Abstract
The objective of this study was to assess the role of anti-retroviral therapy (ART) on the susceptibility of peripheral blood lymphocytes (PBL) from HIV-1-infected individuals to activation-induced apoptosis and in comparison with changes in CD4 lymphocyte counts. Eleven symptomatic HIV+ patients were studied. Ex vivo apoptosis was measured in phytohaemagglutinin (PHA)-stimulated PBL and CD4 subsets by flow cytometry, at baseline and after 1 month (4–6 weeks) and 2/3 months of ART. Six patients had extended studies of the effects of therapy to a maximum of 21 months. Lymphocyte apoptosis was significantly elevated in HIV+ patients at baseline (median 22% compared with 7.5% in HIV− risk-matched controls; P < 0.05). This decreased to control levels on ART (7.4% at 4–6 weeks, P < 0.01, and 6.2% at 8–12 weeks, P < 0.05, compared with baseline). Similar changes occurred in the CD4+ subpopulation. The decrease in apoptosis was maintained for several months, but the effect was rapidly lost if ART was discontinued. CD4 counts showed a reciprocal relationship to changes in apoptosis. The association of changes in apoptosis with those in CD4 counts suggests a link between programmed cell death and lymphocyte depletion. Apoptosis reduced in some individuals without any reduction in viral load, suggesting apoptosis may be influenced by factors in addition to the overall extent of HIV replication.
Keywords: apoptosis, programmed cell death, anti-retroviral therapy, CD4 lymphocyte, HIV
INTRODUCTION
The demonstration of increased susceptibility of lymphocytes from HIV-infected individuals to apoptosis [1,2] led to speculation that this process may be a significant cause of HIV-related CD4 cell depletion and immune dysfunction [3,4]. In vitro, up to 50% of peripheral blood lymphocytes (PBL) from HIV+ individuals undergo apoptosis in response to stimulation with mitogenic lectins or following mobilization of the T cell receptor by anti-CD3 antibody, specific recall antigens or superantigens. The phenomenon appears largely responsible for the impaired lymphocyte proliferation in HIV infection [1].
However, the role of apoptosis in the immunopathogenesis of HIV disease remains controversial. Although the extent of activation-induced apoptosis correlated with disease progression in some cohorts [5,6], other studies, mainly using unstimulated cells, have not been able to confirm an association [7]. Transient increases in apoptosis have been found in other viral diseases, including Epstein–Barr virus infection [8] and in autoimmune conditions [9]. An alternative explanation for the susceptibility to apoptosis is that it is merely a marker of lymphocyte activation [10,11] or of increased lymphopoiesis [12]. Therapeutic interventions, such as IL-12 [13] and glucocorticoids [14], can modulate HIV-associated apoptosis. However, these agents have significant adverse effects and it is important to define the role of programmed cell death in the pathogenesis of HIV infection before embarking on suppression of the phenomenon.
We therefore investigated the relationship between HIV replication, apoptosis and CD4 depletion, by prospective study of the effects of anti-retroviral agents in HIV+ individuals.
PATIENTS AND METHODS
Patients and controls
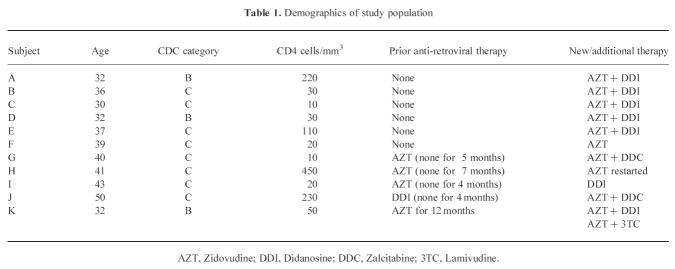
Eleven HIV+ individuals were enrolled. All were Caucasian homosexual men. Three had minor symptoms (Category B disease) and eight major immunodeficiency (Category C disease/AIDS) as defined by 1992 Centre for Disease Control (CDC; Atlanta, GA) criteria. The population demographics and anti-retroviral regimes (all nucleoside analogue inhibitors of viral reverse transcriptase) are shown in Table 1. Six patients were anti-retroviral-naive. Five had prior exposure, in four cases anti-retroviral therapy (ART) had been stopped between 4 and 7 months before commencing new therapy, in one a new agent was added to ongoing treatment. The control group consisted of seven ‘risk-matched’ homosexual men who form part of a cohort followed since 1983 and who are repeatedly HIV− and have normal lymphocyte subpopulations [15].
Table 1.
Demographics of study population
Subjects were clinically assessed at each testing. Any patient receiving medications known to affect apoptosis, such as chemotherapy or systemic corticosteroids, or suffering from an acute opportunist infection, were excluded or study entry postponed for at least 8 weeks after medication was stopped or infection resolved. Those developing minor infections when on the study had testing postponed for 2 weeks. No subject developed a major opportunist (AIDS-defining) infection once on study. Once started, ART was maintained at a stable dosage throughout.
Lymphocyte apoptosis studies and CD4/8 counts were performed at baseline (within 48 h prior to instigation of ART), at 1 month (4–6 weeks maximum limits) and 2–3 months (8–12 weeks maximum limits) of treatment. HIV viral load (VL) was measured at baseline and at 8–12 weeks.
Approval of the local research ethics committee (East London and City Health Authority) was obtained and written informed consent given.
Preparation and culture of peripheral blood mononuclear cells
Mononuclear cells were prepared from heparinized blood by Lymphoprep (Nycomed, Oslo, Norway) centrifugation. Viability of fresh cells by trypan blue exclusion was always > 99%. Cells were cultured at 106/ml in RPMI 1640 medium (Life Technologies) supplemented with 10% fetal calf serum (FCS; TCS Biologicals Ltd), glutamine, penicillin and streptomycin (Sigma, Poole, UK). Activation-induced apoptosis was determined following phytohaemagglutinin stimulation (PHA; Sigma) which was optimal at 10 mg/ml (data not shown). Stimulated cultures were incubated at 37°C, in humidified atmosphere containing 5% CO2.
Measurement of apoptosis
Apoptosis in T cells and CD4/8 subsets was measured by flow cytometry using an adaptation of published methods [16–18]. Cells were repeatedly washed to remove excess PHA and stained with FITC-labelled anti-Leu-3a + 3b (anti-CD4; Becton Dickinson, Cowley, UK). After further washing, cells were fixed for 24 h by gentle re-suspension in cold 50% ethanol. These cells were mixed with a DNA staining solution containing 100 mg/ml propidium iodide (Cambridge BioScience, Cambridge, UK), 10 mm EDTA, 0.1% Triton X-100 and 50 mg/ml RNase (Sigma). Fluorescence was measured using a FACScan (Becton Dickinson) and analysed using Lysis II software. Compensation was used to subtract spectral overlap of both fluorochromes, and doublet discrimination was used to separate single cell events from debris and multicellular aggregates. The apoptotic percentage values (in total lymphocyte populations, or CD4 subpopulations) were derived from the number of events falling below the G0 peak [16,17]. Apoptosis was confirmed by staining fixed cytospin preparations with May–Grünwald–Giemsa stain (BDH, Merck, Poole, UK). Apoptotic cells were recognized by a characteristic shrunken morphology with densely staining nuclei.
Time course of PHA-induced apoptosis
To determine the kinetics of PHA-induced apoptosis, peripheral blood lymphocytes from 14 control subjects and 17 HIV+ individuals were stimulated as above and apoptosis measured prestimulation (0 h) and up to 72 h post-stimulation. This demonstrated that the percentage of cells undergoing apoptosis increased most rapidly in the first 24 h, plateauing by 48–72 h (median values for controls 0.53%, 7.08%, 9.33%, 8.8% and for HIV+ individuals 1.7%, 13.7%, 19.5%, 19.69% at 0, 24 h, 48 h and 72 h, respectively). These observations are similar to those made in controls and HIV+ individuals using other systems of induced apoptosis [19]. A stimulation time of 72 h was used for all subjects in the main study. A single additional patient had studies pre- and post-ART at 24 h post-PHA stimulation to compare with the findings of the longer incubation periods.
To show whether the observed reduction in apoptosis with therapy was reflected in a decrease in overall cell death, concurrent apoptosis and viability (by trypan blue exclusion) were compared at 72 h post-PHA stimulation in five controls and five patients.
Viral load measurement (VL)
HIV VL was quantified by polymerase chain reaction (PCR) using a commercial kit (Amplicor, HIV-1 monitor Test; Roche). PCR amplification of the target cDNA was achieved by reverse transcription of RNA within the patient sample using HIV-specific complementary primers. The amplified products were hybridized to specific probes which were detected colorimetrically. A second target sequence (219 base-pair RNA molecule with primer binding regions identical to that of the HIV-1 target sequence-QS) was added to the patient sample at a known concentration for quantification. The amount of HIV-1 RNA was calculated from the ratio of total optical density (OD) for HIV-1 to the total OD for the QS and input number of QS RNA molecules as follows:
 |
The blood samples were initially taken into heparin and a de-heparinization procedure was required before PCR analysis. This was performed according to the manufacturer's instructions. Plasma (200μl) was mixed with 50 μl buffer (225 mm NaCl, 75 mm Tris–HCl, 15 mm CaCl2, 0.1% bovine serum albumin (BSA), pH 7.5) and 7.5 U heparinase. The sample was incubated at room temperature for 1 h and then processed with the addition of EDTA to the PCR lysis reagent.
These studies were commenced before the widespread availability of VL analysis, therefore only a baseline and single time point could be tested. Eight weeks post-treatment was chosen, as although the most rapid VL decline is in the first 4 weeks, a significant minority of patients respond more slowly. Therefore, testing at 8–12 weeks may better differentiate ‘responders’ from ‘non-responders’.
The interassay variability was 0.3 log10 and a change of 0.5 log10 from baseline was considered significant.
CD4 and CD8 cell counts were performed by flow cytometry using a standard monoclonal panel (Becton Dickinson).
Statistical analysis
Percentage apoptosis, CD4, CD8 counts and VL did not show a normal distribution, therefore median and percentile values (10th and 90th) are used to describe results. Non-parametric statistical analysis (Wilcoxon signed rank test) was used to compare groups (Statview; Abacus Concepts Inc., Berkley, CA) and a significance value of P < 0.05 was used throughout.
RESULTS
Effects of anti-retroviral therapy on lymphocyte apoptosis and viability
In agreement with other studies, the level of activation-induced apoptosis in blood lymphocytes was significantly elevated in symptomatic HIV+ individuals (22% compared with 7.5% in controls; P < 0.05; Table 2 and Fig. 1). Apoptosis was reduced after 4–6 weeks of therapy (median 7.4%, P < 0.01 compared with baseline), the levels declining in 10 of the 11 subjects. This reduction in apoptosis was maintained at 8–12 weeks of treatment (6.2%, P < 0.05 compared with baseline). The levels on ART were no different from those in the HIV− controls. The decline in apoptosis was also reflected in the CD4 subpopulation analysis, levels of 21% at baseline decreasing to 3.1% at 4–6 weeks and 6.3% at 8–12 weeks. The patient in which apoptosis was studied 24 h post-PHA stimulation showed the same response, total lymphocyte apoptosis at 26.45% pre-ART, falling to 5.39% on therapy (figures for CD4 cell apoptosis 33.6% falling to 6.4% and for CD8 cell apoptosis 36.1% falling to 12.09%).
Table 2.
Peripheral blood lymphocyte apoptosis, CD4 counts and viral load changes in relation to anti-retroviral therapy
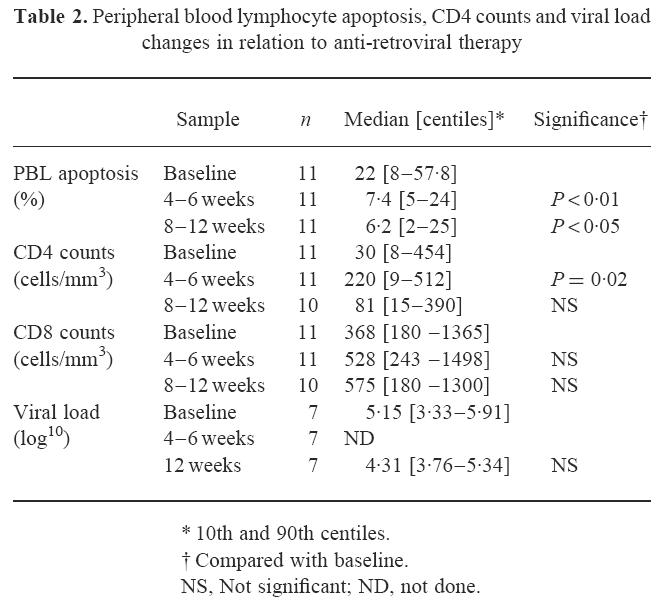
Fig. 1.
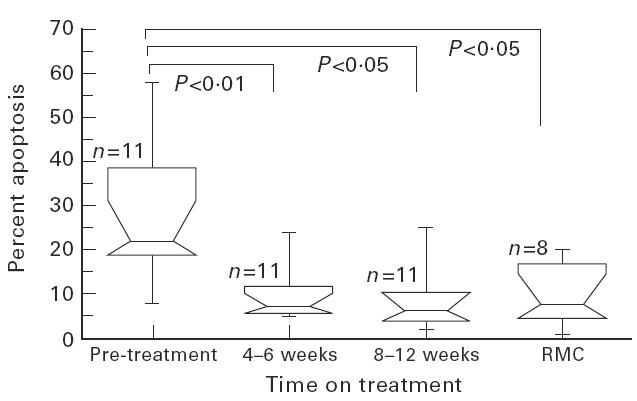
Changes in peripheral blood lymphocyte (PBL) phytohaemagglutinin (PHA)-induced apoptosis at baseline, 4–6 weeks and 8–12 weeks of anti-retroviral therapy (ART). Changes in percentage of PBL undergoing apoptosis following 72 h PHA stimulation are shown in relation to time on ART. Median results with 10th and 90th centiles for 11 patients compared with seven risk-matched controls (RMC) are represented by the boxes, the whiskers representing the ranges.
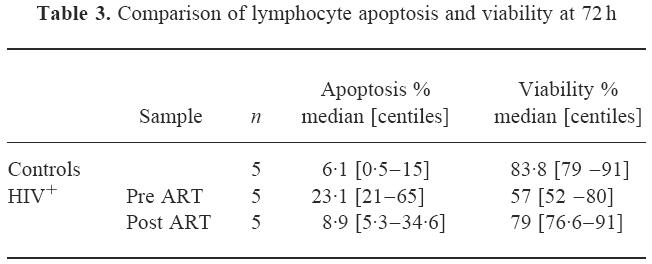
The comparison of viability and apoptosis responses with ART is shown in Table 3. This demonstrated that overall cell death and the apoptosis component decreased together with therapy and that there was not merely a shift from apoptosis to necrosis or metabolic cell death [20], during therapy.
Table 3.
Comparison of lymphocyte apoptosis and viability at 72 h
The effects of anti-retroviral therapy on CD4 and CD8 counts
Over the same time as % apoptosis reduced, CD4 counts showed an initial significant rise (Table 2), from 30 cells/mm3 at baseline, to 220 cells/mm3 (P < 0.05) at 4–6 weeks. However, this fell to 81 cells/mm3 by 8–12 weeks (not significant compared with baseline). The CD8 cell count initially showed a similar pattern to CD4 cells, rising from a median 368–528 cells/mm3 at 4–6 weeks, but being maintained at 575 cells/mm3 at 8–12 weeks. However, none of the CD8 changes reached significance.
Relationship of changes in viral load with lymphocyte apoptosis
Viral load was measured in comparison with apoptosis in seven of the 11 patients, at baseline and at 8–12 weeks of therapy (Table 2). There was an overall reduction in median VL of 0.84 log10, but this was not seen in all patients. Viral load fell > 0.5 log10 in four of the seven individuals; in these subjects PBL apoptosis fell concurrently from a median 30.4% at baseline to 7% at 12 weeks. The three individuals who showed no significant change in VL demonstrated equivalent decreases in lymphocyte apoptosis (median 21.7% at baseline to 7.4% at 12 weeks).
Extended studies of apoptosis and CD4 counts
Six individuals underwent more detailed longitudinal studies of apoptosis, in relation to both the introduction (Fig. 2a) or cessation and re-introduction of ART (Fig. 2b). These show that suppression of apoptosis could be maintained for several months following the initial decrease, but eventually may increase again even when viral suppression appears to be maintained (Fig. 2b, panel vi). Apoptosis increased rapidly on withdrawal of ART but responded to re-introduction or change of drug therapy (Fig. 2b, panels iv, v and vi). Additionally, on an individual patient basis, changes in levels of apoptosis with ART showed a close inverse relationship to changes in CD4 counts (Fig. 2a, b panels i, ii, iii, iv and v).
Fig. 2.
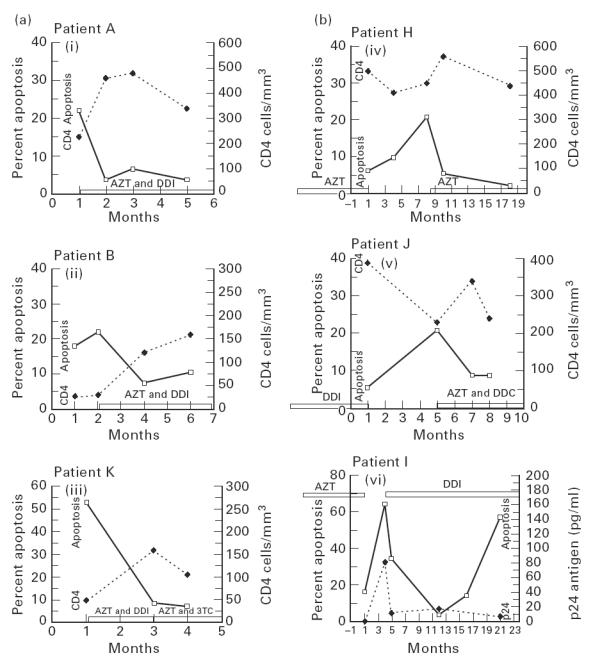
(ai,ii,iii) Longitudinal study of apoptosis and CD4 counts in relation to commencing antiretroviral therapy (ART). Changes in peripheral blood lymphocyte (PBL) apoptosis (after 72 h phytohaemagglutinin (PHA) stimulation) and absolute CD4 counts in relation to the instigation and continuation of ART are shown in three HIV+ patients. (biv,v,vi) Longitudinal study of apoptosis, CD4 counts and HIV p24 antigen in relation to withdrawal and re-introduction of ART. Changes in PBL apoptosis (after 72 h PHA stimulation) and absolute CD4 counts in relation to the withdrawal and re-introduction of ART are shown in two HIV+ patients (panels iv and v) and apoptosis and p24 antigen in one HIV+ patient (panel vi).
DISCUSSION
The study has shown reversal of HIV-associated susceptibility to lymphocyte apoptosis in response to ART. Despite some degree of immunodeficiency, the patients were clinically stable for at least 8 weeks before introduction of therapy. Thus, the changes in apoptosis were unlikely to be due to the effects of intercurrent infections, and the consistent normalization of apoptosis observed in 10 of the 11 subjects suggests that the anti-retroviral drugs were responsible. Can the mechanism of programmed cell death in HIV infection be inferred from our findings? The maximum change in apoptosis with ART occurred within the first 4–6 weeks, over the same time course expected for the most rapid reduction in VL [21,22], suggesting that apoptosis may be directly linked to viral replication. Although VL data were not available for this early period, it is likely that our patients would have shown similar changes to those observed in previous studies, as the degree of VL decline observed at 8–12 weeks was comparable. Direct viral cytopathicity is unlikely to account for in vivo apoptosis, which mainly affects ‘by-stander’ non-infected lymphocytes [23], but soluble viral products may have a role. The regulatory protein tat induces apoptosis [24], partly by down-regulation of the anti-apoptotic molecule bcl-2 [25], which is also cleaved by the virally coded protease [26]. In addition, envelope gp120 cross-links lymphocyte surface CD4, priming through p56lck for apoptosis in response to subsequent signalling through the T cell receptor [27,28], and gp120 presented by monocytes in association with antigen causes a similar effect on lymphocytes [29]. A reduction in the levels of these products as a result of ART could have caused the decrease in activation-induced cell death.
If we propose that the changes in apoptosis are related to viral replication, then an explanation is needed for the observation that apoptosis was reduced even in those individuals who did not show a marked change in VL. This could be because the VL test is too insensitive to detect a minor reduction in virus or its products, that is nevertheless sufficient to reduce priming for apoptosis. Alternatively, selective suppression of more immunopathogenic quasi-species of HIV occurs, which affect apoptosis, but make up too small a proportion of total virus to change overall plasma VL. Finally, it is possible that some individuals had an early decline in VL with associated reduction in apoptosis, but that viral replication rapidly resumed and by 8 weeks the VL had returned to baseline levels.
ART could also influence apoptosis indirectly. Abherent cytokine production leading to Fas receptor up-regulation [30] and dysregulation of CD28 and β-integrin-mediated costimulation, which normally protect or salvage lymphocytes from apoptosis, are suggested causes of programmed cell death in HIV infection [31]. It has been shown that anti-HIV therapy leads to normalization of lymphocyte cytokine production and inositol signalling pathways. However, this appears a less likely explanation for our observations, as such functional improvements occur later in the course of therapy, progressively over a 3–12-month period ([32] and Dr K. Nye, personal communication), whereas the peak of apoptosis response was at 4–6 weeks, with no further improvement.
It is also important to consider whether the drugs could have an anti-apoptosis effect outside of the anti-HIV action. The patients were on nucleoside analogue reverse transcriptase inhibitors which have cross-reactivity with cellular DNA γ-polymerases and could potentially affect cellular mechanisms controlling programmed cell death. However, studies of the effects of protease inhibitors which use a different mechanism to inactivate HIV have shown the same effect on apoptosis with exactly the same time course (personal observations).
The mirroring of changes in apoptosis with peripheral blood CD4 counts during ART is of interest. From these data it could be postulated that the most likely initial rapid increase consistently observed in CD4 counts on ART is due to decreased cell loss from programmed cell death. Alternatively, the increase may be due to mobilization from previously productively infected tissue sites or as a result of enhanced lymphocyte production when the suppressive effects of HIV are removed, with a coincidental reduction in apoptosis due to other factors. However, in other situations where there is an increase in lymphopoiesis, such as following bone marrow engraftment, increases in lymphocyte counts are associated with increased susceptibility to apoptosis in the relatively immature re-populating cells [12]. The increase in CD4 cell counts during the first 4 months of anti-HIV therapy have been shown to be due to increased numbers of CD45RO+ mature memory lymphocytes and presumed to be due to expansion of a pre-existing memory cell repertoire. Re-population with l-selectin+ naive CD45RA+ cells only starts to appear after ≈ 4 months of therapy [33].
This study was limited in that we only investigated patients who had already developed clinical immunodeficiency, as levels of apoptosis in asymptomatic persons may be low [5] and would have made changes with therapy difficult to assess. However, it is important to the understanding of the immunopathogenesis of HIV disease that future investigations are performed to determine the effects of ART on susceptibility to apoptosis earlier in the course of HIV infection. A further limitation of the study was that the numbers of patients were small. A larger group would be needed to clarify the relationship of ART and decreases in VL with changes in apoptosis. More recently developed techniques for the early detection of activation-induced cell death, such as flow cytometric analysis of apoptosis-associated phosphatidlyserine by the binding of FITC-labelled Annexin, may be useful for the study of large numbers of patients. Monitoring of the immunological effects of ART is becoming of increasing importance in guiding therapy for patients who are unable to ‘respond’ to ART in terms of an acceptable reduction in VL, or whose VL is climbing following an initial response. It is recognized that the clinical benefit of ART may outlive the virological effects. Monitoring of immunological parameters, such as apoptosis, may allow the most economical use of the relatively small numbers of combination regimes currently available.
Acknowledgments
Financial support was provided by the Joint Research Board of St Bartholomew's Hospital for N.J. We would like to thank Dr J. Anderson and Professor A. J. Pinching for allowing us to study their patients, Dr L. Riddell and Ms S. Hill for recruiting patients to the study, and Ms J. Norman for performing the viral load assays.
REFERENCES
- 1.Groux H, Torpie RG, Monte D, et al. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992;175:331–40. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyaard L, Otto SA, Jonker RR, et al. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–9. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 3.Ameissen JC, Capron A. Cell dysfunction and deletion in AIDS: the programmed cell death hypothesis. Immunol Today. 1991;12:102–5. doi: 10.1016/0167-5699(91)90092-8. [DOI] [PubMed] [Google Scholar]
- 4.Morrow WJW, Parkin JM. Autoimmune mechanisms in the pathogenesis of HIV infection. In: Morrow WJW, Haigwood NL, editors. HIV molecular organization, pathogenesis and treatment. Amsterdam: Elsevier Science Publishers B.V.; 1993. Chapter 4. [Google Scholar]
- 5.Pandolfi F, Pierdominci M, Oliva A, et al. Apoptosis-related mortality in vitro of mononuclear cells from patients with HIV infection correlates with disease severity and progression. J AIDS. 1995;9:450–8. [PubMed] [Google Scholar]
- 6.Gougeon ML, Lecouer H, Dulioust A, et al. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–20. [PubMed] [Google Scholar]
- 7.Meyaard L, Otto SA, Keet IPM, et al. Programmed death of T cells in human immunodeficiency virus infection. No correlation with progression to disease. J Clin Invest. 1994;93:982–8. doi: 10.1172/JCI117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uehara T, Miyawaki T, Ohta K, et al. Apoptotic cell death of primed CD45RO+ T lymphocytes in Epstein–Barr virus-induced infectious mononucleosis. Blood. 1992;80:452–8. [PubMed] [Google Scholar]
- 9.Emlen W, Niebur J, Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1994;152:3685–92. [PubMed] [Google Scholar]
- 10.Radvanyi LG, Mills GB, Miller RG. Re-ligation of the T cell receptor after primary activation of mature T cells inhibits proliferation and induces apoptotic cell death. J Immunol. 1992;150:5704–15. [PubMed] [Google Scholar]
- 11.Wesselborg S, Jansen O, Kabelitz D. Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells. J Immunol. 1993;150:4338–45. [PubMed] [Google Scholar]
- 12.Donnenberg AD, Margolick JB, Beltz LA, et al. Apoptosis parallels lymphopoiesis in bone marrow transplantation and HIV disease. Res Immunol. 1995;146:11–21. doi: 10.1016/0923-2494(96)80236-7. [DOI] [PubMed] [Google Scholar]
- 13.Estaquier J, Idziorek T, Zou W, et al. T helper type 1/T helper type 2 cytokines and T cell death: preventative effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J Exp Med. 1995;182:1759–67. doi: 10.1084/jem.182.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu W, Salerno-Goncalves R, Yuan J, et al. Glucocorticoids rescue CD4+ T lymphocytes from activation-induced apoptosis triggered by HIV-1: implications for pathogenesis and therapy. AIDS. 1995;9:35–42. doi: 10.1097/00002030-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Lau RKW, Hill A, Jenkins P, et al. Eight year prospective study of HIV infection in a cohort of homosexual men—clinical progression, immunological and virological markers. Int J STD AIDS. 1992;3:261–6. doi: 10.1177/095646249200300406. [DOI] [PubMed] [Google Scholar]
- 16.Nicolettei I, Migliorati G, Pagliacci MC, et al. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1992;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 17.Darzynkiewicz Z, Bruno S, Del Bino G, et al. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- 18.Johnson N, Parkin J. Dysregulation of the interleukin-2 receptor α- and β- chain in CD4 and CD8 T cells in HIV infection. Clin Cytometry. 1997;30:289–95. [PubMed] [Google Scholar]
- 19.Oyaizu N, McCloskey TW, Coronesi M, et al. Accelerated apoptosis in peripheral blood mononuclear cells (PBMCs) from human immunodeficiency virus type 1 infected patients and in CD4 cross-linked PBMCs from normal individuals. Blood. 1993;82:3392–400. [PubMed] [Google Scholar]
- 20.Bofill M, Fairbanks LD, Ruckemann K, Lipman M, Simmonds HA. T-lymphocytes from AIDS pateints are unable to synthesize ribonucleotides de novo in response to mitogenic stimulation. J Biol Chem. 1995;270:29690–7. [PubMed] [Google Scholar]
- 21.Wei X, Ghosh SK, Taylor M, et al. Viral dynamics in human immunodeficiency type 1 infection. Nature. 1995;373:117–22. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 22.Ho DD, Neumann AU, Pereison AS, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–6. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 23.Finkel TH, Tudor-Williams G, Banda NK, et al. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nature Med. 1995;1:129–34. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 24.Li CJ, Friedman DJ, Wang C, et al. Induction of apoptosis in uninfected lymphocytes by HIV tat protein. Science. 1995;268:429–31. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 25.Sastry KJ, Marin MC, Nehete PN, et al. Expression of human immunodeficiency virus type 1 tat results in down-regulation of bcl-2 and induction of apoptosis in haemopoietic cells. Oncogene. 1996;13:487–93. [PubMed] [Google Scholar]
- 26.Strack PR, Frey MW, Rizzo CJ, et al. Apoptosis mediated by HIV protease is preceded by cleavage of bcl-2. PNAS. 1996;93:9571–6. doi: 10.1073/pnas.93.18.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banda NK, Bernier J, Kurahara DK, et al. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J Exp Med. 1992;176:1099–106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corbeil J, Tremblay M, Richman DD. HIV-induced apoptosis requires the CD4 receptor cytoplasmic tail and is accelerated by interaction of CD4 with p56lck. J Exp Med. 1996;183:39–48. doi: 10.1084/jem.183.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cottrez F, Manca F, Dalgleish AG, et al. Priming of human CD4+ antigen-specific T cells to undergo apoptosis by HIV-infected monocytes: a two-step mechanism involving the gp120 molecule. J Clin Invest. 1997;99:257–66. doi: 10.1172/JCI119154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gehri R, Hahn S, Rother M, et al. The Fas receptor in HIV infection: expression on peripheral blood lymphocytes and role in the depletion of T cells. AIDS. 1996;10:9–16. [PubMed] [Google Scholar]
- 31.Ng TT, Kanner SB, Humphries MJ, et al. The integrin-triggered rescue of T lymphocyte apoptosis is blocked in HIV-1-infected individuals. J Immunol. 1997;158:2984–99. [PubMed] [Google Scholar]
- 32.Nye KE, Knox KA, Pinching AJ. Lymphocytes from HIV-infected individuals show aberrant inositol polyphosphate metabolism which reverses after zidovudine therapy. AIDS. 1991;5:413–7. doi: 10.1097/00002030-199104000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostatsis and function in advanced HIV disease. Science. 1997;277:112–6. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]