Abstract
Inducible nitric oxide synthase (iNOS) and TGF-β were localized by immunocytochemistry in skin lesions from patients across the leprosy spectrum, and from patients undergoing reversal reaction. iNOS expression was highest at the tuberculoid pole of the spectrum, and increased during reversal reaction. TGF-β was observed throughout the leprosy spectrum, but was highest at the lepromatous pole. Levels of TGF-β decreased during reversal reaction. Reduced levels of TGF-β may contribute to unregulated inflammatory responses during reactional episodes.
Keywords: leprosy iNOS, transforming growth factor-beta, immunocytochemistry
INTRODUCTION
Leprosy is a chronic infectious disease caused by Mycobacterium leprae, a bacterial pathogen with a predilection for peripheral nerves and skin macrophages. The disease is seen in a variety of clinical forms which reflect differences in the cell-mediated immune response of the infected individual [1]. The tuberculoid (TT), or paucibacillary, pole of the clinical spectrum is characterized by a strong DTH response to M. leprae antigens, which is effective in eliminating most of the bacteria but at the expense of damage to the local nerves. In contrast, lepromatous (LL), or multibacillary, leprosy is characterized by anergy to M. leprae antigens in skin test and lymphocyte proliferation assays, and by lesions containing high numbers of bacteria. Between these two extreme types of disease are the borderline forms, found in borderline tuberculoid (BT), borderline (BB) and borderline lepromatous (BL) leprosy.
Multidrug treatment of leprosy is very effective in killing leprosy bacilli, but changes in immunological responses can result in serious detrimental clinical sequelae during therapy. Amongst the group of patients suffering from borderline forms of disease, ≈ 15% undergo type I reversal reactions [2]. These episodes are associated with increased cell-mediated immunity towards M. leprae antigens and manifest as acute inflammation and neuritis, which can result in severe and irreversible nerve damage. Patients undergoing reactions are generally treated with immunosuppressants, such as corticosteroids [3]. An important goal of leprosy research is to understand the molecular and cellular basis of reversal reactions, with a view to identifying improved treatments for leprosy.
The immunological mechanisms underlying the differences in patients across the leprosy spectrum have been extensively studied. Skin lesions from TT patients are characterized by a predominance of CD4+ T cells, and the presence of mRNA and protein for cytokines such as interferon-gamma (IFN-γ) [4–7] and IL-2 [8,9] generally associated with the Th1 response. LL lesions, on the other hand, contain fewer lymphocytes, a relatively higher proportion of CD8+ T cells, and Th2 cytokines such as IL-4 and IL-10 [10]. Macrophage activation by Th1 cytokines plays a central role in mycobacterial killing [11,12] and the Th2 bias in lepromatous disease is therefore consistent with the observed multibacillary phenotype. Based on these findings, an obvious model for reversal reactions would involve an increase in the expression of Th1 cytokines, with borderline patients moving towards the tuberculoid pole of the spectrum. A key question is whether the enhanced bacterial killing can be achieved without an exacerbation in pathology.
We, and others [13,14], have previously monitored local expression of tumour necrosis factor-alpha (TNF-α) in reactional lesions. TNF-α plays an important role in macrophage activation but, in high concentrations, is also associated with tissue damage [10,15]. Both mRNA and TNF-α protein were found to be elevated in skin and nerve biopsies from patients undergoing reactions [14]. In the present study, we have examined two additional markers: inducible nitric oxide synthase (iNOS) and TGF-β. iNOS is an enzyme responsible for synthesis of reactive nitrogen radicals which, in the mouse model, have been shown to play a role in killing of mycobacteria [12]. Expression of iNOS is induced during macrophage activation by IFN-γ and TNF-α [16]. TGF-β promotes wound healing, stimulating the synthesis and deposition of extracellular matrix components by fibroblasts [17–19]. TGF-β also antagonizes Th1-mediated activation of macrophages, however, and is thought to play a role in suppression of protective immune responses in tuberculosis patients [20]. We describe the immunocytochemical localization of iNOS and TGF-β in skin biopsies from patients across the leprosy spectrum, and from borderline patients undergoing reversal reaction.
PATIENTS AND METHODS
Patients
Skin biopsies were taken from 33 patients with leprosy attending Dhoolpet Research Centre (Hydrabad, India) and classified clinically and histopathologically according to the Ridley–Jopling criteria [1]. The study included patients from across the leprosy spectrum: tuberculoid (TT, n = 4), borderline tuberculoid (BT, n = 6), borderline lepromatous (BL, n = 1), and lepromatous (LL, n = 7). Fifteen additional borderline leprosy patients (BT and BL) were undergoing reversal reaction (RR) at the time the biopsy was taken. Control tissues included in this study were M. tuberculosis-infected lung tissue and normal skin. The specimens were fixed in formalin/mercuric chloride/acetic acid (FMA) [21] and embedded in paraffin. The bacterial load in each specimen was determined by acid-fast staining and expressed on a logarithmic scale as the bacillary index (BI) [21].
Immunocytochemistry
Antibodies used in the study were directed against iNOS (Transduction Labs, Lexington, KY), TGF-β (Pan-specific) (R&D Systems, Abingdon, UK), platelet-derived growth factor (PDGF) and granulocyte-macrophage colony-stimulating factor (GM-CSF; Genzyme, West Malling, UK), human type 1 procollagen (Chemcon International, Temicula, CA) and macrophage marker (Dako Labs, Detroit, MI). Immunocytochemical staining was performed using the streptavidin–biotin technique. Sections of 5 μm thickness were cut and placed on poly-l-lysine-coated slides. The slides were dried in an oven at 37°C overnight and then deparaffinized and hydrated in sequential gradient of ethanol. Endogenous peroxidase was blocked by immersing the slides in 0.6% hydrogen peroxide (Sigma Chemical Co., St Louis, MO) in methanol for 30 min. The sections were washed in PBS pH 7.2 and were trypsinized (0.5% trypsin and 0.5% chemotrypsin; Sigma) for 10 min for type 1 procollagen and 20 min for other antibodies (iNOS, TGF-β, PDGF, GM-CSF and macrophage marker). Sections were washed in distilled water and then incubated with 5% normal rabbit or swine serum according to the secondary antibody for 30 min. The serum was then drained off and the sections covered with the optimal dilution of the primary antibodies against iNOS (N32020, 1/200 equivalent to 2 μg/ml), TGF-β (AB-100-NA, 1/300, equivalent to 4 μg/ml), PDGF-BB (ZP-215, 1/100, equivalent to 10 μg/ml), GM-CSF (ZM-213, 1/200, equivalent to 5 μg/ml); procollagen-1 (MAB 1912, 1/100) and macrophages (CD68) (EBM11, 1/50) for 1 h at room temperature or overnight at 4°C in a humidifying chamber. The sections were washed with PBS and incubated with biotinylated swine anti-rabbit or rabbit anti-mouse or rabbit anti-rat antibodies (Dako) for 30 min, rinsed in PBS and then incubated with peroxidase-labelled streptavidin (Dako) for 30 min at room temperature. Positive staining was visualized with 3,3′-diaminobenzidine (DAB) substrate (Sigma). The sections were counter-stained with Mayer's haematoxylin, mounted with DPX (BDH, Lutterworth, UK). Controls for the specificity of staining included using non-immune serum and omitting the primary antibody. The slides were scored for intensity in a coded fashion with a semiquantitative grading on a scale of 0–4 and the pattern of staining was recorded.
RESULTS
Immunocytochemical localization of iNOS
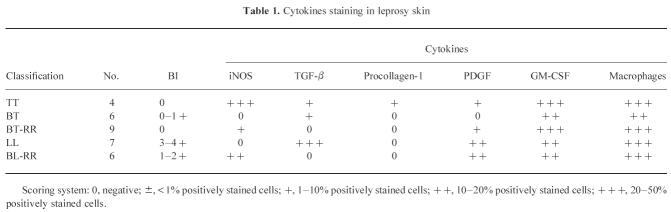
Lesions from TT patients stained strongly for iNOS (Fig. 1a), with up to 50% of cells being positive in sections from four individual patients. Staining was predominantly associated with macrophages and epithelioid cells. In contrast, control biopsies from normal skin, lesions from six BT patients and from seven LL patients (Fig. 1d) were all completely negative with the iNOS antibody. Skin lesions from patients undergoing reversal reactions stained positively for iNOS (Fig. 2a) (13 positive out of 15) and macrophage staining is shown in Fig. 2b. The level of iNOS was broadly similar in biopsies from BT-RR and BL-RR patients, and was lower than that seen in the TT patients. The results are summarized in terms of semiquantitative grading in Table 1.
Fig. 1.
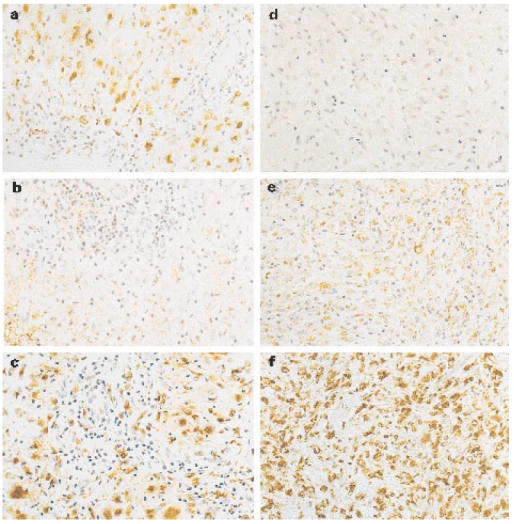
Immunocytochemical staining of inducible nitric oxide synthase (iNOS) and TGF-β in representative lesions from a patient with leprosy. Presence of iNOS (a) strongly positive and TGF-β (b) weakly positive within the tuberculoid granuloma of skin. Absence of iNOS (d) and positive for TGF-β (e) in case of untreated lepromatous leprosy. A section from TT (c) and LL (f) stained with the MoAb PG-M 1 (macrophage marker) indicating large number of positive cells. (× 400.)
Fig. 2.
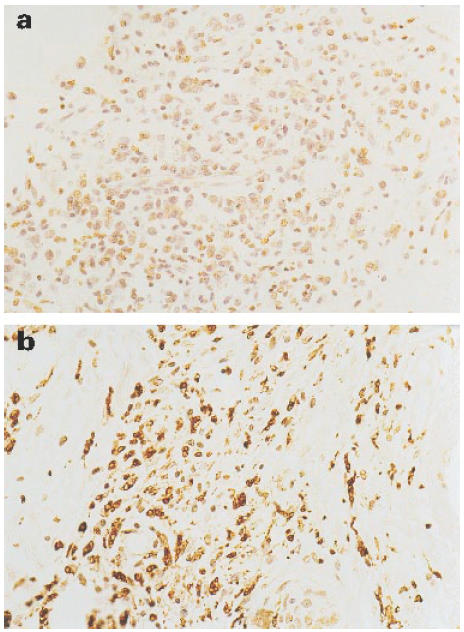
Immunoperoxidase labelling of skin sections from BT in RR patient. (a) Weakly positive staining for inducible nitric oxide synthase (iNOS) in the granuloma. (b) Macrophage marker. (× 400.)
Table 1.
Cytokines staining in leprosy skin
Immunocytochemical localization of TGF-β
While control samples from normal skin were negative, lesions from both TT and LL patients stained positively for TGF-β (Fig. 1b,e). The strongest staining was seen in the LL lesions, with TGF-β localized predominantly within the granuloma rather than the extracellular matrix. Lesions from BT patients were similar to those from TT, with a moderate amount of TGF-β most prominent in the extracellular matrix region. Lesions from BT-RR and BL-RR patients were generally negative for TGF-β, with any staining restricted to the extracellular matrix rather than the granuloma. Interestingly, in two reactional cases, strong TGF-β staining was observed in the perineurium of dermal nerves (Fig. 3a), and macrophage staining is shown in Fig. 3b. The overall results are summarized in Table 1.
Fig. 3.
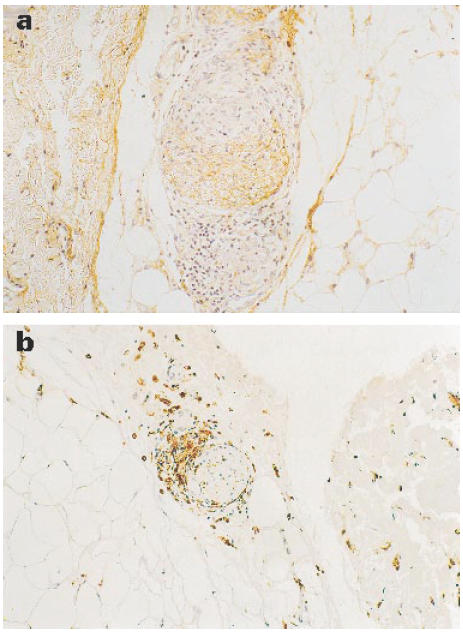
Representative example of dermal nerve showing expression of TGF-β (a) dermal nerve from a BL in RR patient. (b) Macrophage marker. (× 400.)
Analysis of other markers
The extent of macrophage infiltration into the lesions was usually similar throughout the leprosy spectrum (Fig. 1c,f). Antibodies to PDGF and GM-CSF showed similar positive staining in all of the patient biopsies (not shown; data summarized in Table 1). Staining was restricted to the granulomas, and was normally observed in the cytoplasm of mononuclear cells and macrophages in the inflammatory infiltrate. Type-1 procollagen immunostaining was observed in the extracellular matrix of all lesions (not shown; data summarized in Table 1). When uninfected skin was incubated with procollagen antibody, some deposition was observed at perivascular sites; in leprosy lesions, staining was predominantly cellular associated with fibroblasts.
DISCUSSION
iNOS has been shown to play a role in killing of mycobacteria by murine macrophages, but inability to demonstrate its induction in human macrophages has raised questions as to its role in mycobacterial infection in man. A recent report has described expression of iNOS in human alveolar macrophages during tuberculosis infection [22]. The results of our immunocytochemical localization, demonstrating its presence in tuberculoid leprosy lesions, are consistent with a role for iNOS in killing of M. leprae in this form of the disease. Expression of iNOS in reactional lesions is consistent with an activation of bacterial killing mechanisms during reversal reactions. It will be of interest to determine if iNOS expression is activated in cells initially present within the lesion, or whether this is due to an influx of iNOS-positive cells during the reactional episode.
The presence of TGF-β in leprosy lesions has been addressed in two previous studies. Based on analysis of mRNA, it was reported [7] that TGF-β was present in tuberculoid lesions, but absent from lepromatous lesions. In contrast, an immunocytochemical analysis [23] led to the conclusion that TGF-β was present only in the lepromatous form of the disease. Our results suggest that TGF-β can be found throughout the leprosy spectrum, though in greatest abundance at the lepromatous pole. TGF-β has been shown to inhibit iNOS induction by IFN-γ and TNF-α in murine macrophages [24], and its accumulation could play a role in the absence of iNOS in lepromatous lesions. Lesions from patients undergoing reversal reactions were characterized by only very low levels of TGF-β; less than those found at either pole of the spectrum. This finding indicates that TGF-β does not play a direct role in tissue pathology during reactional episodes, although reduction in TGF-β levels might be an important factor in contributing to an unregulated inflammatory response. Two caveats should be pointed out, however. First, we noted isolated instances of TGF-β accumulation in the region of dermal nerves during reaction, and we cannot rule out a role for highly localized TGF-β effects on nerve damage. Second, we have examined biopsies taken during the acute stage of reversal reaction. It is possible that subsequent increases in TGF-β levels could play a role in promoting the late fibrosis that is seen in leprosy-affected peripheral nerves.
The precise molecular events responsible for triggering reversal reactions, and the factors that result in only a subset of patients having to suffer such episodes, remain to be elucidated. Our results would be consistent with a model in which high levels of mycobacterial antigen stimulate expression of high levels of TGF-β and consequent suppression of macrophage activation. A reduction in antigen load as a result of drug treatment could lead to a reduction in TGF-β levels triggering an unregulated inflammatory response. Monitoring of the balance between TGF-β and a proinflammatory cytokine (such as TNF-α) might provide insights into clinical status: a high level of TNF-α in association with a low level of TGF-β might correlate with elevated risk of reaction, for example.
Acknowledgments
We would like to thank all the staff and patients at Dhoolpet Leprosy Research Centre (Hyderabad, India). Dhoolpet Leprosy Research Centre is managed by the UK MRC through Lepra-India. We would like to thank Helen Counihan for excellent technical assistance. This work and S.K-Y. were supported by a grant from LEPRA and Aide aux lepreux Emmaus-Suisse (ALES).
References
- 1.Ridley DS, Jopling WH. Classification of leprosy according to immunity. Int J Lepr. 1966;34:255–73. [PubMed] [Google Scholar]
- 2.Lockwood DNJ, Vinayakumar S, Stanley JNA, McAdam Kpwj, Colston MJ. Clinical features and outcome of reversal (Type 1) reactions in Hyderabad. India Int J Lepr. 1993;61:8–15. [PubMed] [Google Scholar]
- 3.Kiran KU, Stanley JNA, Pearson JMH. The outpatient treatment of nerve damage in patients with borderline leprosy using a semistandardised steroid regimen. Lepr Rev. 1985;56:127–34. doi: 10.5935/0305-7518.19850016. [DOI] [PubMed] [Google Scholar]
- 4.Volc-Platzer B, Stemberger H, Luger T, Radaszkiewicz T, Wiedermann G. Defective intralesional interferon-γ activity in lepromatous leprosy. Clin Exp Immunol. 1988;71:235–40. [PMC free article] [PubMed] [Google Scholar]
- 5.Arnoldi J, Gerdes J, Flad HD. Immunohistological assessment of cytokine production of infiltrating cells in various forms of leprosy. Am J Pathol. 1990;137:749–53. [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper CL, Mueller C, Sinchaisri TA, et al. Analysis of naturally occurring delayed type hypersensitivity reactions in leprosy by in situ hybridisation. J Exp Med. 169:1565–81. doi: 10.1084/jem.169.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–9. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 8.Modlin RL, Hofman FM, Meyer PR, Sharma OP, Taylor CR, Rea TH. In situ demonstration of T lymphocyte subsets in granulomatous inflammation: leprosy, rhinoscleroma and sarcoidosis. Clin Exp Immunol. 1983;51:430–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Longley J, Haregewoin A, Yemaneberhan T, Van Diepen TW, Nsibami J, Knowles D, Smith KA, Godal T. In vivo responses to Mycobacterium leprae; antigen presentation, interleukin-2 production, and immune cell phenotypes in naturally occurring leprosy lesions. Int J Lepr. 1985;53:385–94. [PubMed] [Google Scholar]
- 10.Yamamura M, Wang XH, Ohmen JD, Uyemura K, Rea TH, Bloom BR, Modlin RL. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992;149:1470–5. [PubMed] [Google Scholar]
- 11.Flesch IE, Kaufmann SHE. Mycobacterial growth inhibition by interferon-γ activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987;138:4408–13. [PubMed] [Google Scholar]
- 12.Denis M. Interferon-γ treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991;132:150–7. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan L, Sano S, Pirmez C, et al. Expression of adhesion molecules in leprosy lesions. Infect Immun. 1991;59:4154–60. doi: 10.1128/iai.59.11.4154-4160.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khanolkar-Young S, Rayment N, Brickell PM, Katz DR, Vinayakumar S, Colston MJ, Lockwood DNJ. Tumour necrosis factor-alpha (TNF-α) synthesis is associated with the skin and peripheral nerve pathology of leprosy reversal reactions. Clin Exp Immunol. 1995;99:196–202. doi: 10.1111/j.1365-2249.1995.tb05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kindler V, Sappino AP, Grau GE, Piguet PF, Vasalli P. The inducing role of tumour necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–40. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 16.Flesch IE, Hess JH, Oswald IP, Kaufmann SHE. Growth inhibition of Myco. bovis by IFN-γ stimulated macrophages: regulation by endogenous TNF-α and IL-10. Int Immunol. 1994;6:693–700. doi: 10.1093/intimm/6.5.693. [DOI] [PubMed] [Google Scholar]
- 17.Pierce GF, Mustoe TA, Lingelbach J, et al. PDGF and TGF-β enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989;109:429–40. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalil N, Bereznay M, Sporn M, Greenberg AH. Macrophage production of TGF-β and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989;170:727–37. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Border W, Ruoslahti E. TGF-β in disease: the dark side of tissue repair. J Clin Invest. 1992;90:1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toosi Z, Gogate P, Shiratsuchi H, Young T, Ellner J. Enhanced production of TGF-β by blood monocytes from patients with active tuberculosis and presence of TGF-β in tuberculous granulomatous lung lesions. J Immunol. 1995;154:465–73. [PubMed] [Google Scholar]
- 21.Ridley DS. Skin biopsy in leprosy. 2. Basle: CIBA-GEIGY Ltd; 1985. l. [Google Scholar]
- 22.Nicholson S, Bonecini-Almeida M, Lepa e Silva J, et al. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goulart I, Figueiredo F, Coimbra T, Foss NT. Detection of transforming growth factor-β in dermal lesions of different clinical forms of leprosy. Am J Pathol. 1996;148:911–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Vodovotz Y, Bogdon C, Paik J, Xie Q, Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor-β. J Exp Med. 1993;178:605–13. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]