitory effect of different compounds on MoAbs. The inhibitory effect of these compounds depended upon both the iodine content and its arrangement on the molecule. The highly iodinated compound (T4) inhibited the immunoreactivity of MoAb 42C3 by 50% at a very low concentration (0.0005 μg/ml). T3 inhibited the binding of the same MoAb to Tg by 50% at a concentration of 0.1 μg/ml, rT3 inhibited this reaction at a concentration of 1 μg/ml, and T2 inhibited this antibody by 50% only at an estimated concentration of 500 μg/ml. The slope of the inhibition curve provided by T2 differed from those of the other iodinated thyronines. Thyronine with no iodine failed to inhibit this antibody at any concentration tested.
Fig. 3.
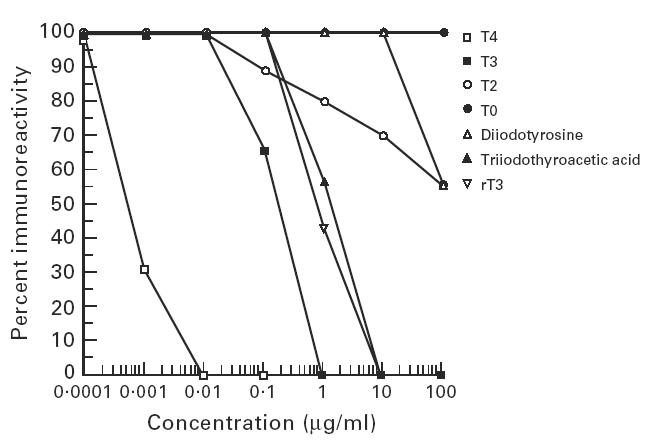
Inhibition of the immunoreactivity of binding of MoAb 42C3 to Tg by different concentrations of T4, T3, rT3, T2, T0, triiodothyroacetic acid and diiodotyrosine. Monoclonal antibody 42C3 was incubated with different concentrations of each compound at room temperature for 14 h. The T4 (thyroxine), T3 (triiodothyronine), rT3 (reverse T3), T2 (diiodothyronine), T0 (thyronine), diiodotyrosine, and triiodothyroacetic acid-treated antibody was then added to an ELISA plate coated with Tg. As control, untreated antibody was added to the plate. The amount of immunoglobulin bound to Tg was then measured by ELISA. Details of the ELISA are discussed in Materials and Methods. The figure shows inhibitory effect of each compound at concentrations of 0.0001 μg/ml to 100 μg/ml on binding of MoAb 42C3 to Tg in comparison with untreated (control) antibody.
We also determined the dissociation constants (KD) of binding of these iodinated thyronines to MoAb 42C3 by the method of Friguet et al. [13]. The results are shown in Table 1. They confirm the data presented in Fig. 3 that the affinity of MoAb 42C3 for T4 is much greater than its binding affinity for other iodinated thyronines or tyrosine.
Table 1.
Dissociation constant (KD)* of binding of iodinated compounds to MoAbs 42C3 and 133B1
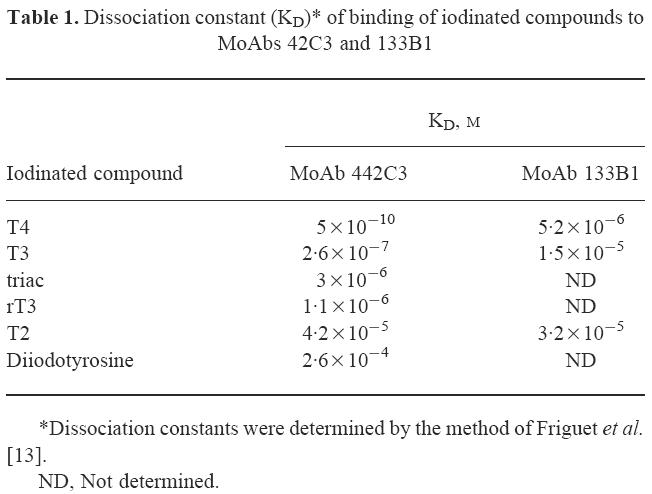
Since not all iodinated sites may contain thyronines, we conducted an experiment to find out if iodinated tyrosine would inhibit MoAb 42C3. Different concentrations of diiodotyrosine were incubated overnight with MoAb 42C3 and the inhibition experiment continued the same way as for T4, T3, rT3, T2 and thyronine. Diiodotyrosine inhibited MoAb 42C3 by 50% only at an estimated concentration of 500 μg/ml (Fig. 3), similar to T2, which also inhibited MoAb 42C3 by 50% at this concentration. Although the KD of diiodotyrosine is 10-fold higher than T2 (Table 1), these results suggest that diiodinated derivatives of these compounds with either one (diiodotyrosine) or two aromatic rings (T2) have the same weak inhibitory effect on the binding of MoAb 42C3 to normal Tg.
Structural similarity of MoAb 42C3 to T3 and T4 receptor
Surks et al. [14] reported that the T3 and T4 receptor bound to an iodinated derivative of thyronine. This receptor is present in nuclear membranes of most cells and is required for the uptake of these hormones. Since MoAb 42C3 was inhibited by T3 and T4, this MoAb may have structural similarity to the T3 or T4 receptor. The order of binding of these compounds to the receptor was triac > ′3-isopropyltriiodothyronine > T3 > T4 > rT3, while T2 and monoiodothyronine did not bind to the receptor. Since the receptor bound strongly to triac, we tested the inhibitory effect of this compound on binding of MoAb 42C3 to Tg. In contrast to the receptor, MoAb 42C3 was inhibited by these compounds in the order of T4 > T3 > rT3 > triac > T2 (Fig. 3). These data show that triac, a derivative of T3 that binds strongly to the receptor, required a concentration 10-fold greater than T3 to inhibit MoAb 42C3 by 50%. Thus, it is unlikely that the 42C3 binding site mimics the T3 and T4 receptor.
Inhibitory effect of iodinated thyronines and tyrosine on the binding of MoAb 133B1
In a previous study [12] we found that MoAb 133B1 was inhibited only at high concentrations of T4, but not T3. This MoAb also reacted with N-Tg and INeg-Tg, but it did not react with this Tg after iodination in vitro (I+-Tg) [10]. Since MoAb 133B1 showed a different specificity from MoAb 42C3, we studied the effect of T4, T3, T2 and T0 on the binding of MoAb 133B1 to normal Tg, and the results are shown in Fig. 4. Although, in contrast to our earlier report [12], MoAb 133B1 was inhibited by T3, the concentrations of these compounds needed to inhibit this MoAb by 50% were much higher than the concentrations of the compounds to inhibit MoAb 42C3 (Figs 3 and 4). T4 inhibited MoAb 133B1 by 50% at a concentration of 20 μg/ml. T3 and T2 required concentrations of > 200 μg/ml for 50% inhibition and T0 did not inhibit this MoAb at any concentration tested. The KDs of these iodinated thyronines on the binding of MoAb 133B1 were also higher than the KDs of these compounds on the binding of MoAb 42C3 (Table 1). Since T4, T3, rT3 and T2 inhibited MoAb 133B1 only at high concentrations, we did not test the effect of the other compounds (rT3, triac and diiodotyrosine) on the binding of this MoAb to Tg.
Fig. 4.
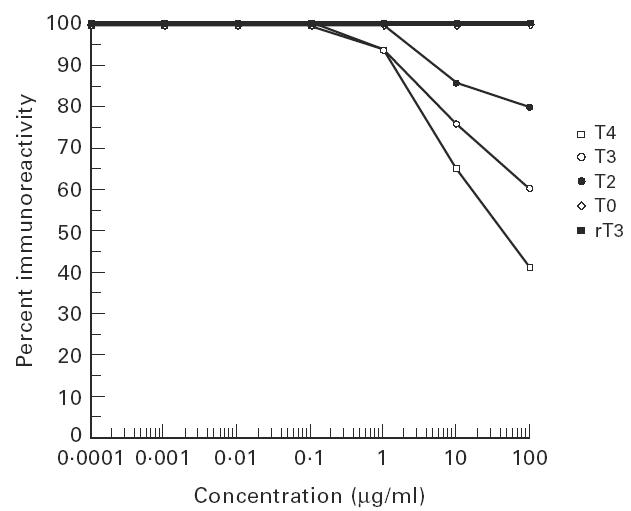
Inhibition of the immunoreactivity of binding of MoAb 133B1 to Tg by different concentrations of T4, T3, T2 and T0. Monoclonal antibody 133B1 was incubated with different concentrations of each compound at room temperature for 14 h. T4 (thyroxine), T3 (triiodothyronine), T2 (diiodothyronine) and T0 (thyronine)-treated antibody was then added to an ELISA plate coated with Tg. As control, untreated antibody was added to the plate. The amount of immunoglobulin bound to Tg was then measured by ELISA. Details of ELISA are discussed in Materials and Methods. The figure shows the inhibitory effect of each compound at concentrations of 0.0001 μg/ml to 100 μg/ml on binding of MoAb 133B1 to Tg in comparison with untreated (control) antibody.
The iodinated thyronines (T4, T3 and T2) did not inhibit the binding of anti-BSA to I+-BSA at any concentration tested, confirming the specificity of the competition assays. As a further test for specificity of this reaction, we also expanded the competitive inhibition of I+-BSA on binding of MoAb 42C3 to Tg. I+-BSA was incubated with MoAb 42C3 and the mixture was then added to an ELISA plate coated with Tg. I+-BSA did not reduce the binding of MoAb 42C3 to Tg at concentrations from 50 ng to 500 μg/ml, showing that iodotyrosines of I+-BSA are unable to compete for binding sites of MoAb 42C3.
DISCUSSION
Based on our previous studies, the binding of MoAb 42C3 to Tg has the following characteristics: (i) it binds to a relatively conserved region, (ii) the binding is inhibited by both T4 and T3, and (iii) the corresponding epitope is recognized by autoantibodies found in the sera of many normal individuals as well as patients with thyroid disease [1]. Most important, the binding of MoAb 42C3 to Tg varies according to the levels of iodine of naturally iodinated Tg [9]. Other studies showed that the epitope of MoAb 42C3 is not affected by reduction and may therefore not be dependent upon its conformation [15].
We found that iodinated thyronines (T4, T3, rT3 and T2) and iodinated tyrosine but not non-iodinated thyronine (T0) inhibits the binding of MoAb 42C3 to Tg (Fig. 3). This finding suggests that one of these iodinated compounds is present in the binding site of Tg. Furthermore, T4, T3, rT3, T2 and T0 blocked the binding of MoAb 42C3 to Tg in proportion to their iodine content, indicating that the content of iodine substantially increases the affinity of the antigen–antibody complex. When the affinities were calculated by the method of Friguet et al. [13], they were greatest with T4. The affinities decreased with the other derivatives of thyronines and tyrosine (Table 1). Based on these results, we suggest that MoAb 42C3 binds most strongly to an epitope containing T4.
Iodination of Tg by the in vitro method, which contains N-chloro-benzenesulphonamide as the oxidizing agent, may iodinate most of the 134 tyrosines of Tg. However, chemical iodination results in few T4 residues compared with the number of monoiodotyrosines (MIT) or diiodothyrosines (DIT) in contrast to enzymatically iodinated Tg [16]. Other amino acids such as histidine and cysteine of the Tg molecule could be iodinated as well. The reactivity of MoAb 42C3 appears specific to the Tg molecule, as this antibody did not react with the unrelated iodinated protein, I+-BSA (Fig. 2). Although BSA does not contain thyronines, we would expect I+-BSA to contain iodinated tyrosines, histidines and cysteines, since the iodinated BSA contained 24 atoms of iodine/molecule of BSA. Even high concentrations of I+-BSA were unable to bind MoAb 42C3.
We observed that the more iodine substitutions, the higher the binding affinity of the iodinated thyronines for the MoAb 42C3. This cannot be accounted for by alteration in the shape of the Tg molecule, since the only differences were the number of iodines on the thyronines. Reverse T3 inhibited the binding of 42C3 (Fig. 3), so the orientation of the iodines on the molecule matters less than the number of iodines. However, orientation does play a role in the epitope binding of MoAb 42C3, since rT3 has a 10-fold lower binding affinity than T3. Other parts of the molecule may have a minor influence on the binding affinity. Triac contains the same number of iodines with the same orientation as T3, but lacks the amino group of T3 (Fig. 1). Again, the binding affinity of triac to MoAb 42C3 was 10-fold less than that of T3 for this MoAb (Fig. 3).
Another MoAb directed to Tg, MoAb 133B1, was also inhibited by T4 [12]. It is clearly different in specificity from MoAb 42C3, because MoAb 133B1 bound to a human-specific epitope whereas MoAb 42C3 bound to a cross-reactive epitope shared by Tg of all species tested [12]. A concentration of 20 μg/ml of T4 was required to inhibit MoAb 133B1, in contrast to only 0.0005 μg/ml of T4 needed to inhibit MoAb 42C3. We conclude therefore that iodothyronines contribute little to the binding affinity of MoAb 133B1, but play a major role in the binding affinity of MoAb 42C3.
Based on the foregoing data, we suggest that the antibody binding site of MoAb 42C3 has the best fit with an epitope that contains T4. If fewer iodines are present the fit is less perfect, but binding will still occur. An alternative explanation is that iodine may increase electrostatic binding of the Tg molecule and thus enhance its antibody binding. A precedent for this suggestion is the report by Yuhasz et al. [17], who showed that a hapten-specific binding could be enhanced by iodination of the hapten. The enhanced binding occurred not by additional interactions of the antibody with the iodine, but by stabilizing the resonance form of the hapten that interacts most favourably with the antibody.
In conclusion, in this study and the companion article [10], we have found that iodine has an important function in immunoreactivity of Tg with its respective antibodies. On one hand, the presence of iodine in the Tg molecule, inserted either physiologically or artificially, affects its conformation so that cryptic epitopes appear and other epitopes are lost. On the other hand, iodinated thyronine, a derivative of tyrosine, an amino acid unique to Tg, determines the binding specificity of certain antibodies. Thus, MoAb 42C3 will provide a unique tool to evaluate the influence of iodine on antigenic properties of Tg.
Acknowledgments
This study was funded in part by NIH grant DK-42174 and NIEHS Center Grant ES03819.
References
- 1.Bresler HS, Burek CL, Hoffman WH, Rose NR. Autoantigenic determinants on human thyroglobulin: II. Determinants recognized by autoantibodies from patients with chronic autoimmune thyroiditis compared to autoantibodies from healthy subjects. Clin Immunol Immunopathol. 1990;54:76–86. doi: 10.1016/0090-1229(90)90007-d. [DOI] [PubMed] [Google Scholar]
- 2.Caturegli P, Mariotti S, Kuppers RC, Burek CL, Pinchera A, Rose NR. Epitopes on thyroglobulin: a study of patients with thyroid disease. Autoimmunity. 1994;18:41–9. doi: 10.3109/08916939409014678. [DOI] [PubMed] [Google Scholar]
- 3.Rasooly L, Rose NR, Saboori AM, Ladenson PW, Burek CL. Iodine is essential for human T cell recognition of human thyroglobulin. Autoimmunity. 1998;27:313–9. doi: 10.3109/08916939808993833. [DOI] [PubMed] [Google Scholar]
- 4.den Hartog MT, De Boer M, Veenboer GJ, de Vijlder JJ. Generation and characterization of monoclonal antibodies directed against noniodinated and iodinated thyroglobulin, among which are antibodies against hormonogenic sites. Endocrinology. 1990;127:3160–5. doi: 10.1210/endo-127-6-3160. [DOI] [PubMed] [Google Scholar]
- 5.Mallet B, Lejeune PJ, Ruf J, Piechaczyk M, Marriq C, Carayon P. Tyrosine iodination and iodotyrosyl coupling of the N-terminal thyroid hormone forming site of human thyroglobulin modulate its binding to auto- and monoclonal antibodies. Mol Cell Endocrinol. 1992;88:89–95. doi: 10.1016/0303-7207(92)90012-u. [DOI] [PubMed] [Google Scholar]
- 6.Hutchings P, Cooke A, Dawe K, et al. A thyroxine-containing peptide can induce murine experimental autoimmune thyroiditis. J Exp Med. 1992;175:869–72. doi: 10.1084/jem.175.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong YC, McCormick DJ, Wan Q, et al. Primary hormonogenic sites as conserved autoepitopes on thyroglobulin in murine autoimmune thyroiditis. Secondary role of iodination. J Immunol. 1995;155:5847–54. [PubMed] [Google Scholar]
- 8.Wan Q, Motte RW, McCormick DJ, et al. Primary hormonogenic sites as conserved autoepitopes on thyroglobulin in murine autoimmune thyroiditis. Clin Immunol Immunopathol. 1997;85:187–94. doi: 10.1006/clin.1997.4443. [DOI] [PubMed] [Google Scholar]
- 9.Saboori AM, Rose NR, Kuppers RC, Butscher WG, Bresler HS, Burek CL. Immunoreactivity of multiple molecular forms of human thyroglobulin. Clin Immunol Immunopathol. 1994;72:121–8. doi: 10.1006/clin.1994.1115. [DOI] [PubMed] [Google Scholar]
- 10.Saboori AM, Rose NR, Bresler HS, Talor M, Burek CL. Iodination of human thyroglobulin (Tg) alters its immunoreactivity I. Iodination alters multiple epitopes of human Tg. Clin Exp Immunol. 1997;113:297–302. doi: 10.1046/j.1365-2249.1998.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saboori AM, Rose NR, Burek CL. Amino acid sequence of a tryptic peptide of human thyroglobulin reactive with sera of patients with thyroid disease. Autoimmunity. 1995;22:87–94. doi: 10.3109/08916939508995304. [DOI] [PubMed] [Google Scholar]
- 12.Bresler HS, Burek CL, Rose NR. Autoantigenic determinants on human thyroglobulin: I. Determinant specificities of murine monoclonal antibodies. Clin Immunol Immunopathol. 1990;54:64–75. doi: 10.1016/0090-1229(90)90006-c. [DOI] [PubMed] [Google Scholar]
- 13.Friguet B, Chaffotte AF, Djavadi-Ohaniance L, Goldberg ME. Measurements of the true affinity constant in solution of antigen–antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods. 1985;77:305–19. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- 14.Surks MI, Koerner DH, Oppenheimer JH. In vitro binding of L-triiodothyronine to receptors in rat liver nuclei. Kinectics of binding, extraction properties, and lack of requirement for cytosol proteins. J Clin Invest. 1975;55:50–60. doi: 10.1172/JCI107917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saboori AM, Burek CL, Rose NR, Bresler HS, Talor M, Kuppers RC. Tryptic peptides of human thyroglobulin: I. Immunoreactivity with murine monoclonal antibodies. Clin Exp Immunol. 1994;98:454–8. doi: 10.1111/j.1365-2249.1994.tb05512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taurog A, Dorris ML, Lamas L. Comparison of lactoperoxidase- and thyroid peroxidase-catalyzed iodination and coupling. Endocrinology. 1974;94:1286–94. doi: 10.1210/endo-94-5-1286. [DOI] [PubMed] [Google Scholar]
- 17.Yuhasz SC, Ysern X, Strand M, Amzel M. Structural analysis of affinity maturation: the three dimensional structure of complexes on an anti-nitrophenol antibody. Mol Immunol. 1995;32:1143–56.. doi: 10.1016/0161-5890(95)00063-1. [DOI] [PubMed] [Google Scholar]


