Abstract
Human Tg, the site of synthesis of thyroid hormones, thyroxine (T4) and triiodothyronine (T3), is one of the major autoantigens in autoimmune thyroiditis. The degree of iodination of Tg may have a major impact on its immunological properties by changing its antigenicity with respect to antibody binding. We have previously prepared a panel of MoAbs that bind to different epitopes of the Tg molecule. In the present study, we show that iodination alters the conformation of Tg molecule in such a way that it is recognized differently by different MoAbs. Monoclonal antibody 137C1 recognizes Tg regardless of its iodine content. Monoclonal antibody 42C3 recognizes Tg only if the Tg is iodinated either in vitro or in vivo. Monoclonal antibody 133B1 recognizes both in vivo iodinated Tg and non-iodinated Tg, but this MoAb did not recognize Tg following in vitro iodination. Monoclonal antibody 41A5 recognizes intact Tg and tryptic peptides of normal (in vivo) iodinated and non-iodinated Tg, but did not bind the tryptic peptides of artificially (in vitro) iodinated Tg. From the results of these experiments, we conclude that iodination of Tg by either in vivo or in vitro methods changes its conformation in such a way that some natural epitopes are ‘lost’ and some ‘new’ epitopes are generated. The generation of new epitopes may be important in the generation of autoimmune responses leading to autoimmune disease.
Keywords: thyroglobulin, autoimmunity, monoclonal antibody, iodine
INTRODUCTION
Tg is one of the major proteins synthesized by the thyroid epithelial cell and is used for the storage of iodine with subsequent synthesis of the thyroid hormones, thyroxine (T4) and triiodothyronine (T3) [1]. Several epidemiological and clinical reports suggest that the incidence of autoimmune thyroid disease has risen concomitantly with increased iodine consumption [2–6]. Furthermore, experimental evidence in animal models showed that increased dietary iodine enhanced the development of autoimmune thyroiditis in genetically susceptible CS and OS chickens [7,8], BB/W and BUF rats [9,10], NOD-H2h4 mice [11], and hamsters [12]. Additional work has been performed in vitro. Champion et al. have shown that certain T cell hybridomas responded to Tg in direct proportion to its iodine content [13]. The T4-containing peptides recognized by these hybridomas have also been shown to induce thyroiditis experimentally [14]. Further work by Kong et al. [15] confirmed that certain iodinated peptides heightened the proliferative responses of cultures from mice immunized with Tg. However, they also demonstrated that all peptides were not dependent upon iodine to induce T cell proliferation, since one peptide that contained a hormonogenic site induced proliferation equally well with or without iodine. Thus, it appears that for T cell recognition, some peptides are dependent upon the presence of iodine while others are not. In a recent report, we also demonstrated that human T cells responded more vigorously in vitro to iodinated Tg than the same non-iodinated Tg [16].
The mechanism by which iodine promotes autoimmune thyroiditis has not been clarified. Iodine may have multiple effects, one of which, suggested by various animal studies, is that iodination of the Tg molecule changes its antigenic characteristics. We have considered two possibilities to account for these changes. The insertion of iodine may alter the stereochemical configuration of the Tg molecule and thus affect the manner by which it is recognized by the immune system. The second possibility is that iodine may create novel binding sites by its presence on a particular antigenic determinant. The latter possibility is dealt with in a companion paper [17]. In the present study, we present evidence that the presence of iodine alters multiple epitopes of the Tg molecule, indicating both a direct involvement of iodine as well as a major conformational change.
Monoclonal antibodies raised against Tg have provided a useful tool to map various epitopes of the Tg molecule [18–21]. For this study, we selected four MoAbs on the basis of their differing specificity for Tg. Three of the four MoAbs were inhibited by T4, whereas the fourth MoAb was not [22]. We compared the immunoreactivity of these MoAbs with naturally and in vitro iodinated Tg and Tg containing no detectable iodine. In addition to the intact molecule, we evaluated the reactivity of peptide fragments of Tg produced by limited proteolytic digestion to test for hidden determinants.
MATERIALS AND METHODS
Chemicals and the method for protein assay used in this study were described in a previous communication [23].
Murine MoAbs to Tg
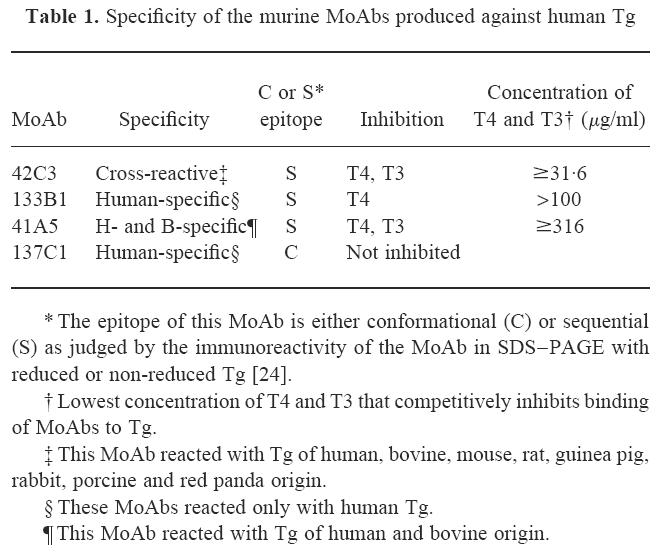
Preparation and characterization of the MoAbs were described by Bresler et al. [22]. The specificity and inhibition properties of the MoAbs used in this study are shown in Table 1. We also indicated that the MoAb reacted with a sequential (S) or conformational (C) determinant as judged by binding to reduced versus non-reduced Tg [24].
Table 1.
Specificity of the murine MoAbs produced against human Tg
Thyroglobulin preparation and trypsinization of Tg
Preparation and trypsinization of Tg have been described in detail in the previous publication [24]. Thyroglobulin with no detectable iodine was obtained from a patient with non-toxic goitre (INeg-Tg) and this Tg was later iodinated with iodobeads.
Iodination of Tg
Iodobeads were washed with 100 mm phosphate buffer pH 7.0, and dried on filter paper. Twenty beads were used for iodination. The beads were put in 1.5 ml of 1.5 mm potassium iodide for 5 min at room temperature with continuous shaking. Protein (3 mg) was added to the beads and incubated at room temperature for 30 min with continuous shaking. The supernatant, containing the iodinated protein, was dialysed against four changes (each of 8 h at 4°C) of 500 ml of 100 mm phosphate buffer pH 7.0. The iodine content of the sample was determined by a method described previously [25]. As a control, the procedure was carried out with Tg omitting the potassium iodide. The in vitro iodinated Tg (I+-Tg) had 150 atoms of iodine/molecule of Tg.
Dot blot analysis
For the dot blot experiment, 5 μl of 5 μg/ml of Tg with different levels of iodination, or the tryptic peptides of these Tg were applied to nitrocellulose (NC) membranes. The membranes were incubated for 2 h at room temperature with different MoAbs, diluted with 1% bovine serum albumin (BSA) in PBS containing 0.05% Tween-20 (PBS–T). At the end of the incubation period, the membranes were washed with PBS–T and incubated with alkaline phosphatase-labelled goat anti-mouse antibody for 2 h at room temperature. The membranes were then washed with PBS–T, and incubated with alkaline phosphatase substrate (5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium) according to the method of Sambrook et al. [26].
Gel electrophoresis and Western immunoblotting
Methods for gel electrophoresis and Western immunoblotting were described in detail previously [27].
RESULTS
Dot blots of iodinated and non-iodinated Tg and their tryptic peptides
The MoAbs listed in Table 1 were tested by dot blot for their reaction with iodinated and non-iodinated preparations of Tg and their tryptic peptides. Each of these MoAbs recognized a different epitope, since the binding of one MoAb to Tg did not inhibit the binding of the other MoAbs [22]. Monoclonal antibody 42C3, which is inhibited by T3 and T4, reacted with sequential determinant shared by naturally iodinated Tg of all species tested. This MoAb failed to react with INeg-Tg (Fig. 1a) and its tryptic peptides (Fig. 1b). It did react, however, when this preparation was iodinated in vitro (I+-Tg).
Fig. 1.
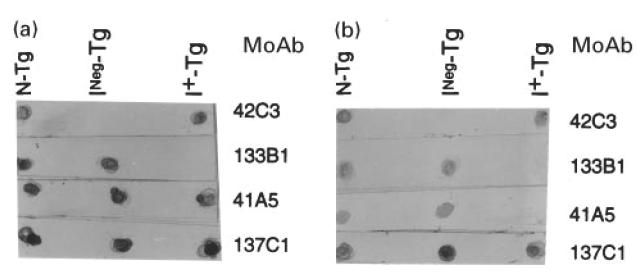
Dot blot analysis of thyroglobulins with different levels of iodination and their tryptic peptides reacted with MoAbs 137C1, 41A5, 42C3 and 133B1. The normal Tg (N-Tg) contained 19 atoms of iodine/molecule of Tg. The INeg-Tg had no detectable iodine. The I+-Tg was made from NTG-Tg that was iodinated to the level of 150 atoms of iodine/molecule. Five microlitres of 5 μg/ml of Tg or 2.5 μl of 100 μg/ml of tryptic peptides of Tg were applied into NC membrane. The protein on the membrane was treated with the appropriate MoAb. Details are given in Materials and Methods. The figure shows the immunoreactivity of these MoAbs with intact Tg (a) or tryptic peptides of Tg (b).
Monoclonal antibody 133B1 is human-specific and is inhibited by T4 but not by T3 (Table 1). This MoAb also recognized a sequential determinant but differed from the determinant recognized by MoAb 42C3. In contrast to MoAb 42C3, MoAb 133B1 reacted with INeg-Tg but failed to react with this preparation after in vitro iodination (Fig. 1a). It even failed to react with the tryptic peptides generated from in vitro iodinated Tg (Fig. 1b), showing that the corresponding sequential epitope was not available even on the peptide fragments.
Monoclonal antibody 41A5 was reactive only with human and bovine Tg and inhibited by T4 and T3. This MoAb reacted with all three Tg preparations regardless of their iodine content (Fig. 1a). It failed, however, to react with the tryptic peptides of in vitro iodinated Tg (Fig. 1b), suggesting that a sequential epitope of the iodinated product was altered or destroyed by proteolytic digestion. Although each of the three MoAbs was inhibited by T4, they clearly differed in competitive inhibition assays [22], suggesting that each MoAb reacts with a different determinant of Tg. Thus, the presence of iodine is associated with changes in multiple epitopes.
Monoclonal antibody 137C1 is a human-specific MoAb that was not inhibited by T3 or T4. This MoAb bound to a conformational epitope that was not affected by the presence of iodine in the Tg molecule. This MoAb also reacted with the whole Tg and tryptic peptides generated from iodinated and non-iodinated Tg (Fig. 1a, b). These findings indicate that the presence of iodine does not affect the immunoreactivity to other epitopes of Tg.
Western immunoblots of intact Tg
We then tested the immunoreactivity of the four MoAbs, 42C3, 133B1, 41A5 and 137C1, by Western immunoblot with Tg having different levels of iodination. Monoclonal antibody 42C3 reacted with the 330-kD monomer of normal Tg (N-Tg) but failed to react with INeg-Tg (Fig. 2a), thus confirming the results of the dot blot experiments shown in Fig. 1. This MoAb also reacted with some low molecular weight peptides of N-Tg, suggesting that the sequential epitope recognized by this MoAb is present in naturally occurring low molecular weight fragments of Tg. Monoclonal antibody 42C3 reacted with I+-Tg, the Tg preparation iodinated in vitro, by Western immunoblot. In contrast to N-Tg, however, I+-Tg did not produce distinct bands. It showed a smeared pattern rather than the distinct bands of N-Tg.
Fig. 2.
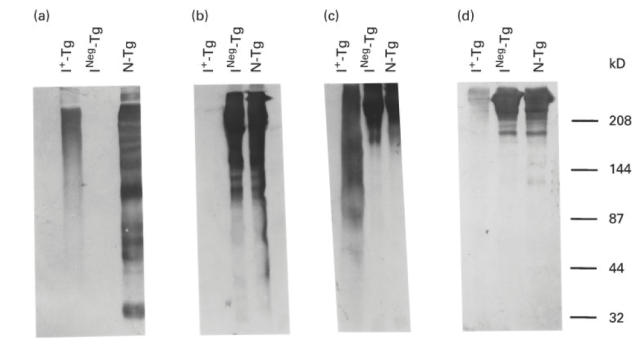
Western immunoblot patterns of binding of MoAbs 42C3, 133B1, 41A5 and 137C1 to intact thyroglobulins. Thirty micrograms of normal Tg (N-Tg), non-toxic goitre-Tg (INeg-Tg), and iodinated-NTG-Tg (I+-Tg) were analysed on a 7.5% SDS–PAGE. The proteins on the gel were transferred into an NC membrane. The membrane was treated with either MoAb 42C3 (a), 133B1 (b), 41A5 (c) or 137C1 (d). The molecular weights of protein standards are shown on the right.
When analysed on SDS–PAGE and stained for protein by coomassie blue, I+-Tg failed to resolve into distinct bands (Fig. 3a). This pattern of I+-Tg may be due to the addition of iodine on this Tg or to treatment of the Tg with iodobeads used for iodination. To rule out the latter possibility, we treated INeg-Tg with iodobeads without the added iodine. The iodobeads-treated INeg-Tg showed distinct bands on SDS–PAGE (Fig. 3b). Hence the smeared pattern of I+-Tg on either SDS–PAGE stained with coomassie blue (Fig. 3a) or Western immunoblot with MoAbs 42C3 and 41A5 (Fig. 2) is not due to the treatment of this Tg with iodobeads. We attributed this finding to an alteration in the conformation of in vitro iodinated Tg molecule, as will be discussed later.
Fig. 3.
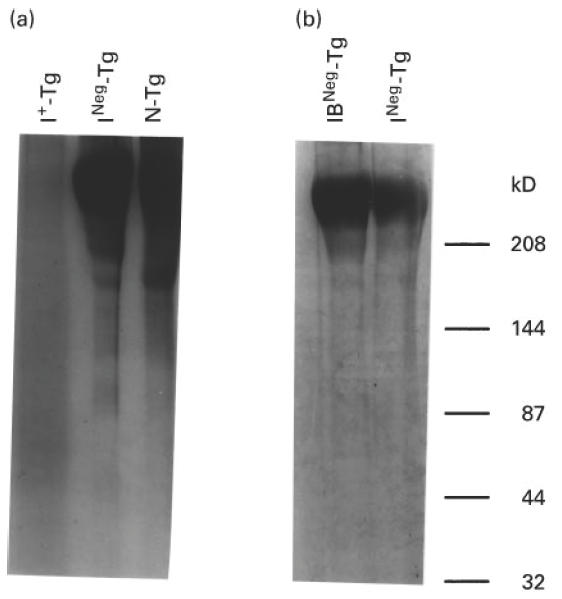
Electrophoretic gel pattern of Tg with different levels of iodination. Fifty micrograms of normal Tg (N-Tg) (with 19 atoms of iodine/molecule of Tg), INeg-Tg (with no detectable amounts of iodine), and I+-Tg (with 150 atoms of iodine/molecule of Tg) were analysed on a 7.5% SDS–PAGE and the gel was stained with coomassie blue (a). (b) Fifty micrograms of Tg of either control (not treated with iodobeads) (INeg-Tg) or Tg treated with iodobeads (IB-INeg-Tg) were analysed on 7.5% SDS–PAGE and stained with coomassie blue. The molecular weights of protein standards are shown on the right.
Figure 2b shows the Western immunoblot pattern of the three Tg with MoAb 133B1. This MoAb reacted with N-Tg and INeg-Tg, but not with INeg-Tg after in vitro iodination, confirming the results of dot blot shown in Fig. 1. Thus, in vitro iodination of INeg-Tg changes its structure in such a way that it is no longer recognized by MoAb 133B1.
Figure 2c shows the immunoblot pattern of N-Tg, INeg-Tg and I+-Tg with MoAb 41A5. In contrast to the immunoreactivity of MoAbs 133B1 and 42C3, MoAb 41A5 reacted with all three Tg. Similar to the Western immunoblot pattern of MoAb 42C3 with I+-Tg, the Western immunoblot of MoAb 41A5 showed a smeared pattern.
Figure 2d shows the immunoblot patterns of MoAb 137C1. This MoAb reacted to both N-Tg and INeg-Tg. A faint band was seen in the immunoblot of this MoAb with I+-Tg, indicating some diminished reactivity compared with N-Tg. This reduced reaction may be attributed to an altered configuration of the corresponding epitope on iodinated Tg. In contrast to MoAb 42C3 (Fig. 2a), MoAb 137C1 did not react with naturally occurring low molecular weight peptides of Tg (Fig. 2d), suggesting that this conformational epitope is not present in the low molecular weight fragments of Tg.
Western immunoblot of tryptic peptides
Since both physiologically and in vitro iodinated Tg reacted with MoAb 42C3 (Figs 1 and 2), we attempted to identify the iodinated fragments that reacted with this MoAb. For this purpose, the tryptic peptides of the three Tg used in Fig. 2 were analysed by Western immunoblot for reactivity with MoAbs 42C3, 133B1, 41A5 and 137C1 (Fig. 4a–d, respectively).
Fig. 4.
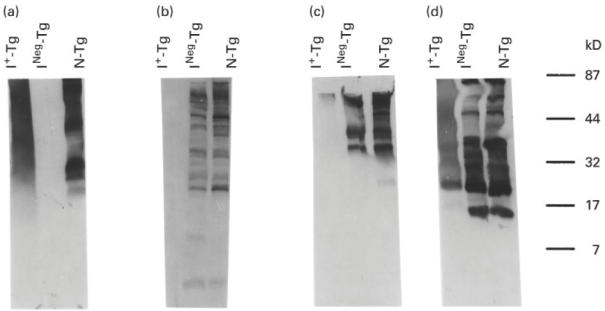
Western immunoblot pattern of immunoreactivity of binding of MoAbs 42C3, 133B1, 41A5 and 137C1 to tryptic peptides of normal Tg (N-Tg), INeg-Tg and I+-Tg. Thirty micrograms of 4-h tryptic peptides of N-Tg, INeg-Tg, and I+-Tg were analysed on a 5–20% SDS–PAGE and the peptides were transferred into a NC membrane. The membrane was then treated with either MoAb 42C3, 133B1, 41A5 or 137C1. (a,b,c,d) The immunoblot patterns of MoAbs 42C3, 133B1, 41A5, and 137C1, respectively. The molecular weights of protein standards are shown on the right.
Monoclonal antibody 42C3 (Fig. 4a) failed to react with tryptic peptides of INeg-Tg, but did react with multiple peptides with molecular weights from 20 to 100 kD of N-Tg and I+-Tg. The electrophoretic patterns of tryptic peptides of N-Tg and I+-Tg differed, suggesting that the structure of I+-Tg changed during the process of trypsin cleavage.
Figure 4b shows the Western immunoblot pattern of MoAb 133B1 with the tryptic peptides of the three Tg. This MoAb reacted with the peptides of N-Tg and INeg-Tg, but not with I+-Tg, confirming the data presented in Figs 1 and 2. Thus, the epitope of MoAb 133B1 was not available even on the tryptic peptides of I+-Tg.
The Western immunoblot pattern of MoAb 41A5 with tryptic peptides of I+-Tg differed markedly from the pattern of the other two MoAbs, 137C1 and 42C3. In the dot blot experiment presented in Fig. 1b, MoAb 41A5 reacted with peptides of normal and INeg-Tg but failed to react with I+-Tg (Fig. 4c). Similarly, the immunoblot pattern of this MoAb with I+-Tg showed faint bands in comparison with the distinct bands produced by this Tg before iodination (Fig. 4). Since in Fig. 1 this MoAb did not react with the tryptic peptides of I+-Tg in the dot blot experiment, the faint band seen in Western immunoblot of the MoAb might be due to reduced reactivity of this MoAb with this Tg.
Monoclonal antibody 137C1 (Fig. 4d) reacted similarly to tryptic peptides of both normal and INeg-Tg. The reactivity of this MoAb to tryptic peptides of I+-Tg, however, differed from the pattern of INeg-Tg and N-Tg; peptides of these Tg showed distinct bands, in contrast to the extended band produced by peptides of I+-Tg. Furthermore, a prominent low molecular weight band (Mpa of 15 kD) in the immunoblot pattern of Tg was substantially reduced or absent in the peptides generated from I+-Tg, indicating that in vitro iodination altered the conformation of the Tg molecule and changed the gel pattern of the tryptic fragments.
DISCUSSION
The major finding in this study is that iodinated Tg differs from non-iodinated Tg in its reactivity with a panel of MoAbs, indicating a loss of some epitopes and the gain of others. Although MoAb 42C3 failed to react with Tg that lacked detectable iodine, it reacted with this same preparation of Tg if it were iodinated in vitro. In our previous experiments, we used Tg preparations from different individuals to obtain Tg with various levels of iodination [24]. A limitation of these studies was that allotypic or post-translational differences may be responsible for the differences seen [28]. These problems were avoided by starting with a Tg preparation that had no detectable iodine; we then iodinated this preparation in vitro. Our present study shows that addition of iodine to the Tg molecule enabled it to bind to MoAb 42C3. Thus, Tg reacted with this MoAb if the protein were iodinated, either in vivo or in vitro. This indicates that iodine but not other post-translational modifications are involved in the binding of MoAb 42C3 to Tg.
We have considered two possible explanations for these findings: (i) MoAb 42C3 binds directly to an epitope that contains iodine, such as iodothyronine, or (ii) the presence of iodine alters the conformation of the Tg molecule. We found evidence supporting both of these possibilities. Considering the first alternative, certain of our experimental data favour the concept that the binding site of MoAb 42C3 contains iodinated thyronine or tyrosine. This issue is considered in detail in a companion paper [17]. Other findings support the view that iodination produces profound changes in the configuration of the Tg molecule, eliminating some determinants and perhaps revealing hidden or cryptic determinants [29].
Work by Dunn et al. [30] demonstrated that major changes occurred in the stereochemical configuration of Tg when iodinated. These authors, however, did not investigate changes in immunoreactivity of the iodinated Tg. They did show that increased iodination had dramatic effects on the naturally derived fragments of Tg. The observations of Dunn and colleagues are similar to those we found by SDS–PAGE and Western immunoblot experiments (Figs 2,3 and 4). For the experiment with MoAb 42C3, we used either a 7.5% gel with 3% stacking gel to analyse the whole I+-Tg or 5–20% gel with no stacking gel to analyse the tryptic peptides of this in vitro-iodinated Tg. We did not see any protein remaining on the top of the gel, therefore intact Tg or its tryptic peptides entered the gels. We concluded therefore that the smeared pattern of I+-Tg was not due to aggregation of this Tg on the top of the gel. The broad band in the gel is consistent with the production of many fragments from the intact iodinated molecule due to changes in enzymatic degradation.
Additional evidence supports the concept that iodine affects Tg by changing the shape or stability of the molecule. Electron microscopy showed that iodinated Tg has a compact appearance, whereas Tg with no iodine had a porous cylindrical structure [31]. Furthermore, iodine-induced changes in the conformation of Tg molecule may make it more resistant to enzymatic degradation, as Lamas & Ingbar showed that highly iodinated Tg was less susceptible than normally iodinated Tg to hydrolysis by intrathyroidal proteases [32]. Changes in the conformation of Tg molecule following iodination might result from addition of iodine to thyronines, tyrosines, and even other amino acids such as cysteines and histidines [33].
The reactivity of the three other MoAbs (137C1, 41A5 and 133B1) allowed us to look for specific alterations as well as more general changes in the shape of the Tg molecule. An example is illustrated by the 15-kD peptide recognized by MoAb 137C1. This peptide was present in Western immunoblot pattern of normal Tg and INeg-Tg, but was absent from the immunoblot pattern of I+-Tg (Fig. 4), suggesting that iodination in vitro altered the configuration of this conformational epitope.
Monoclonal antibodies 133B1 and 41A5 also showed differences in recognizing INeg-Tg after iodination. These MoAbs differed from MoAb 42C3 in their precise specificity, since these MoAbs were only partly inhibitable by T3 or T4 [22]. In contrast to MoAb 42C3, MoAb 133B1 reacted with INeg-Tg but failed to react with this Tg if it were iodinated in vitro. Both MoAbs, 42C3 and 133B1, however, recognized physiologically iodinated Tg. These results suggest that in vitro iodination of Tg changes its conformation in such a way that it can still be recognized by MoAb 42C3 but not by MoAb 133B1 (Figs 1, 2 and 4).
Our findings may help us to understand how increased dietary iodine can bestow greater autoantigenic potency to Tg. Iodination of Tg may change the conformation of Tg in a way that affects its uptake and processing by antigen-presenting cells (APC). B cells may be particularly important as APC in recognition of Tg. The binding of Tg to some B cell receptors, like the binding to MoAb 42C3, may be enhanced by iodination [34].
Acknowledgments
This study was funded in part by NIH grant DK-42174 and NIEHS Center Grant ES03819.
References
- 1.Dunn JT. Thyroglobulin: chemistry and biosynthesis. In: Braverman LE, Utiger RD, editors. The thyroid. 7. Philadelphia: Lippincott-Raven; 1996. pp. 85–95. [Google Scholar]
- 2.Beierwaltes WH. Iodine and lymphocytic thyroiditis. Bull All India Med Sci. 1969;3:145. [Google Scholar]
- 3.Weaver DK, Batsakis JG, Nishiyama RH. Relationship of iodine to ‘lymphocytic goiters’. Arch Surg. 1969;98:183–6. doi: 10.1001/archsurg.1969.01340080075014. [DOI] [PubMed] [Google Scholar]
- 4.Hay ID. Thyroiditis: a clinical update. Mayo Clin Proc. 1985;60:836–43. doi: 10.1016/s0025-6196(12)64789-2. [DOI] [PubMed] [Google Scholar]
- 5.Tajiri J, Higashi K, Morita M, Umeda T, Sato T. Studies of hypothyroidism in patients with high iodine intake. J Clin Endocrinol Metab. 1986;63:412–7. doi: 10.1210/jcem-63-2-412. [DOI] [PubMed] [Google Scholar]
- 6.Braverman LE. Effects of iodine on thyroid function in man. (Review) Trans Am Clin Climatol Assoc. 1990;102:143–51. [PMC free article] [PubMed] [Google Scholar]
- 7.Bagchi N, Brown TR, Urdanivia E, Sundick RS. Induction of autoimmune thyroiditis in chickens by dietary iodine. Science. 1985;230:325–7. doi: 10.1126/science.4048936. [DOI] [PubMed] [Google Scholar]
- 8.Sundick RS, Herdegen DM, Brown TR, Bagchi N. The incorporation of dietary iodine into thyroglobulin increases its immunogenicity. Endocrinology. 1987;120:2078–84. doi: 10.1210/endo-120-5-2078. [DOI] [PubMed] [Google Scholar]
- 9.Allen EM, Appel MC, Braverman LE. The effect of iodide ingestion on the development of spontaneous lymphocytic thyroiditis in the diabetes-prone BB/W rat. Endocrinology. 1986;118:1977–81. doi: 10.1210/endo-118-5-1977. [DOI] [PubMed] [Google Scholar]
- 10.Allen EM, Braverman LE. The effect of iodine on lymphocytic thyroiditis in the thymectomized buffalo rat. Endocrinology. 1990;127:1613–6. doi: 10.1210/endo-127-4-1613. [DOI] [PubMed] [Google Scholar]
- 11.Rasooly L, Burek CL, Rose NR. Iodine-induced autoimmune thyroiditis in NOD-H-2h4 mice. Clin Immunol Immunopathol. 1996;81:287–92. doi: 10.1006/clin.1996.0191. [DOI] [PubMed] [Google Scholar]
- 12.Follis RH. Further observations on thyroiditis and colloid accumulation in hyperplastic thyroid glands of hamsters receiving excess iodine. Lab Invest. 1964;13:1590–9. [PubMed] [Google Scholar]
- 13.Champion BR, Page KR, Parish N, et al. Identification of a thyroxine-containing self-epitope of thyroglobulin which triggers thyroid autoreactive T cells. J Exp Med. 1991;174:363–70. doi: 10.1084/jem.174.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchings P, Cooke A, Dawe K, et al. A thyroxine-containing peptide can induce murine experimental autoimmune thyroiditis. J Exp Med. 1992;175:869–72. doi: 10.1084/jem.175.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong YC, McCormick DJ, Wan Q, et al. Primary hormonogenic sites as conserved autoepitopes on thyroglobulin in murine autoimmune thyroiditis. Secondary role of iodination. J Immunol. 1995;155:5847–54. [PubMed] [Google Scholar]
- 16.Rasooly L, Rose NR, Saboori AM, Ladenson PW, Burek CL. Iodine is essential for human T cell recognition of human thyroglobulin. Autoimmunity. 1998;27:213–9. doi: 10.3109/08916939808993833. [DOI] [PubMed] [Google Scholar]
- 17.Saboori AM, Rose NR, Burek CL. Iodination of human thyroglobulin (Tg) alters its immunoreactivity. II. Fine specificity of a monoclonal antibody that recognizes iodinated Tg. Clin Exp Immunol. 1998;113:303–8. doi: 10.1046/j.1365-2249.1998.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry M, Zanelli E, Piechaczyk M, Pau B, Malthiery Y. A major human thyroglobulin epitope defined with monoclonal antibodies is mainly recognized by human autoantibodies. Eur J Immunol. 1992;22:315–9. doi: 10.1002/eji.1830220205. [DOI] [PubMed] [Google Scholar]
- 19.Henry M, Malthiery Y, Zanelli E, Charvet B. Epitope mapping of human thyroglobulin. Heterogeneous recognition by thyroid pathologic sera. J Immunol. 1990;145:3692–8. [PubMed] [Google Scholar]
- 20.Bresler HS, Burek CL, Hoffman WH, Rose NR. Autoantigenic determinants on human thyroglobulin: II. Determinants recognized by autoantibodies from patients with chronic autoimmune thyroiditis compared to autoantibodies from healthy subjects. Clin Immunol Immunopathol. 1990;54:76–86. doi: 10.1016/0090-1229(90)90007-d. [DOI] [PubMed] [Google Scholar]
- 21.Malthiery Y, Henry M, Zanelli E. Epitope mapping of human thyroglobulin reveals a central immunodominant region. FEBS Letters. 1991;279:190–2. doi: 10.1016/0014-5793(91)80146-t. [DOI] [PubMed] [Google Scholar]
- 22.Bresler HS, Burek CL, Rose NR. Autoantigenic determinants on human thyroglobulin: I. Determinant specificities of murine monoclonal antibodies. Clin Immunol Immunopathol. 1990;54:64–75. doi: 10.1016/0090-1229(90)90006-c. [DOI] [PubMed] [Google Scholar]
- 23.Saboori AM, Rose NR, Burek CL. Amino acid sequence of a tryptic peptide of human thyroglobulin reactive with sera of patients with thyroid disease. Autoimmunity. 1995;22:87–94. doi: 10.3109/08916939508995304. [DOI] [PubMed] [Google Scholar]
- 24.Saboori AM, Burek CL, Rose NR, Bresler HS, Talor M, Kuppers RC. Tryptic peptides of human thyroglobulin: I. Immunoreactivity with murine monoclonal antibodies. Clin Exp Immunol. 1994;98:454–8. doi: 10.1111/j.1365-2249.1994.tb05512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saboori AM, Rose NR, Butscher WG, Burek CL. Modification of a nonincinerative method for determination of iodine in iodoproteins. Anal Biochem. 1993;214:335–8. doi: 10.1006/abio.1993.1500. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch EF, Maniatis T. Anonymous molecular cloning, a laboratory manual. 2. New York: Cold Spring Harbor Laboratory Press; 1989. Use of chromogenic subsrates with enzyme-coupled antibodies; pp. 18–74. [Google Scholar]
- 27.Saboori AM, Rose NR, Kuppers RC, Butscher WG, Bresler HS, Burek CL. Immunoreactivity of multiple molecular forms of human thyroglobulin. Clin Immunol Immunopathol. 1994;72:121–8. doi: 10.1006/clin.1994.1115. [DOI] [PubMed] [Google Scholar]
- 28.Dunn JT, Ray SC. Variations in the structure of thyroglobulins from normal and goitrous human thyroids. J Clin Endocrinol Metabolism. 1978;47:861–9. doi: 10.1210/jcem-47-4-861. [DOI] [PubMed] [Google Scholar]
- 29.Ametani A, Sercarz EE. The nature of B- and T-cell determinants. Immunol Series. 1993;59:13–28. [PubMed] [Google Scholar]
- 30.Dunn JT, Kim PS, Dunn AD, Heppner DG JR, Moore RC. The role of iodination in the formation of hormone-rich peptides from thyroglobulin. J Biol Chem. 1983;258:9093–9. [PubMed] [Google Scholar]
- 31.Berg G, Ekholm R. Electron microscopy of low iodinated thyroglobulin molecules. Biochim Biophys Acta. 1975;386:422–31. doi: 10.1016/0005-2795(75)90285-8. [DOI] [PubMed] [Google Scholar]
- 32.Lamas L, Ingbar SH. The effect of varying iodine content on the susceptibility of thyroglobulin to hydrolysis by thyroid acid protease. Endocrinology. 1978;102:188–97. doi: 10.1210/endo-102-1-188. [DOI] [PubMed] [Google Scholar]
- 33.Malthiery Y, Lissitzky S. Primary structure of human thyroglobulin deduced from the sequence of its 8448-base complementary DNA. Eur J Biochem. 1987;165:491–8. doi: 10.1111/j.1432-1033.1987.tb11466.x. [DOI] [PubMed] [Google Scholar]
- 34.Champion BR, Hutchings P, Rayner DC, et al. In vitro regulation of thyroglobulin (Tg) autoantibody production by Tg-specific T-cell lines and hybridomas. Immunology. 1991;73:415–20. [PMC free article] [PubMed] [Google Scholar]


