Abstract
To investigate the role of GM-CSF in asthmatic airways inflammation, we have targeted GM-CSF transgene to the airway cells in a mouse model of ovalbumin (OVA)-induced allergic airways inflammation, a model in which there is marked induction of endogenous IL-5 and IL-4 but not GM-CSF. Following intranasal delivery of a replication-deficient adenoviral gene transfer vector (Ad), transgene expression was found localized primarily to the respiratory epithelial cells. Intranasal delivery of 0.03 × 109 plaque-forming units (PFU) of AdGM-CSF into naive BALB/c mice resulted in prolonged and compartmentalized release of GM-CSF transgene protein with a peak concentration of ≈ 80 pg/ml detected in bronchoalveolar lavage fluid (BALF) at day 7, but little in serum. These levels of local GM-CSF expression per se resulted in no eosinophilia and only a minimum of tissue inflammatory responses in the lung of naive mice, similar to those induced by the control vector. However, such GM-CSF expression in the airways of OVA-sensitized mice resulted in a much greater and sustained accumulation of various inflammatory cell types, most noticeably eosinophils, both in BALF and airway tissues for 15–21 days post-OVA aerosol challenge, at which times airways inflammation had largely resolved in control mice. While the levels of IL-5 and IL-4 in BALF and the rate of eosinophil apoptosis were found similar between different treatments, there was an increased number of proliferative leucocytes in the lung receiving GM-CSF gene transfer. Our results thus provide direct experimental evidence that GM-CSF can significantly contribute to the development of allergic airways inflammation through potentiating and prolonging inflammatory infiltration induced by cytokines such as IL-5 and IL-4.
Keywords: GM-CSF, airways inflammation, eosinophilia, apoptosis, gene transfer
INTRODUCTION
It is now well recognized that airways inflammation is a central feature of bronchial asthma. The severity of asthma is correlated with the degree of inflammation, particularly the degree of eosinophil and lymphocyte infiltration [1–4]. While eosinophilia is considered a prominent feature of airways inflammation [1,2,5,6], the numbers of other types of leucocytes, including macrophages, lymphocytes and neutrophils, are also increased in asthmatic airways [1,7–9]. The mechanisms underlying the sustained accumulation of these inflammatory cells remain incompletely understood. Among a vast array of contributing soluble mediators are GM-CSF and IL-5. While these two cytokines are two members of the same haematopoietic growth factor family, GM-CSF differs from IL-5 in that it can be released from and act upon a much wider range of inflammatory cell types [10]. Thus, GM-CSF has been considered to play an important role in the initiation, co-ordination and maintenance of these cellular responses. Indeed, abundant in vitro studies have demonstrated a powerful anti-apoptotic and activating effect by GM-CSF on eosinophils, neutrophils and monocytes as well as lymphocytes [10–14], and markedly elevated levels of GM-CSF were often detected, along with IL-5, IL-4 and IL-3, in bronchoalveolar lavage fluid (BALF), serum and lung tissues of asthmatic patients [15–20]. However, the precise mode by which GM-CSF contributes to the pathogenesis of allergic airways inflammation in vivo remains largely speculative.
Recently, a number of experimental models have been established for dissection of molecular mechanisms of asthmatic airways inflammation. In this regard, a mouse model of ovalbumin (OVA)-induced allergic airways inflammation has been increasingly utilized. Using this model, we recently characterized both cellular and cytokine responses in the lung, peripheral blood and bone marrow [21]. We found that while this model duplicated many aspects of human asthmatic airways inflammation, including increased contents of IL-5 and IL-4 in BALF and peripheral blood upon antigen sensitization and challenge, little GM-CSF was induced [21]. In our current study, we have chosen to use this mouse model as a pseudo-GM-CSF-deficient model for investigation of the role of GM-CSF in allergic airways inflammation by means of transferring murine GM-CSF transgene into airways lining epithelial cells in OVA-sensitized and challenged mice. This gene transfer approach allows us to achieve prolonged continuous expression of GM-CSF by the airway cells, thus closely mimicking the events occurring during asthmatic airways inflammation.
MATERIALS AND METHODS
Animals
Female BALB/c mice (6–8 weeks of age) were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN) and maintained under Level B pathogen-free housing conditions in a 12-h light–dark cycle. Level B is an access-restricted area where all cages, food and bedding are autoclaved, and all manipulations of mice were carried out in a laminar flow hood by gloved and masked personnel only. All the experiments described herein were approved by the Animal Research Ethics Board of McMaster University.
Antigen sensitization and challenge protocol
The sensitization and challenge protocols that we used were described previously [21]. Briefly, mice were sensitized intraperitoneally with 0.5 ml of 8 μg of OVA (Sigma Chemical Co., St Louis, MO) which was adsorbed overnight at 4°C to 4 mg of aluminium hydroxide (alum) (Aldrich Chemical Co., Milwaukee, WI) in PBS at days −17 and −12. Twelve days after the second sensitization (day 0), the mice were placed in a Plexiglas chamber (10 × 15 × 25 cm) and exposed to aerosolized OVA (5 mg/ml in 0.9% saline) challenge twice 4 h apart, with each lasting for 1 h. The aerosolized OVA was produced by a Bennet nebulizer at a flow rate of 10 l/min.
Delivery of adenoviral constructs into the lung
Recombinant replication-deficient adenoviral constructs that were used in this study include Ad5E1PACCMVmGM-CSF (AdGM-CSF) expressing murine GM-CSF transgene, AdCMVLacZ (AdLacZ) expressing β-galactosidase (β-Gal), and Add170-3 which does not contain any transgene and was used as a control vector. The construction of these vectors has previously been described by us [22–24]. AdGM-CSF, Add170-3 or AdLacZ were diluted into desired concentrations in a total volume of 30 μl in PBS. Intranasal (i.n.) delivery was carried out as previously described [25]. Briefly, mice were anaesthetized and half of 30-μl virus suspension was applied slowly to the mouse nasal orifice so that the mouse could breathe in the suspension naturally. The second half was applied in the same way thereafter. Dose responses and kinetic studies of adenoviral constructs given intranasally were done in normal mice. For OVA-sensitized mice, adenoviral constructs were delivered intranasally 1 day before the mice received OVA aerosol challenge (day −1). AdLacZ was delivered intranasally into normal mice to determine the localization of adenoviral vector-mediated transgene expression.
Cytochemical staining of lung for β-Gal
β-Gal staining was carried out as previously described [26]. Briefly, 1 day after AdLacZ i.n. delivery, mice were killed and the whole lungs were taken out with trachea and were instilled with 2% formaldehyde containing 0.2% glutaraldehyde in PBS through the trachea. The end of the trachea was ligated with suture and the lungs were placed into a 10-ml tube with the same fixative. After keeping on ice for 60 min, the lungs were washed with PBS twice and then stained with X-gal by instilling 0.3 ml of freshly made staining solution through the trachea at 37°C for 6 h or overnight. Blue stain identified positive reaction product for β-Gal. The lungs were then sliced and put in 70% ethanol for paraffin embedding and sectioning. The sections were counterstained with nuclear fast red.
Sample collection and assessment
At various time points after virus delivery or after antigen challenge, mice were killed and BALF, blood and lung tissues were collected following a previously described procedure [21]. Briefly, blood samples were obtained by retroorbital bleeding and serum was prepared for cytokine measurement by ELISA. For BALF, the lungs were dissected and the trachea was cannulated with a polyethylene tube (Becton Dickinson, Sparks, MD). The lungs were lavaged twice with 0.25 ml and 0.20 ml of PBS, respectively, and ≈ 0.4 ml of the instilled fluid was consistently recovered. After centrifugation of the BALF, the supernatants were collected and stored at −70°C for cytokine measurement. The cell pellet was resuspended with PBS and total cell numbers (TCN) were counted with a haemocytometer. Cytospin smears were prepared from BAL cells and were stained with Hemat-Tek 2000 Slide Stainer (Miles Inc., Elkhart, IN). Differential cell counts in BAL cells were determined by counting at least 500 leucocytes, using standard haemocytologic procedures. The lung tissue was fixed with 10% formalin and embedded in paraffin. Tissue sections were stained with haematoxylin and eosin (H–E) for routine histopathological assessment.
Cytokine measurement
ELISA kits for murine GM-CSF, IL-4 and IL-5 were purchased from Amersham (Aylesbury, UK). The sensitivity of detection for all of these cytokines was 5 pg/ml.
DNA labelling technique and flow cytometry analysis of apoptosis and proliferation
Twelve hours before sacrifice, the mice were injected intraperitoneally with 0.5 ml of 3 mg/ml 5-bromo-2-deoxyuridine (BrdU; Sigma). At days 3 and 9 after OVA challenge when neutrophil infiltration in the lung was minimal, BAL cells from mice receiving either AdGM-CSF or Add170-3 or PBS were pooled, washed and placed into plastic dishes to allow macrophages to adhere to plastic. Non-adherent cells were enriched with eosinophils. One million of these cells from each group were harvested and fixed with ice-cold 70% ethanol at −20°C for at least 60 min. Cell proliferation and apoptosis were analysed by flow cytometry following staining with anti-BrdU–FITC (Becton Dickinson) and propidium iodide (PI; Sigma), respectively [27]. Briefly, 70% ethanol-fixed BAL cells were centrifuged and ethanol removed without disturbing the pellet. The cell membrane was permeabilized and nuclear DNA denatured into single-stranded form with 2 n HCl in 0.5% (v/v) Triton-100 (Sigma) solution for 30 min at room temperature. Excess HCl was neutralized with 0.1 m Na2B4O7 (pH 8.5) and cells were washed with 0.5% Tween 20/1.0% bovine serum albumin (BSA)/PBS solution. The cells were stained with anti-BrdU–FITC for 30 min at room temperature and then with 0.5 ml of 5 μg/ml PI–PBS solution at room temperature for 5 min before analysis. The BrdU immunoreactivity (i.e. anti-BrdU–FITC fluorescence) and total DNA content (i.e. PI fluorescence) were quantitatively measured using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). Apoptotic cells were identified as cells with DNA content less than that of G1 (diploid DNA) cells. BrdU positivity reflects the proliferative state of a cell population in terms of percentage of cells entering cell cycle. The data were presented as percentage of cells undergoing apoptosis as reflected by hypodiploid DNA content and percentage of proliferating cells as reflected by BrdU positivity in the cell population obtained above.
Statistical analysis
Data were expressed as mean ± s.e.m. for groups of 4–6 mice. The significance of differences between experimental groups was determined using a Minitab statistical software package (State College, PA). The difference was considered statistically significant when P ≤ 0.05.
RESULTS
Localization of adenoviral vector-mediated transgene expression in mouse lung
To examine the cell types that could be transfected to express the transgene in mouse lung, an adenoviral vector expression β-Gal was intranasally delivered into mouse lung, and lung tissues were processed for β-Gal histochemical staining 24 h post-gene transfer. Microscopically, transgene protein was mainly localized to bronchial and alveolar epithelial cells and to a lesser degree, alveolar macrophages (data not shown). These findings are consistent with those we have previously observed in rat lung [26]. No staining was found in lungs from mice treated with the control vector Add170-3.
Compartmentalized GM-CSF transgene protein in normal mice lung
To best study the local effect of GM-CSF transgene protein on OVA-induced airways inflammation, we set out to achieve compartmentalized release of GM-CSF transgene protein within the lung without significant leakage into the circulation. These local levels of GM-CSF should be significant but small, so that they by themselves do not induce significant inflammatory responses including airways eosinophilia. We have previously shown that airways and peripheral blood eosinophilia may occur in response to high levels of GM-CSF transgene protein in the lung and significant leakage of local GM-CSF to the circulation [22]. To this end, AdGM-CSF at various doses was delivered intranasally into normal BALB/c mice and both BALF and serum were collected at various time points after virus delivery and assayed for GM-CSF by ELISA. Based on this dose–response study (data not shown), a dose of 0.03 × 109 plaque-forming units (PFU)/mouse was chosen for delivering AdGM-CSF or Add170-3 throughout this study. This dose of AdGM-CSF led to a primarily compartmentalized GM-CSF transgene protein release in the lung for 10–14 days, with a peak of 80 pg/ml measured in the BALF at day 7 and only negligible amounts of GM-CSF measured in the peripheral blood compartment (Fig. 1a). These levels of GM-CSF transgene protein in the lung were considered very significant when compared with the minimal levels of endogenous GM-CSF elicited during OVA-induced allergic airways inflammation (Fig. 1b).
Fig. 1.

(a) Bronchoalveolar lavage fluid (BALF) and serum levels of GM-CSF in normal mice at various time points after intranasal delivery of AdGM-CSF or Add170-3. ○, BALF from AdGM-CSF-treated mice; •, serum from AdGM-CSF-treated mice; □, BALF from Add170-3-treated mice. (b) BALF levels of GM-CSF at various time points in ovalbumin (OVA)-sensitized mice after OVA aerosol challenge. Results are expressed as mean ± s.e.m. from four to six mice per point.
Cellular responses in BALF after i.n. delivery of AdGM-CSF into normal mice
To ensure that the compartmentalized levels of GM-CSF transgene protein we achieved would not induce significant inflammatory responses, we compared cellular responses in BALF collected from mice receiving i.n. delivery of 0.03 × 109 PFU of AdGM-CSF or Addl70-3. Total cell numbers in BALF retrieved from mice receiving AdGM-CSF were similar to those in BALF from mice treated with the same dose of control vector Add170-3 (Fig. 2). Examination of both differential cell counts and lung histology confirmed that this dose of viral vector per se did not cause significant viral-mediated inflammation, and GM-CSF release mediated by AdGM-CSF did not elicit an eosinophilic response in normal mouse lung. Thus, this dose of gene transfer vector was chosen for studying the effect of GM-CSF on allergic airways inflammation in OVA-sensitized/challenged mice.
Fig. 2.

Total cell number (TCN) in bronchoalveolar lavage (BAL) from AdGM-CSF- or Add170-3-treated normal mice. Results are expressed mean ± s.e.m. from four to six mice per point.
Effect of GM-CSF transgene protein on cellular responses in the lung of OVA-sensitized/challenged mice
To examine the effect of GM-CSF on allergic airways inflammation, a mouse model of OVA-induced allergic airways inflammation was set up as previously described [21,25] (Fig. 3). Sensitized mice were instilled intranasally with 0.03 × 109 PFU of AdGM-CSF or Add170-3 or PBS 1 day before OVA aerosol challenge (day −1), and at days 1, 5, 14 and 21 after OVA challenge mice were killed and various samples were collected and analysed (Fig. 3). As shown in Fig. 4a, total cell counts in BAL were significantly higher in mice receiving GM-CSF gene transfer compared with Add170-3- or PBS-treated mice at days 5, 14 and 21 post-OVA aerosol challenge. The control vector had little effect on TCN, which were very similar to those in the PBS-treated group. The number of macrophage/monocytes, lymphocytes or neutrophils in BALF was significantly higher in mouse lung expressing GM-CSF than in PBS- or Add170-3-treated lungs, particularly at days 14 and 21 (Fig. 4b, c, d). Noticeably, GM-CSF transgene gene expression led to a far greater number of eosinophils in BALF than in Add170-3- or PBS-treated mice at day 5 post-OVA challenge (> 100% increase) (Fig. 4e). By days 14 and 21, while the number of eosinophils in BALF of PBS- or Add170-3-treated mice declined close to background, the absolute number of eosinophils was still statistically significantly higher in the lung of mice expressing GM-CSF transgene (Fig. 4e).
Fig. 3.
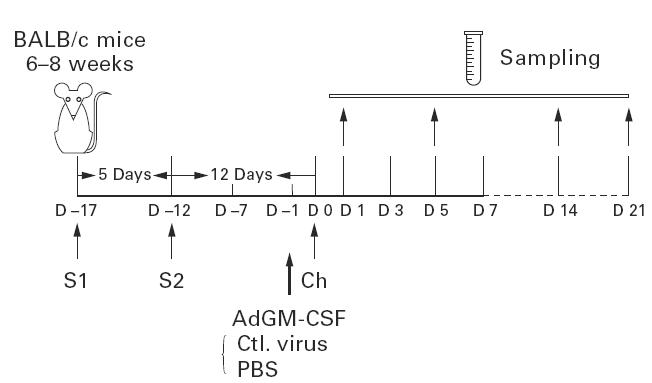
Schematic illustration of experimental protocol. BALB/c mice were intraperitoneally sensitized twice with ovalbumin (OVA) 17 and 12 days before OVA aerosol challenge (S1and S2), and OVA aerosol-challenged 12 days after S2 (day 0; Ch). AdGM-CSF, Add170-3 or PBS was intranasally administered into these mice 1 day before OVA aerosol challenge. Bronchoalveolar lavage fluid (BALF) and lung tissue samples were collected at days 1, 5, 14 and 21 post-OVA aerosol challenge.
Fig. 4.

Effects of AdGM-CSF over-expression in ovalbumin (OVA)-sensitized/challenged mice. Data demonstrate changes in inflammatory cells in bronchoalveolar lavage fluid (BALF) at various time points after OVA aerosol challenge. (a) Total cell number (TCN). (b) Macrophages/monocytes (macro/mono.). (c) Lymphocytes. (d) Neutrophils. (e) Eosinophils. Data are expressed as mean ± s.e.m. from four to six mice/time point. Statistical analysis was performed using Minitab software; *P ≤ 0.05.
Histopathology in the lung after i.n. delivery of AdGM-CSF into OVA-sensitized/challenged mice
To examine histological changes in the lung of OVA-sensitized and challenged mice post-gene transfer, lung tissues were processed and examined. In line with observations with BALF, at day 5 after OVA challenge (or 6 days after i.n. delivery of AdGM-CSF or Add170-3 or PBS), greater airways eosinophilia as well as greater magnitude of inflammatory responses of other cell types were seen in the lung of mice receiving GM-CSF gene transfer than in the lung of PBS- or Add170-3-treated mice (Fig. 5a–c). The tissue responses in PBS-treated mice were similar to those in Add170-3-treated mice. At day 14 after OVA challenge (or 15 days after i.n. delivery of AdGM-CSF or Add170-3), when airways eosinophilia and the overall inflammatory responses largely subsided in the lung of PBS- or Add170-3-treated mice, we still observed substantially greater cellular responses with noticeable eosinophilia in the lungs of mice receiving AdGM-CSF (Fig. 5d,e).
Fig. 5.

Light photomicrographs of paraffin-embedded sections of lung tissues stained with haematoxylin–eosin obtained from ovalbumin (OVA)-sensitized/challenged mice at 6 days (a,b,c) and 15 days (d,e,f) after intranasal delivery of PBS (a,d), or Addl70-3 (b,e), or AdGM-CSF (c,f), i.e. 5 days and 14 days after OVA aerosol challenge. Some of eosinophils are marked by arrows. (Mag. × 200.)
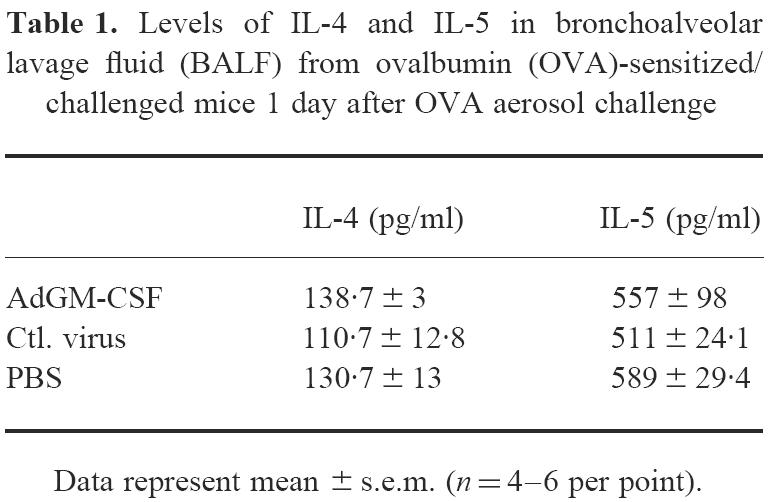
IL-4 and IL-5 levels in BALF from OVA-sensitized/challenged mice after i.n. delivery of AdGM-CSF
To examine whether GM-CSF transgene expression enhanced inflammatory responses through up-regulation of IL-4 and IL-5, the level of IL-4 or IL-5 in BALF collected at day 1 post-OVA challenge was measured. We have previously shown that IL-5 or IL-4 content in BALF peaks at this time post-OVA challenge [21]. We found that IL-4 and IL-5 levels in BALF were not significantly different among different groups (Table 1), suggesting that the effect of GM-CSF was unlikely to be mediated through up-regulation of IL-4 and IL-5 expression.
Table 1.
Levels of IL-4 and IL-5 in bronchoalveolar lavage fluid (BALF) from ovalbumin (OVA)-sensitized/challenged mice 1 day after OVA aerosol challenge
Apoptosis and proliferation of BALF cells from OVA-sensitized/challenged mice after i.n. delivery of AdGM-CSF
Since GM-CSF has been shown to inhibit apoptosis of eosinophils, neutrophils and lymphocytes in vitro [10–14], we examined whether GM-CSF potentiated airways inflammation by inhibiting apoptosis and/or stimulating proliferation of these cells, particularly lymphocytes in the lung of OVA-sensitized/challenged mice. To this end, at days 3 and 9 after OVA aerosol challenge, BALF cells were pooled and non-adherent cells were collected after adherence to plastic dishes. The resulting non-adherent cells consisted of eosinophils (65–75%) and lymphocytes (25–35%) with a minimal number of neutrophils. As shown in Table 2, the extent of apoptosis in these cells from mice receiving GM-CSF gene transfer was similar to that in cells from Add170-3-treated mice at days 3 and 9 after aerosolized OVA challenge. The relatively increased rate of apoptosis at day 9 compared with that at day 3 suggests that apoptosis is one important mechanism by which airways inflammation resolves. Indeed, inflammation begins to wane significantly by 2 weeks after OVA challenge. Thus, the ability of GM-CSF to enhance airways inflammation in this model was unlikely to be mediated through the prevention of apoptosis. However, we observed a > 100% increase in proliferative cells by day 9 in the cell preparation from the lung receiving GM-CSF gene transfer (Table 3). Since eosinophils present in this cell preparation were the end-stage cell types incapable of replication, the cells undergoing proliferation we observed by FACS were probably lymphocytes.
Table 2.
Apoptosis in eosinophil-enriched bronchoalveolar lavage (BAL) cells from ovalbumin (OVA)-sensitized/challenged mice with different treatments (%)

Table 3.
Proliferative responses in eosinophil-enriched bronchoalveolar lavage (BAL) cells from ovalbumin (OVA)-sensitized/challenged mice with different treatments (%)

DISCUSSION
GM-CSF is a member of the haematopoietic cytokine family. In vitro data have shown that GM-CSF is able to promote the proliferation, maturation, survival and activation of myeloid progenitor cells and/or mature granulocytes and monocytes [10,13,14,28–30]. The level of GM-CSF in asthmatic patients was correlated with airways hyperreactivity and eosinophil activation. Systemic steroid treatment significantly reduced this GM-CSF expression [31]. Studies with samples obtained from patients with mild atopic asthma and allergic subjects after segmental endobronchial allergen challenge also demonstrated elevated GM-CSF contents in BALF [15,16,18,19]. Therefore, this cytokine has been thought to play an important role in the pathogenesis of allergic airways inflammation characteristic of asthma. However, how GM-CSF contributes to the pathogenic processes of asthmatic airways inflammation remains to be fully understood.
In order to elucidate the pathogenesis of allergic airways inflammation, we recently established and characterized a mouse model of OVA-induced allergic airways inflammation using Th2-prone BALB/c mice [21]. This model reproduces many important features of asthmatic inflammation, including bronchial hypersensitivity, airways eosinophilic inflammation, IgE response and the generation of Th2 cytokines IL-5 and IL-4. However, different from asthmatic inflammation in humans, the airways inflammatory response in this model is self-limiting, which largely resolves by day 14 post-OVA challenge and, importantly, very little GM-CSF was induced throughout the entire course of OVA sensitization and challenge [21], suggesting that GM-CSF plays a very small, if any, role in the pathogenesis of airways inflammation in this model. While the self-limiting nature of airways inflammation and the lack of GM-CSF response represent two drawbacks with this mouse model, this model provides us with a unique opportunity to study the role of GM-CSF in allergic airways inflammation upon expressing GM-CSF in airways cells by using a transgene approach.
We took advantage of natural tropism of adenoviral vectors for the respiratory epithelium and delivered an adenoviral vector harbouring the murine GM-CSF transgene intranasally into mouse lung. We achieved a significant compartmentalized transgene expression primarily by airways lining cell types which resulted in small, but significant, clinically relevant levels of GM-CSF transgene protein being measurable for about 14 days, mimicking the events occurring during airways inflammation in the lung [22,32]. Of importance, these amounts of GM-CSF by themselves did not induce significant inflammation. These features allowed us to investigate the effect of such GM-CSF expression on allergic airways inflammation induced by IL-5 and IL-4.
We observed that transgenic GM-CSF expression in the lungs of mice that had been sensitized to and subsequently challenged with OVA antigen, led to a much greater and more sustained airways inflammatory response with increased numbers of macrophages, lymphocytes and neutrophils, and most noticeably, eosinophils. These effects of GM-CSF were probably not mediated through further enhancement of IL-4 or IL-5 production, since the BALF contents of IL-4 and IL-5 were similar between different groups. Nor were they mediated through significant inhibition of apoptosis of eosinophils and lymphocytes. The latter finding does not seem to lend support to the contention from in vitro findings [10–14] that the primary role of GM-CSF in airways inflammation is to prolong eosinophil survival. The markedly enhanced and prolonged airways eosinophilia by GM-CSF transgene expression at levels which by themselves did not induce eosinophil accumulation in naive mouse lung, probably occurred due to the direct and indirect effects of GM-CSF on eosinophil recruitment. We have previously shown that two components are critically required for the development of airways eosinophilia, the presence of eosinophil-activating/chemoattractive cytokines locally in the lung, and a peripheral blood eosinophilia caused by circulating eosinopoietic cytokine IL-5 or GM-CSF [21,22,25,32]. Thus, when GM-CSF is only present locally in the lung but not in the circulation, it does not induce airways eosinophilia, as we showed in naive mice expressing compartmentalized GM-CSF. However, when significant GM-CSF is present in the lung during the course of OVA sensitization and challenge, it may directly induce or potentiate airways eosinophilia, since OVA induces markedly heightened circulatory IL-5 and peripheral blood eosinophilia in this model [21,25]. Furthermore, GM-CSF may potentiate airways eosinophilia induced by IL-5 or eosinophil chemokines by priming eosinophils [33]. In this regard, Warringa et al. have reported that at picomolar concentration in vitro, GM-CSF potentiates eosinophil chemotaxis by other chemotactic factors and at nanomolar concentration it has a direct chemotactic effect on eosinophils [29]. GM-CSF is also chemotactic to neutrophils [14]. At this point in time, we cannot rule out the effect of GM-CSF on C-X-C and C-C chemokines, which have been suggested to play an important role locally in the lung in the elicitation of allergic airways inflammation [8]. It is possible that GM-CSF transgene expression enhanced chemokine release during OVA-induced allergic airways inflammation. Such chemokine release may be from activated macrophages and/or lymphocytes. Indeed, we have previously shown that GM-CSF transgene expression in the lung can activate alveolar macrophages [34], and in our current study we have observed an increased number of proliferative lymphocyte populations in the lung post-GM-CSF gene transfer. The effect of GM-CSF on lymphoid cells is probably indirectly mediated through its effect on antigen-presenting cells (APC) and antigen presentation processes. GM-CSF is a potent cytokine capable of enhancing differentiation and activation of APC, particularly dendritic cells [35–37]. Indeed, we have recently observed that GM-CSF over-expression in mouse lung induces a marked increase and activation of dendritic cells. In addition to enhanced lymphocytic proliferation, GM-CSF-activated APC and activated lymphocytes may release enhanced amounts of chemokines active on eosinophils and other leucocytes, thus enhancing airways inflammatory accumulation of various leucocyte subsets that we have observed in our current study.
In conclusion, we have investigated the effect of GM-CSF transgene expression on airways inflammation in a mouse model of antigen-induced asthmatic inflammation where there is marked induction of endogenous IL-5 and IL-4, but not GM-CSF. We found that GM-CSF transgenic expression markedly enhanced and prolonged airways inflammation induced by endogenous Th2 cytokines. These data provide direct experimental evidence that GM-CSF may play a significant role in the development and perpetuation of asthmatic airways inflammation.
Acknowledgments
The technical help of Susanna Goncharova, Rajka Borojevic, Duncan Chong, Xueya Feng and the secretarial assistance of Sara DeSilvio are gratefully acknowledged. This work was funded by the Medical Research Council (MRC) of Canada, Ontario Thoracic Society and Astra Draco AB (Sweden). M.R.S. is holder of a Fellowship of MRC/Canadian Lung Association, M.J. is a Career Scientist of the Ontario Ministry of Health and Z.X. is a scholar of MRC, Canada.
References
- 1.Bochner BS, Undem BJ, Lichtenstein LM. Immunological aspects of allergic asthma. Ann Rev Immunol. 1994;12:295–335. doi: 10.1146/annurev.iy.12.040194.001455. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Chanez P, Vignola AM, Lacoste J-V, Michel FB. Eosinophil inflammation in asthma. Am J Respir Crit Care Med. 1994;150:S33–S38. doi: 10.1164/ajrccm/150.5_Pt_2.S33. [DOI] [PubMed] [Google Scholar]
- 3.Robinson DS, Bently AM, Hartnell A, Kay AB, Durham SR. Activated memory-T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma—relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26–32. doi: 10.1136/thx.48.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker C, Kaegi MK, Braun P, Blaser K. Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. J Allergy Clin Immunol. 1991;88:935–42. doi: 10.1016/0091-6749(91)90251-i. [DOI] [PubMed] [Google Scholar]
- 5.Weller PF. The immunobiology of eosinophils. N Engl J Med. 1991;324:1110–8. doi: 10.1056/NEJM199104183241607. [DOI] [PubMed] [Google Scholar]
- 6.Seminario M-C, Gleich GJ. The role of eosinophils in the pathogenesis of asthma. Curr Opin Immunol. 1994;6:860–4. doi: 10.1016/0952-7915(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 7.Howarth PH, Bradding P, Montefort S, Peroni D, Djukanovic D, Carroll MP, Holgate ST. Mucosal inflammation and asthma. Am J Respir Crit Care Med. 1994;150:S18–S22. doi: 10.1164/ajrccm/150.5_Pt_2.S18. [DOI] [PubMed] [Google Scholar]
- 8.Lukacs NW, Strieter RM, Kunkel SL. Leukocyte infiltration in allergic airway inflammation. Am J Respir Cell Mol Biol. 1995;13:1–6. doi: 10.1165/ajrcmb.13.1.7598934. [DOI] [PubMed] [Google Scholar]
- 9.Poston R, Litchfield P, Chanez P, Lacoste JY, Lee TK, Bousquet J. Immunohistochemical characterization of the cellular infiltration of asthmatic bronchi. Am Rev Respir Dis. 1992;145:918–21. doi: 10.1164/ajrccm/145.4_Pt_1.918. [DOI] [PubMed] [Google Scholar]
- 10.Metcalf D. The granulocyte-macrophage colony stimulating factors. Cell. 1985;43:5–6. doi: 10.1016/0092-8674(85)90004-2. [DOI] [PubMed] [Google Scholar]
- 11.Lopez AF, Williamson J, Gamble JR, et al. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature neutrophil and eosinophil functions, surface receptor expression, and survival. J Clin Invest. 1986;78:1220–8. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owen WF, Rothenberg ME, Silberstein DS, Gasson JC, Stevens RL, Austen KF, Soberman RJ. Regulation of human eosinophil viability, density and function by granulocyte/macrophage colony-stimulating factor in the presence of 3T3 fibroblasts. J Exp Med. 1987;166:129–41. doi: 10.1084/jem.166.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agro A, Jordana M, Chan C, Cox G, Richards C, Stepien H, Stanisz A. Synoviocyte-derived GM-CSF mediates the survival of human lymphocytes. J Rheumatol. 1992;19:1065–9. [PubMed] [Google Scholar]
- 14.Gasson JC. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991;77:1131–45. [PubMed] [Google Scholar]
- 15.Broide DH, Firestein GS. Endobronchial allergen challenge in asthma. Demonstration of cellular source of granulocyte macrophage colony-stimulating factor by in situ hybridization. J Clin Invest. 1991;88:1048–53. doi: 10.1172/JCI115366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broide DH, Paine MM, Firestein GS. Eosinophils express interleukin 5 and granulocyte macrophage-colony stimulating factor mRNA at sites of allergic inflammation in asthmatics. J Clin Invest. 1992;90:1414–24. doi: 10.1172/JCI116008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan S, Broide DH. Compartmentalization of eosinophil granulocyte-macrophage colony-stimulating factor expression in patients with asthma. J Allergy Clin Immunol. 1996;97:966–76. doi: 10.1016/s0091-6749(96)80072-8. [DOI] [PubMed] [Google Scholar]
- 18.Woolley KL, Adelroth E, Woolley MJ, Ellis R, Jordana M, O'Byrne PM. Effects of allergen challenge on eosinophils, eosinophil cationic protein, and granulocyte-macrophage colony-stimulating factor in mild asthma. Am J Respir Crit Care Med. 1995;151:1915–24. doi: 10.1164/ajrccm.151.6.7767540. [DOI] [PubMed] [Google Scholar]
- 19.Kato M, Liu MC, Stealey BA, Friedman B, Lichtenstein LM, Permutt S, Schleimer RP. Production of granulocyte/macrophage colony-stimulating factor in human airways during allergen-induced late-phase reaction in atopic subjects. Lymphokine Cytokine Res. 1992;11:287–92. [PubMed] [Google Scholar]
- 20.Brown PH, Crompton GK, Greening AP. Proinflammatory cytokines in acute asthma. Lancet. 1991;338:590–3. doi: 10.1016/0140-6736(91)90605-o. [DOI] [PubMed] [Google Scholar]
- 21.Ohkawara Y, Lei X-F, Stämpfli MR, Marshall JS, Xing Z, Jordana M. Cytokine and eosinophil responses in the lung, peripheral blood, and bone marrow compartments in a murine model of allergen-induced airways inflammation. Am J Respir Cell Mol Biol. 1997;16:510–20. doi: 10.1165/ajrcmb.16.5.9160833. [DOI] [PubMed] [Google Scholar]
- 22.Xing Z, Ohkawara Y, Jordana M, Graham FL, Gauldie J. Transfer of granulocyte-macrophage colony-stimulating factor gene to rat lung induces eosinophilia, monocytosis, and fibrotic reactions. J Clin Invest. 1996;97:1102–10. doi: 10.1172/JCI118503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Addison CL, Braciak T, Ralston R, Muller WJ, Gauldie J, Graham FL. Intra-tumoral injection of an adenovirus expressing IL-2 induces regression and immunity in a murine breast cancer model. Proc Natl Acad Sci USA. 1995;92:8522–6. doi: 10.1073/pnas.92.18.8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bett AJ, Haddara W, Prevec L, Graham FL. An efficient and flexible system for construction of adenovirus with insertion of deletion in early regions 1 and 3. Proc Natl Acad Sci USA. 1994;91:8802–6. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei X-F, Ohkawara Y, Stämpfli MR, Mastruzzo C, Marr RA, Snider D, Xing Z, Jordana M. Disruption of antigen-induced inflammatory responses in CD40 ligand knock out mice. J Clin Invest. 1998;101:1342–53. doi: 10.1172/JCI1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing Z, Braciak T, Jordana M, Croitoru K, Graham FL, Gauldie J. Adenovirus-mediated cytokine gene transfer at tissue sites: overexpression of IL-6 induces lymphocytic hyperplasia in the lung. J Immunol. 1994;153:4059–69. [PubMed] [Google Scholar]
- 27.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 28.Rivier A, Chanze P, Pène J, Michel FB, Godard Ph, Dugas B, Bousquet J. Modulation of phenotypic and functional properties of normal human mononuclear phagocytes by granulocyte-macrophage colony-stimulating factor. Int Arch Allergy Immunol. 1994;104:27–32. doi: 10.1159/000236705. [DOI] [PubMed] [Google Scholar]
- 29.Warringa RAJ, Koenderman L, Kok PTM, Kreukniet J, Bruijnzeel PLB. Modulation and induction of eosinophil chemotaxis by granulocyte-macrophage colony-stimulating factor and interleukin-3. Blood. 1991;77:2694–700. [PubMed] [Google Scholar]
- 30.Ebisawa M, Liu MC, Yamada T, Kato M, Lichtenstein LM, Bochner BS, Schleimer RP. Eosinophil transendothelial migration induced by cytokines. II. Potentiation of eosinophil transendothelial migration by eosinophil-active cytokine. J Immunol. 1994;152:4590–5. [PubMed] [Google Scholar]
- 31.Lai CKW, Ho SS, Chan CHS, Leung R, Lai KN. Gene expression of interleukin-3 and granulocyte macrophage colony-stimulating factor in circulating CD4+ T cells in acute severe asthma. Clin Exp Allergy. 1996;26:138–46. doi: 10.1111/j.1365-2222.1996.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 32.Xing Z, Braciak T, Ohkawara Y, et al. Gene transfer for cytokine functional studies in the lung: the multifunctional role of GM-CSF in pulmonary inflammation. J Leukoc Biol. 1996;59:481–8. doi: 10.1002/jlb.59.4.481. [DOI] [PubMed] [Google Scholar]
- 33.Resnick MB, Weller PF. Mechanisms of eosinophil recruitment. Am J Respir Cell Mol Biol. 1993;8:349–55. doi: 10.1165/ajrcmb/8.4.349. [DOI] [PubMed] [Google Scholar]
- 34.Xing Z, Tremblay GM, Sime PJ, Gauldie J. Overexpression of GM-CSF induces pulmonary granulation tissue formation and fibrosis by induction of TGF beta1 and myofibroblast accumulation. Am J Pathol. 1997;150:59–66. [PMC free article] [PubMed] [Google Scholar]
- 35.Peters JH, Gieseler R, Thiele B, Steinbach F. Dendritic cells: from ontogenetic orphans to myelomonocytic descendants. Immunol Today. 1996;17:273–7. doi: 10.1016/0167-5699(96)80544-5. [DOI] [PubMed] [Google Scholar]
- 36.Fischer H-G, Frosch S, Reske K, Reske-Kunz AB. GM-CSF activates macrophages derived from bone marrow cultures to synthesis of MHC II molecules and to augmented antigen presentation function. J Immunol. 1988;141:3882–8. [PubMed] [Google Scholar]
- 37.Chang C-H, Furue M, Tamaki K. B7-1 expression of Langerhans cells is up-regulated by proinflammatory cytokines, and is down-regulated by interferon-gamma or by IL-10. Eur J Immunol. 1995;25:394–8. doi: 10.1002/eji.1830250213. [DOI] [PubMed] [Google Scholar]


